Abstract
Background: Genetic polymorphisms in the TPMT gene cause inter-individual variability in 6MP metabolism and tolerability. In the context of ALL maintenance therapy (SJCRH Total XII) that was heavily reliant upon 6MP and methotrexate (MTX) and without individualizing 6MP based on TPMT genetics, dose-limiting toxicity due to TPMT defects resulted in interruptions of 6MP and MTX administration, which have been associated with worse ALL outcomes in some studies (McLeod, Br J Haematol 1999; Welch, Cancer Chemother Pharmacol 1996). Overall 6MP dose intensity (i.e. prescribed dose /protocol dose) was 83% (Relling, Blood 1999).
Here we characterized the dose intensity (DI) and tolerance of 6MP among patients with different TPMT genotypes in a more contemporary ALL regimen that included multiple agents in addition to 6MP and MTX. Our goal was to determine the tolerability of 6MP in patients with heterozygous vs wild-type TPMT in the context of multi-agent ALL therapy.
Method: 388 children with ALL on the SJCRH Total XV protocol were evaluable for this study. TPMT genotype was analyzed at day 5 of remission induction using PCR and exome sequencing. Of the 388 patients, 347 (89%) were classified as wild-type and 41 (11%) carried a low activity allele, including *2, *3A, *3C and 677G>A (Udaka, Genet Test 2005). No homozygous deficient patients were identified.
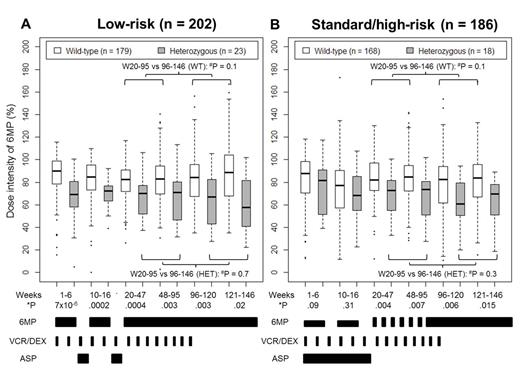
It was generally recommended that patients with TPMT heterozygosity receive a starting 6MP dose of 50-60 mg/m2/d regardless of risk arm. Wild-type patients on the low-risk (LR) arm (n = 202) received daily 6MP at 75 mg/m2, while those on the standard/high-risk (SHR) arm (n = 186) started with 50 mg/m2/d in weeks 1-16, then increased to 75 mg/m2/d until week 120 (girls) or week 146 (boys). No 6MP was given during reinductions I (weeks 7-9) and II (weeks 17-19). The daily dosage, days of treatment, and DI of 6MP were analyzed for each phase of maintenance therapy.
6MP dosages were re-evaluated every 8-16 weeks to maintain a desired degree of leukopenia. Dosage was decreased if patients had myelosuppression (WBC < 1000/mm3, ANC < 300/mm3 and platelet < 5 × 104/mm3). Dosage increases were considered if patients missed less than 25% of therapy but had persistently high WBC (> 4000/mm3) and ANC (> 1000/mm3).
In addition to the two reinductions, SHR patients received weekly asparaginase during weeks 1-19 and monthly cyclophosphamide/cytarabine until week 68. After week 20, patients received daily 6MP and weekly MTX with addition of monthly vincristine/dexamethasone (VCR/DEX) pulses until week 96, after which only 6MP and MTX were given.
Result: The median cumulative DI was 83% (range 14-135%) in wild-type and 68% (range 5-102%) in heterozygotes, similar to that reported for SJCRH Total XII (Relling, Blood 1999). The DI of each phase differed by TPMT genotype (Fig 1). During week 10-16, wild-type patients on the SHR arm had lower median DI (77%, range 12-173%) than those on the LR arm (85%, range 0-110%; P = 6.4 × 10-5) despite the lower protocol dose in the SHR arm, supporting the practice of lowering the protocol 6MP dose during the early intensive weeks of therapy for the SHR arm, and suggesting the higher dose of 75 mg/m2/d is well tolerated even during early intensive therapy for the LR arm.
The DI was similar in weeks 20-95 (6MP/MTX plus monthly VCR/DEX pulses) and in weeks 96-146 (6MP/MTX only) after stratifying by genotype and risk arm (Fig 1), suggesting that VCR/DEX pulses do not alter 6MP tolerance.
TPMT heterozygotes received lower median daily 6MP doses (61 mg/m2) than wild-type (73 mg/m2; P = 3.2 × 10-7) during maintenance therapy. By using the lowered daily dose in heterozygotes, we prevented dose interruptions: the median percentage of days with no 6MP therapy was similar in heterozygotes and wild-type (12% vs 11% missed, P = 0.5).
Conclusion: Using the approach of lowering the 6MP dose from 75 to 50 mg/m2/d during early intensive therapy for SHR patients results in reasonable DI in both groups. The lower tolerated daily dose of 6MP 60 mg/m2 in heterozygotes, compared to 75 mg/m2 in TPMT wild-type patients, supports the recommendation for a lower starting dose of 6MP for TPMT heterozygotes. 6MP DI was similar during phases that did vs did not include VCR/DEX, and similar in this contemporary regimen that includes VCR/DEX pulses compared to older studies without VCR/DEX pulses.
*P-value of comparison between TPMT genotypes, and #P-value of comparison between weeks 20-95 and weeks 96-146.
Evans:St. Jude: In accordance with institutional policy (St. Jude), I and/or my spouse have in the past received a portion of the income St. Jude receives from licensing patent rights related to TPMT polymorphisms as clinical diagnostics. Patents & Royalties. Relling:St. Jude: In accordance with institutional policy (St. Jude), I and/or my spouse have in the past received a portion of the income St. Jude receives from licensing patent rights related to TPMT polymorphisms as clinical diagnostics. Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal