Abstract
PR1 is an HLA-A2-retricted, nonameric peptide that is derived from the azurophil granule proteases neutrophil elastase (NE) and proteinase 3 (P3). PR1 has been targeted successfully in acute (AML) and chronic (CML) myeloid leukemia using anti-PR1/HLA-A2 antibody (8F4), PR1-peptide vaccine and PR1-specific cytotoxic T lymphocytes (PR1-CTL). We have previously reported that NE and P3 are cross-presented by normal B cells and dendritic cells (DC), leading to PR1 expression by HLA-A2. Since multiple myeloma (MM) is a B cell malignancy, we investigated whether MM cells can cross-present PR1 as a possible target for immunotherapy.
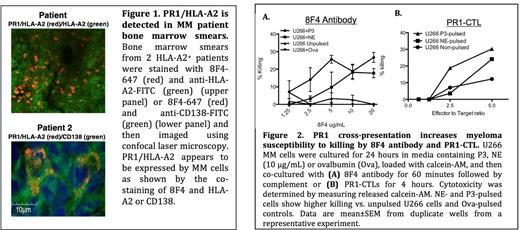
To study whether PR1 is presented by MM cells, patient bone marrow (BM) was stained with 8F4 antibody and then imaged using confocal microscopy. PR1/HLA-A2 was detected on the surface of CD138+ MM cells in BM samples from 3 of 6 HLA-A2+ MM patients (Fig. 1). We then investigated whether cellular immunity to PR1 is detected in peripheral blood (PB) from patients with MM. PB samples from MM patients who had undergone allogeneic (allo) (n=9) and autologous (auto) (n=2) stem cell transplantation (SCT) were stained with PR1/HLA-A2 dextramer in addition to standard lineage markers. PR1-specific CD8+CTL were detected in PB of 10 of 11 patients (range=0.02%-2.9%).
Because P3 and NE expression is limited to myeloid cells, we sought to determine the mechanism of PR1 presentation in MM. We performed RT-PCR and western blotting on seven MM cell lines, including U266, ARK, ARP-1, OPM-2, LP-1, IM-9 and RPMI 8226. Neither NE nor P3 were detected in the MM cell lines studied at either the mRNA or proteins levels. We then investigated whether MM cells took up NE and P3. We cultured MM cells for 30 hours with 10 ug/mL of soluble NE and P3 or irradiated HLA-A2 negative PMN, the latter as a source for cell-associated NE and P3. Cells were then stained intracellularly for NE and P3 at different time points. Flow cytometry analysis showed that all the cell lines analyzed took up NE and P3. Uptake was seen as early as 1 hour after co-culture with soluble NE and P3 and was higher for soluble P3. Additionally, more uptake was seen in the cells that were co-cultured with irradiated PMN in comparison with soluble NE and P3.
We then investigated whether PR1 expression in MM was through NE and P3 cross-presentation. We focused our studies on the HLA-A2+ U266 MM cell line. U266 cells were co-cultured with soluble NE, P3 or irradiated PMN, as described in the previous section, and then surface stained with 8F4. We detected PR1/HLA-A2 on the surface of U266 cells by flow cytometry as early as 6 and 24 hours after co-culture with soluble NE and P3, respectively. Cross-presentation was also seen in the cells that were co-cultured with irradiated PMN to a similar extent in comparison with the cells that were cultured with soluble NE and P3, however, cross-presentation occurred at an earlier time point (1 hour) in the cells that were cultured with PMNs. Cross-presentation was abrogated by lactacystin, a proteasome inhibitor, and brefeldin A, an ER/Golgi transport inhibitor, indicating that NE and P3 cross-presentation occurs through conventional cross-presentation mechanisms. Furthermore, because immune modulatory drugs and proteasome inhibitors are part of the standard of care therapy for MM, and since both have been shown to affect cross-presentation by DC, we tested whether lenalidomide and bortezomib altered PR1 cross-presentation by U266 and normal DC. U266 and DC were cultured with irradiated PMN in the presence of increasing doses of bortezomib or lenalidomide and then stained with PR1/HLA-A2. Flow-cytometry analysis showed a significant inhibition of PR1-cross-presentation by U266 after addition of bortezomib, but not lenalidomide. PR1 cross-presentation by DC was not affected by either drug.
Finally, we tested whether PR1 cross-presentation caused MM cells to become susceptible to PR1-targeting immunotherapies. U266 cells were cultured with NE or P3 for 24 hours, the time point that showed the maximal cross-presentation, followed by addition of 8F4 antibody or co-culture with PR1-CTL. Using calcein AM cytotoxicity assays, we showed dose dependent killing of U266 by 8F4 and PR1-CTL following PR1 cross-presentation (Fig. 2).
Together our data show that PR1 is cross-presented by primary MM cells and cell lines. These findings lay the foundation for the future applications of PR1-targeting immunotherapies in MM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal