Abstract
CD4+CD25+Foxp3+ regulatory T cells (Treg) play a critical role in establishment of immune tolerance and prevention of graft versus host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). The recovery and maintenance of Treg after HSCT is dependent on homeostatic factors including the generation of naïve Treg from hematopoietic precursor cells, the proliferation and expansion of mature Treg and the survival of Treg in vivo. We previously reported that Treg in healthy donors are more susceptible to mitochondrial apoptotic priming than conventional T cells (Tcon). Mitochondrial priming is increased after hematopoietic stem cell transplantation in all T cell subsets and particularly in patients with chronic GVHD. Interleukin-2 (IL-2) is a critical homeostatic cytokine that is required for Treg development, expansion, activity and survival. We previously demonstrated that daily administration of low-dose IL-2 resulted in selective expansion of Treg in vivo, clinical improvement of chronic GVHD (Koreth et al. NEJM 2011), increased Treg Bcl2 expression and increased Treg resistance to apoptosis in vitro (Matsuoka et al. SciTM, 2013). These observations led us to undertake a more detailed analysis of apoptotic pathways in functionally and phenotypically distinct Treg subsets including naïve Treg, recent thymic emigrants (RTE) Treg and memory Treg.
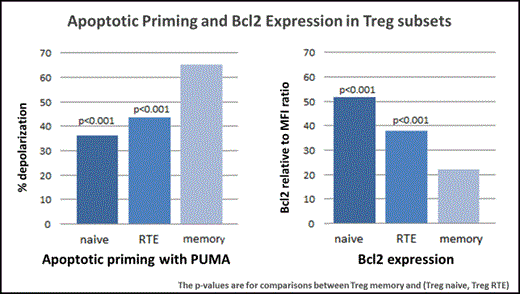
To examine the mechanisms whereby IL-2 affects susceptibility to apoptosis in each Treg subset, we used a functional flow cytometry-based assay (apoptotic profiling) to measure mitochondrial membrane depolarization in response to a panel of pro-apoptotic BH3 peptides (BIM, BID, BAD, NOXA, PUMA, BMF, HRK). This assay allowed us to compare "priming", which we define as susceptibility to BH3 peptide-induced mitochondrial membrane depolarization, in naïve, RTE and memory subsets. We also examined cytoplasmic expression of Bcl2 as an additional measure of susceptibility to apoptosis in each subset. In resting blood obtained from healthy donors (n=25), memory Treg were more "primed" than either naïve or RTE subsets when exposed to several BH3 peptides (BID, NOXA, PUMA, BMF and the combination of BAD+NOXA) (Figure 1). Memory Treg were also found to have decreased expression of Bcl2 compared to naïve or RTE subsets. Thus, memory Treg in healthy individuals are more susceptible to apoptosis than other Treg subsets through the mitochondrial pathway (Figure 1).
To examine the functional effects of IL-2 on T cell homeostasis, Treg and Tcon were purified by cell sorting and cultured for 5 days with different concentrations of IL-2 (0, 10, 100, 1000 U/ml). Low-concentration IL-2 (10 U/ml) lowered apoptotic priming and increased Bcl2 expression in all Treg subsets. Similar effects were observed in all Tcon subsets, but higher concentrations of IL-2 (≥100 U/ml) were required to decrease apoptotic priming and increase Bcl2 expression in Tcon subsets. The peak effect of IL-2 on Treg priming occurred on day 3 but these effects appeared more rapidly in naïve and RTE Treg subsets than in memory Treg. However, the effect of IL-2 on apoptotic priming persisted for a longer period in memory Treg than in naïve and RTE Treg subsets. The peak effect of IL-2 on Bcl2 expression occurred on day 3 in all Treg subsets. To compare the effect of IL-2 on Treg and Tcon subsets, we examined apoptotic priming and Bcl2 expression after 3 day incubation with different IL-2 concentrations (0.001 - 1000 U/ml). Very low-concentration IL-2 (1 U/ml) lowered priming and increased Bcl2 expression in memory Treg, but higher concentrations of IL-2 (≥10 U/ml) were required to increase Bcl2 expression and decrease priming in naïve and RTE Treg. All Tcon subsets showed a similar pattern of response but higher concentrations of IL-2 (≥100 U/ml) were required.
Memory Treg are the predominant regulatory population in patients with cGVHD and the high level of priming in this subset contributes to the inability to maintain adequate numbers of CD4 Treg after HSCT. In healthy donors, low concentrations of IL-2 are sufficient to reduce mitochondrial priming and increase Bcl2 expression in memory Treg. IL-2 has similar effects on Tcon but 10-100 fold higher concentrations of IL-2 are required. These concentration-dependent effects of IL-2 likely contribute to the selective expansion and persistence of Treg in patients with cGVHD receiving daily low-dose IL-2 therapy after HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal