Abstract
Introduction: Chemoimmunotherapy has become a standard approach in previously untreated and also in pretreated CLL. Addition of Rituximab to FC in fit patients has proven superior to chemotherapy alone and more recently aCD20 treatment was shown to improve outcomes in patients treated with Chlorambucil, suggesting that immunotherapy may be of benefit, independent of the chosen chemotherapy backbone. In follicular and mantle cell lymphoma rituximab maintenance treatment has become a clinical standard. While we and others have presented Phase II data on the feasibility of Rituximab maintenance after chemoimmunotherapy induction, there are currently no available randomized data on the efficacy of such an approach.
Study design: Patients were recruited after informed consent at the end of any Rituximab-containing induction treatment in 1st or 2nd line that achieved at least a PR. Excluded were patients with uncontrolled bacterial or viral infections and conditions that might severely affect life-expectancy (such as other malignancy, heart disease etc). The trial was registered at clinicaltrials.gov with the identifier NCT01118234. Randomization was stratified by country, line of treatment, induction response and type of induction regimen. Primary endpoint was PFS and a planned sample-size of 256 patients was calculated. All patients were recruited in participating centers between September 2009 and December 2013. An interim analysis was planned to be conducted after half of the planned events (i.e. 46) were observed and is presented here.
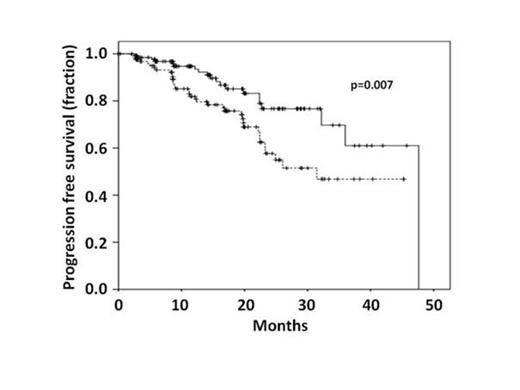
Results: The current analysis includes 263 patients enrolled into the trial. Patients had a median age of 63 years, 28.9% were female and 80.6% were enrolled after 1st induction treatment. Hierarchical FISH cytogenetic risk was available in 221 patients: del17p 3.1%, del11q 27.6%, tris 12 10.8%, del13q 36.2%, and normal FISH karyotype 21.2%. IgVH Mutation state was available in 161 patients at time of interim analysis and 67% were unmutated. The induction regimen was FCR in 73.5% and BR in 20.2%, the response to induction treatment was CR/CRi in 58% and PR in 41.8% of patients and 57% scored negative in an 8-colour MRD flowcytometric analysis after induction. Rituximab treatment was allocated to 134 patients and 129 were in the observation arm. No significant imbalances in randomization were found in the above-mentioned parameters. Median observation time is currently 17.3 months. Regarding toxicities the current state of monitoring allows an analysis on the level of SAEs only. SAE causes were well balanced between arms with the exception of infectious SAEs - 32 in the rituximab and 22 in the observation arm, 3 deaths were attributed to infections (1 in the rituximab arm and 2 in the observation arm) - and secondary malignancies (8 in the rituximab arm vs. 1 in the observation arm). Four of the neoplasms in the rituximab arm were localized non-melanoma skin cancers and the 2 deaths from malignomas occurred one in each arm. Regarding efficacy, currently 27.9% have progressed in the observation arm and 14.9% in the rituximab arm. Ten patients died in the observation arm and 7 in the rituximab arm. The primary endpoint (PFS) is significantly in favour of rituximab maintenance (see Fig) with a p-value of 0.007 and a PFS at 17.3 months of 85.1% vs.75.5% in rituximab vs. observation arms, respectively. To account for toxicities and secondary neoplasms an EFS was calculated counting secondary malignancies, termination of treatment due to toxicities, progression or death as events. In this analysis the benefit was preserved, albeit with a lower p-value of 0.03. The observed benefit seemed independent from response after induction (CR vs. PR), but associated with positive MRD state after induction. Further factors that influenced the benefit in exploratory subgroup analyses were sex, cytogenetics, IgVH and B-symptoms at diagnosis.
Conclusions: Rituximab maintenance after chemoimmunotherapy induction in CLL seems feasible, although with an increase in infectious complications, and shows signs of efficacy in this interim analysis. The presented interim analysis refutes the alternative hypothesis and allows the trial to continue. Exploratory analyses suggest that with longer follow-up it may be possible to define subpopulations with larger benefit from extended immunotherapy.
Greil:Roche: Honoraria; Pfizer: Honoraria, Research Funding; Boehringer-Ingelheim: Honoraria; Astra-Zeneca: Honoraria; Novartis: Honoraria; Genentech: Honoraria, Research Funding; Janssen-Cilag: Honoraria; Merck: Honoraria; Mundipharma: Honoraria, Research Funding; Eisai: Honoraria; Amgen: Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Cephalon: Consultancy, Honoraria, Research Funding; Bristol-Myers-Squibb: Consultancy, Honoraria; Sanofi Aventis: Honoraria; GSK: Research Funding; Ratiopharm: Research Funding. Off Label Use: Rituximab in Maintenace Treatment of CLL. Kozak:Roche: Honoraria, Travel grants Other. Girschikofsky:Pfizer: Honoraria, Research Funding; Mundipharm: Consultancy, Honoraria. Petzer:Celgene: Honoraria, unrestricted grant Other. Egle:Roche: Honoraria, Research Funding, Travel Grants Other.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal