Abstract
Background: The randomized PETHEMA/GEM phase III trial GEM05menos65 (www.clinicaltrials.gov NCT00461747) demonstrated that pretransplant induction therapy with VTD resulted in a significantly higher CR rate both, pretransplant and postransplant and in a significantly longer progression-free survival (PFS) when compared with thalidomide/dexamethasone (TD) and combination chemotherapy plus bortezomib (VBMCP/VBAD/B) (Rosiñol et al, Blood 2012). We report here the long-term results of the trial, five years after the last patient was included.
Methods: From April 6, 2006 to August 5, 2009, 386 patients younger than 65 years with newly diagnosed symptomatic multiple myeloma (MM) were randomized to receive three different induction regimens: six 4-week cycles of TD (thalidomide 200 mg daily; dexamethasone 40 mg on days 1-4 and 9-12) vs. six 4-week cycles of VTD (TD at identical doses plus i.v. bortezomib 1.3 mg/m2 on days 1, 4, 8 and 11) vs. combination chemotherapy plus bortezomib (4 cycles of alternating VBMCP and VBAD chemotherapy followed by two cycles of i.v. bortezomib at the usual dose of 1.3 mg/m2 on days 1,4,8,11 every 3 weeks). The duration of the induction therapy was 24 weeks in all arms. All patients were planned to undergo ASCT with high-dose melphalan at 200 mg/m2 followed by maintenance therapy with thalidomide/bortezomib (TV) vs. thalidomide (T) vs. alfa-2b-interferon (alfa2-IFN) for 3 years. One-hundred and thirty patients were allocated to VTD, 127 to TD and 129 to VBMCP/VBAD/B. Seventy out of the 330 patients (21%) with cytogenetic studies had high-risk cytogenetics [t(4;14), t(14;16) and/or 17p deletion]. Patient characteristics at diagnosis and prognostic factors such as ISS, cytogenetics and maintenance arm were similarly distributed in the 3 arms.
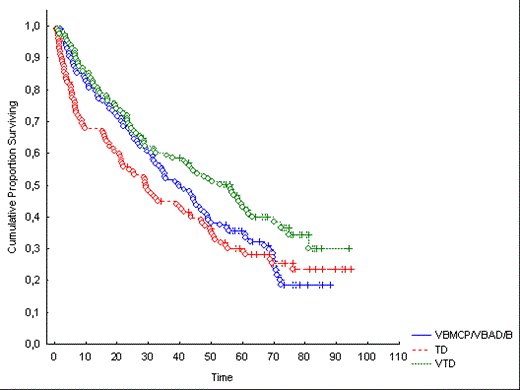
Results: After a median follow-up of 70.6 months, VTD resulted in a significantly longer PFS when compared with TD and VBMCP/VBAD/B (56.1 vs 29.2 vs 39.9 months, p=0.005) (Figure 1). The estimated overall survival (OS) at 8 years was 60% with no significant differences among the 3 arms.
In the overall series, the PFS was significantly shorter in patients with high-risk cytogenetics compared with patients with standard-risk (15.7 vs. 44.3 months, p=0.003). In the TD and in the VBMCP/VBAD/B arm patients with high-risk cytogenetics had a significantly shorter PFS than patients with standard-risk (8.9 vs 32.8 months, p=0.04 in TD group; 14.1 vs. 43.3 months, p=0.05 in VBMCP/VBAD/B group). However, there was no significant difference in the VTD arm (23.6 vs 56.1 months, p=0.2). Patients with high-risk cytogenetics had a significantly shorter OS in the overall series (median 42.1 months vs not reached, p=0.00001) and this was observed in the three treatment arms: VTD median 37.1 months vs not reached (p=0.001), TD median 54.2 months vs not reached (p=0.06), VBMCP/VBAD/B median 30.2 months vs not reached (p=0.007).
The achievement of a deeper response at the end of induction was associated with a longer PFS and OS. Thus, patients achieving CR at the end of induction had a significantly longer PFS than patients achieving a lower degree of response (median 62 vs. 28 months, p=0.00001), irrespective of the treatment arm. Furthermore, on an intention to treat basis, patients who were in postrasplant CR had a significantly longer PFS (p<0.00001) and OS (p<0.00001) than those who did not reach CR after ASCT (p<0.001).
In the overall series the OS after progression was 30.5 months and was not significantly different among the 3 arms (VTD 25.4 months, TD 50 months, VBMCP/VBAD/B 30.2 months, p=0.4). Patients with high-risk cytogenetics had a significantly shorter OS after relapse in the overall series (13.3 months vs. 37.5 months, p=0.001), in the VTD arm (13.3 vs 33.9, p=0.01) and in the VBMCP/VBAD/B arm (8.5 vs 38 months, p=0.01).
Conclusions: Our long-term results confirm that induction with VTD results in a significantly longer PFS when compared with TD and VBMCP/VBAD/B. Patients with high-risk cytogenetics had a worse outcome even with the use of novel drugs. Finally, the PFS of 56 months achieved with VTD is the longest ever reported in the first line treatment of younger patients with MM elegible for ASCT and support the use of VTD as the standard of care for pretransplant induction therapy.
Rosiñol:Janssen: Honoraria; Celgene: Honoraria. Oriol:Celgene Corporation: Consultancy. De La Rubia:Janssen: Honoraria; Celgene: Honoraria. Gutierrez:Janssen: Honoraria; Celgene: Honoraria. Martinez-Lopez:Janssen: Honoraria; Celgene: Honoraria. Alegre:Janssen: Honoraria; Celgene: Honoraria. Lahuerta:Janssen: Honoraria; Celgene: Honoraria. San Miguel:Janssen: Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal