Abstract
Introduction:
Our lab has developed novel methods, using an engineered form of the Notch ligand Delta-1, for the ex vivo expansion of cord blood CD34+ hematopoietic stem and progenitor cells (HSPC) for clinical applications. These same methods can be used to expand primary murine HSPC. We have previously demonstrated that transplantation of completely mismatched Notch-expanded lin-/sca-1+/c-kit+ (LSK) cells can prolong survival in mice that received lethal total body irradiation (TBI). The mismatched LSK cells rapidly reconstitute myeloid cells in irradiated recipients that are short-lived, with low-level persistence of lymphoid cells (CD4/CD8) for more than 90 days. This long-term chimerism was directly proportional to the TBI dose, with higher levels of persistent donor engraftment with increasing TBI. We hypothesized that the residual donor lymphoid cells were de novo generated, tolerant by virtue of host education, and might convey immune tolerance. Herein, we now demonstrate the ability of engrafted mismatched Notch-expanded LSK cells to induce immune tolerance toward the same mismatched donor using a skin allograft technique.
Methods:
Bone marrow LSK cells from Ly5a mice (H-2b, CD45.1) were cultured for 14 days in the presence of engineered Notch ligand, Delta1ext-IgG. The progeny were harvested and cryopreserved and later transplanted into Balb/c (H-2d, CD45.2) mice that were lethally irradiated with 7.5 Gy at a dose of 5x106 cells per mouse. Control animals received 2x105 fresh Balb/c bone marrow cells. 60 days post-transplant, reconstitution and donor chimerism was assessed in the peripheral blood of the recipient mice and bilateral allogeneic and autologous skin grafting was performed. Chimerism ranged from 0 to 12%, with a mean of 3%. Mice were evenly distributed by chimerism into the following 3 groups: mice receiving autologous Balb/c skin graft on the left flank and either a mismatched Ly5a or third-party C3H (H-2k, CD45.2) skin graft on the right flank, or the combination of Ly5a and C3H. Seven days after skin grafting, the dressings were removed and the grafts were scored daily for a period of 30 days. The day of rejection was defined as > 80% of the graft being necrotic, scabbed, or dislodged from the graft bed.
Results:
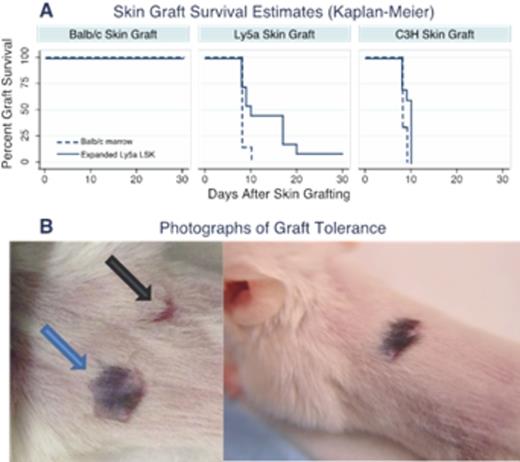
Control animals (those reconstituted with Balb/c marrow, Figure 1A, dashed line) had 0% rejection of Balb/c grafts (0/5) and 100% rejection of C3H grafts (6/6) and Ly5a skin grafts (7/7) within the first 10 days. Experimental animals (those reconstituted with expanded Ly5a cells, Figure 1A, solid line) also had 0% rejection of Balb/c skin grafts (0/12). As with Control animals, chimeric mice also had 100% rejection of the C3H grafts (10/10) within the first 10 days after implantation. Interestingly, chimeric animals that received Ly5a skin grafts showed a slower rate of rejection over a period of 30 days; by day 10 only 45.5% had rejected (5/11), another 45.5% rejected between day 10 and 21 (5/11) and 9% (1/11) had complete engraftment of the Ly5a skin without any signs of rejection to present day. Figure 1B shows complete engraftment of Ly5a skin on Balb/c mice that received expanded Ly5a cells. The black arrow shows a contracted scar of the C3H graft rejection and blue arrow shows tolerance to the Ly5a. T test of the graft survival times between mice receiving Balb/c marrow and expanded Ly5a LSK that received Ly5a skin grafts was significant (p=0.05) as was the comparison between Ly5a skin and C3H skin in animals that received expanded Ly5a LSK (p=0.05). Of note, the one animal with no rejection of Ly5a skin had the highest hematopoietic donor chimerism within that group at the time of skin grafting.
Conclusion:
Preliminary data from these experiments provide strong evidence that Delta1ext-IgG-expanded Ly5a LSK cells, in addition to improving hematopoietic reconstitution, are immunomodulatory and can convey immune tolerance. The results suggest that the level of donor chimerism may correlate with the degree of induced tolerance. Further experiments are being conducted with higher doses of TBI, which we have demonstrated previously result in higher persistent donor chimerism of the expanded Ly5a cells, to investigate the relationship between level of donor engraftment and immune tolerance. The ability to induce immune tolerance could be very important for solid organ transplantation, by enabling the discontinuation of antirejection medicines in transplant recipients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal