Abstract
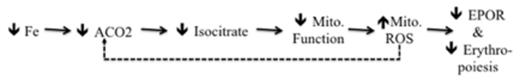
Iron and erythropoietin (Epo) are intimately linked regulators of erythropoiesis. Moderate iron restriction suppresses erythropoiesis at the Epo-dependent, CFU-E stage, without induction of apoptosis and without suppression of other hematopoietic cell lineages. Iron modulates Epo bioactivity in patients with iron deficiency anemia (IDA) and patients with anemia of chronic disease and inflammation (ACDI). To conserve iron when supplies are low, this erythroid iron restriction response reduces iron consumption by suppressing erythropoiesis. The erythroid iron sensor is unknown. Aconitases are multifunctional iron-sulfur cluster proteins localized in the cytosol (Aco1) and mitochondria (Aco2) that convert citrate into isocitrate. We have shown that iron restriction inhibits Aco2 enzymatic activity leading to suppression of erythropoiesis in vitro, and these effects are reversed by isocitrate. Isocitrate corrects IDA in mice and ACDI in rats (Bullock GC, et al. Blood. 2010;116:97-108; Richardson CL, et al. J Clin Invest. 2013 Aug 1;123(8):3614-3623). Iron restriction also alters the cross-talk between transferrin receptor and Epo receptor signaling pathways. These results suggest that Aco2 is an iron-responsive regulator of erythropoiesis. We are investigating the downstream molecular signaling mechanisms by which iron restriction induced-inactivation of Aco2 suppresses erythropoiesis. Our novel preliminary data show that mitochondrial oxidative metabolism rates change over time during erythropoiesis and that iron restriction reduces erythroid mitochondrial metabolism 4 to 7-fold compared to iron replete controls. This iron restriction induced change in respiration is associated with a significant, 1.5 to 3-fold, increase in mitochondrial superoxide production without a corresponding increase in hydrogen peroxide. Importantly, these mitochondrial alterations are reproduced by direct inhibition of aconitase with fluoroacetate (FA) and are not due to changes in mitochondrial number. Further, isocitrate reverses the effects of iron restriction or aconitase inhibition on mitochondrial metabolism and attenuates superoxide production. Based on these data and the known role of reactive oxygen species (superoxide/hydrogen peroxide) in Epo signaling, we propose the overarching hypothesis that iron restriction inhibits mitochondrial aconitase which, in turn, alters erythroid mitochondrial metabolism and ROS signaling resulting in suppression of erythropoiesis (Figure 1 ). We show for the first time bioenergetics profiles from iron restricted and iron replete primary human erythroid progenitor cells undergoing erythropoiesis. We also show that moderate levels of iron restriction cause mitochondrial dysfunction and alterations in mitochondrial ROS in differentiating erythroid progenitors. The clinical relevance of this project lies in its potential for the development of new iron-free agonists and antagonists of red blood cell production. Agonists may benefit patients with anemia due to iron deficiency or chronic inflammation and antagonists may benefit patients with myeloproliferative neoplasms.
Figure 1:Proposed mechanism of iron-dependent regulation of erythropoiesis
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal