Abstract
Patients with acute myeloid leukemia (AML) experience sustained and profound neutropenia during induction chemotherapy; infections remain the leading cause of morbidity and mortality in myelosuppressed leukemia patients. The current standard of care is to provide patients with broad-spectrum antibiotic, antifungal and antiviral therapy; however resistance to available anti-infective agents is increasing. Transfusion of functional non-irradiated allogeneic granulocytes to neutropenic leukemia patients may treat, delay or prevent infections in patients with leukemia, and importantly may also have anti-leukemia benefits.
Patients treated at MD Anderson Cancer Center with a diagnosis of AML undergoing front-line AML therapy were eligible. Patients were required to be free of signs and symptoms of infection at the time of study entry, and have sufficient volunteer donors to administer prophylactic white cell transfusions approximately twice a week for six weeks. Allogeneic white cell transfusions (≥ 4 x 10¹⁰ cells per transfusion) were administered every 3-4 days during the induction chemotherapy cycle, so long as patients remained neutropenic with an absolute neutrophil count < 500. Prophylactic transfusions continued until the time of sustained ANC recovery, initiation of a new treatment regimen, or after the completion of 6 weeks on protocol.
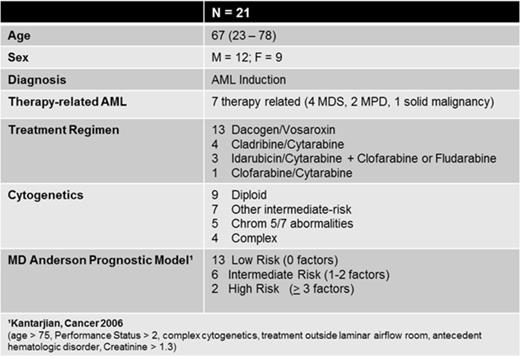
Herein we provide the results of the first 21 patients. Median age was 67 (range 23-78); 12 patients were male and 9 were female. All patients were enrolled at the time of initiation of AML induction therapy. Seven patients had a diagnosis of therapy-related AML (6 with an antecedent hematologic disorder and 1 with prior solid malignancy). Various treatment regimens were administered and are displayed in Table 1, along with cytogenetic and MD Anderson prognostic model classification.
Patients received a median of 5 transfusions (range 0 – 11). Of 21 patients, 1 withdrew consent prior to the first transfusion, and 3 patients discontinued transfusions prior to the end of study (due to a diagnosis of pneumonia in one with potential concern for increased pulmonary toxicity with continued transfusions, and two at patient requests due to fevers and myalgias post transfusions).
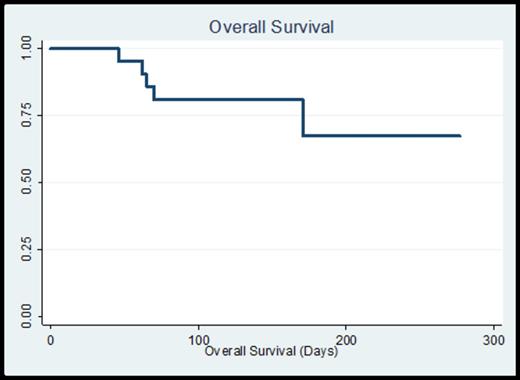
Median time to ANC recovery was 38 days (range 14 – 137) and platelet recovery 29 days (range 12 – 160), consistent with the elderly population and the allotted treatment regimens. All patients experienced at least one neutropenic fever, often within 48 hours of white cell administration. Documented infections included 2 instances of bacteremia (1 streptococcal and 1 E. coli), 2 urinary tract infections (both coagulase negative staphylococci), 8 cases of pneumonia (1 documented E. coli, 7 culture-negative and presumed fungal pneumonia per imaging). There was one patient with neutropenic colitis per imaging. There were no induction-related deaths. 8-week mortality was 5% (n=1) and 12-week mortality was 20% (n=4). With a median follow-up time on study of 20.3 weeks, overall survival is 67% with a median survival which has not yet been reached (Figure 1). Overall response to initial induction chemotherapy was 67% (9 CR, 1 CRi, 4 PR). A total of 15 patients (71%) ultimately attained a CR (after the first cycle or with additional cycles of therapy), and 4 patients received a stem cell transplant at the time of CR/CRi.
In conclusion, prophylactic transfusion of allogeneic non-irradiated white blood cells for newly diagnosed AML patients during induction chemotherapy was associated with a decreased incidence of life-threatening infections, decreased induction mortality (6-week OS of 100%), and may also lead to improved remission rates and overall survival. This feasibility study has been expanded to enroll a total of 50 patients, with updated results to be provided at the annual meeting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal