Abstract
Thymopoiesis is a complex process involving crosstalk between developing thymocytes and the non-hematopoietic stromal microenvironment, which includes thymic epithelial cells (TECs), fibroblasts and endothelial cells (ECs). Despite its importance, the thymus is exquisitely sensitive to cellular insults, including cytoreductive chemo- and radiation therapy required for successful hematopoietic stem cell transplantation; therefore identification of thymic repair mechanisms will offer promising therapeutic targets for immune regeneration. Recent studies in tissues such as liver, lung and bone marrow have revealed that ECs not only passively deliver oxygen and nutrients to tissues, but also actively produce distinct paracrine factors that can orchestrate their repair. The role of thymic ECs in thymopoiesis beyond their contribution of local circulation and the importation of lymphoid progenitors has not been comprehensively studied.
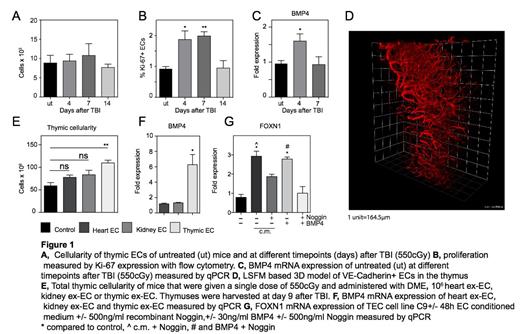
In order to evaluate the role of ECs in thymic regeneration, we closely examined the kinetics of thymic recovery following total body irradiation (TBI, 550cGy) in young C57BL/6 mice. Although we observed a dramatic decline in total thymic cellularity, we found no significant change in the number of thymic ECs, suggesting they are extremely radio-resistant (Fig. 1A). Interestingly, although thymic ECs appeared to be resistant to the cytoreductive effects of TBI, we revealed an increase in their proliferation as measured by expression of Ki67 shortly after injury, indicating a role in the endogenous regeneration of the thymus (Fig. 1B).
Given their radioresistance and cycling after TBI, we hypothesized that thymic ECs can provide instructive signals supporting thymic regeneration. To explore potential regenerative signals stemming from ECs during thymic regeneration, we performed a transcriptome analysis of highly purified ECs of untreated mice and at days 4 and 7 following TBI. Among the 288 genes that were altered in ECs after TBI (131 upregulated and 157 downregulated), we found significant upregulation in the expression of BMP4 (which has previously been implicated in thymic regeneration) and was validated using quantitative PCR (Fig 1C). In addition, we have developed a novel light sheet fluorescence microscopy approach to visualize the thymic vasculature in 3D at high resolution (Fig. 1D).
Consistent with their potential role in aiding thymic regeneration, administration of ex vivo expanded thymic ECs (ex-EC) could significantly enhance thymic regeneration when given after TBI. Intriguingly, this regenerative effect of ex-ECs was tissue specific as ex-EC derived from heart or kidney did not exhibit a similar regenerative effect (Fig. 1E). Similar to our findings in the endogenous regeneration setting, we found that thymic ex-ECs demonstrated a significantly higher BMP4 expression compared to cardiac and kidney ex-ECs (Fig. 1F). Previous reports have suggested that BMP4 signaling is capable of directly regulating the key transcription factor of TEC function, FOXN1. We found that incubation of the TEC cell line C9 with thymic ex-EC conditioned medium induced an increase in FOXN1 expression but was abrogated in the presence of Noggin, a potent BMP signaling inhibitor (Fig. 1G). These findings support the hypothesis that BMP4 mediates the regenerative effect of ECs in thymic regeneration.
In summary, we found that thymic ECs are capable of mediating endogenous thymic regeneration, likely via their expression of BMP4. Adoptive transfer of ex-ECs leads to enhanced thymic regeneration after immune injury. Thus, adoptive transfer of thymic ECs represents a novel strategy to improve immune function in immunocompromised patients.
Ginsberg:Angiocrine Bioscience: Employment. Rafii:Angiocrine Biosciences: Founder Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal