Abstract
Paraneoplastic thrombocytosis is associated with many solid tumors and often correlates with reduced survival. Recent studies suggest that a pathogenic feed back loop may be operative between platelets and tumor cells, with reciprocal interactions between tumor growth/metastasis and thrombocytosis/platelet activation. Specific molecular pathways have been identified in which tumors can stimulate platelet production and activation; activated platelets can, in turn, promote tumor growth and metastasis. Taken together, these findings provide exciting new potential targets for therapeutic intervention.
Introduction
Normally quiescent platelets in the circulation respond to localized breaches in vascular integrity by providing hemostasis with swiftness and precision. They do so by mobilizing their remarkable repertoire of capabilities to respond to injury: adhesion, activation, secretion, aggregation, recruitment, and wound healing. However, only in recent years have we begun to appreciate the functional versatility of platelets beyond their role in hemostasis; for example, in defending their human hosts against microbial invasion, participating in inflammatory and immune responses, and even contributing to the development and regeneration of organs.1,2 Now, however, it is coming to light that a malignant tumor can use platelets to promote its growth and metastasis. In turn, the growing tumor can enhance the production and activation of platelets, thereby potentially creating a positive feedback loop to fuel the tumor’s growth.
The hijacking of platelet functions by malignant tumors
Tumor metastasis consists of tissue invasion by the tumor cells, bloodstream entry, an intravascular phase, extravasation of the tumor cells from the capillaries, and growth at a distant site. Tumor cells activate platelets, and the metastatic potential of tumor cells correlates with their efficacy in inducing platelet aggregation.3,4 Tumor cells generate thrombin, a potent platelet activator agonist, either by direct contact with platelets or indirectly by stimulating tissue factor-mediated activation of the coagulation system that generates thrombin within the tumor microenvironment.5 Egan et al showed that ovarian cancer-induced platelet activation is mediated by adenosine 5′-diphosphate released from tumor cells and can be blocked by adenosine 5′-diphosphate receptor (P2Y12 and P2Y1) antagonists.6 More recently, Mitrugno et al demonstrated that tumor cells could directly induce platelet activation and secretion of dense granules containing adenine nucleotides via the platelet Fcγ receptor IIa.7 Platelet activation by tumors throughout all phases of the metastatic cascade leads to the release of platelet-derived factors stored in their granules that then mediates the inflammatory, proliferative, and proangiogenic activities of platelets to promote tumor growth, tissue invasion, and metastasis.8,9
Conversely, platelets activated by tumor cells play major roles in aiding and abetting tumor progression. First, platelets can help tumor cells survive immune surveillance in the blood circulation. Activated platelets may act as protective “cloaks” for circulating tumor cells, shielding them from immune destruction by natural killer cells.10,11 This process is medicated by platelet-derived growth factor and transforming growth factor β.12,13 Hematogenous dissemination of tumor cells can also be facilitated by “platelet mimicry,” in which tumor cells acquire a phenotype that closely resembles platelets and expresses platelet/megakaryocytic gene products such as γIIb/β3, protease-activated receptors, and platelet endothelial cell adhesion molecule 1.5,14 Second, platelet-derived transforming growth factor β stimulates the proliferation of ovarian cancer cells in vitro and in vivo15 and promotes epithelial-to-mesenchymal transition in tumor metastasis.16 Third, platelets, leukocytes, and vascular endothelium may facilitate tumor cell extravasation and seeding through adhesion molecules P- and L-selectins, a hypothesis supported by the observation that tumor metastases are reduced in mice lacking them.17-19 More recently, Schumacher et al showed that platelet-dense granule-derived adenine nucleotides facilitate the transmigration of tumor cells across endothelium through activation of the endothelial adenosine triphosphate receptor P2Y2.20 Finally, for tumors to grow to sizes larger than 2 mm, they must establish their own blood supply through angiogenesis, a process regulated by platelets and their α granules that contain both proangiogenic and antiangiogenic proteins, including more than 80% of circulating vascular endothelial growth factor.21 Contact with tumor cells activates platelets to preferentially release proangiogenic proteins. However, the mechanism of this selective release process through the possible organization of pro- and antiangiogenic proteins into separate α granules or platelet populations remains ill-defined.22
Mechanisms of paraneoplastic thrombocytosis
Malignant tumors not only hijack platelet functions but can also increase their production. During normal hematopoiesis, platelet production can be stimulated at different hierarchical levels, primarily through thrombopoietin (TPO) and its receptor.23 Inappropriately high levels of TPO generated by a variety of clinical disorders, such as chronic inflammation and infection, lead to secondary (reactive) thrombocytosis of diverse etiologies. This is in contrast to the primary thrombocytosis of myeloproliferative neoplasms that is associated with normal or low TPO levels.23 TPO usually is not overexpressed by solid tumors, but several other cytokines, including interleukin 1 (IL-1), IL-3, IL-6, IL-11, leukemia inhibitory factor, KitL, and oncostatin M, likely contribute to tumor-stimulated thrombopoiesis.24 IL-6 in particular, acting as an autocrine growth factor, is overproduced in a variety of malignancies, including gastrointestinal, renal cell, prostate, epithelial ovarian, and lung cancer, as well as Kaposi’s sarcoma and glioblastoma multiforme.25 The IL-6 effect is mediated through induction of TPO mRNA expression and protein production in the liver, and TPO-neutralizing antibodies can abrogate the paraneoplastic thrombocytosis.25 Importantly, increased serum levels of IL-6 correlate with platelet counts and anti-IL-6 antibody administration abolishes thrombocytosis in cancer patients.25-29 Clinically, thrombocytosis may precede the diagnosis of malignancy by months or even years,30 and accumulating evidence suggests it is highly prevalent in many solid tumors at the time of diagnosis and correlates with significantly reduced survival and/or response to surgery and chemotherapy.24 Table 131-42 summarizes all retrospective, prospective, and meta-analytic studies of more than 300 patients that correlate thrombocytosis at the time of diagnosis with survival and treatment response in solid tumors.
Summary of studies with more than 300 patients correlating thrombocytosis with survival in solid tumors
| Cancer type, N . | Cutoff (per mm3) . | Prevalence, n (%) . | Study type . | Survival results (thrombocytosis vs normal) . | Reference . |
|---|---|---|---|---|---|
| Lung | |||||
| 398 | 400 000 | 86 (21.6) | Retrospective | OS: HR, 1.58 (P = .006, mva) | 31 |
| 317 | 400 000 | 64 (20.2) | Prospective | Time to progression: (P = .2, uva) | 32 |
| Chemotherapy response: 32.8% vs 56% (P = .001) | |||||
| 611 | 400 000 | 98 (16) | Retrospective | OS: HR, 1.29 (P = .0348, mva) | 33 |
| 1115 | 400 000 | 358 (32.1) | Retrospective | OS: HR, 4.24 (P < .001, mva) | 34 |
| Mesothelioma | |||||
| 336 | 400 000 | 184 (54.8) | Retrospective | OS: HR, 1.57 (P < .001, mva) | 35 |
| Breast | |||||
| 4300 | 400 000 | 161 (3.7) | Retrospective | OS: HR, 1.73 (P = .0064, mva) | 36 |
| Gynecologic | |||||
| 578 | 450 000 | 129 (22.3) | Retrospective | DFS: HR, 1.38 (P = .02, mva) | 37 |
| OS: HR, 1.45 (P = .003, mva) | |||||
| Stage I/II (n = 127); OS: HR, 5.06 (P = .008, mva) | |||||
| 3490 | Various | 709 (20.3) | Meta-analysis | OS: RR, 1.62 (P < .0001, mva) | 38 |
| Colorectal | |||||
| 453 | 300 000 | 226 (49.9) | Retrospective | OS: HR, 1.64 (P = .039, mva) | 39 |
| 636 | 370 000 | 77 (12.1) | Retrospective | OS: HR, 3.04 (P < .001, mva) | 40 |
| DFS: HR, 2.54 (P < .001, mva) | |||||
| Gastric | |||||
| 369 | 400 000 | 42 (11.4) | Retrospective | OS: HR, 2.48 (P = .015, mva) | 41 |
| Renal | |||||
| 804 | 450 000 | 63 (7.8) | Retrospective | OS: OR, 1.8 (P < .0001, mva) | 42 |
| Cancer type, N . | Cutoff (per mm3) . | Prevalence, n (%) . | Study type . | Survival results (thrombocytosis vs normal) . | Reference . |
|---|---|---|---|---|---|
| Lung | |||||
| 398 | 400 000 | 86 (21.6) | Retrospective | OS: HR, 1.58 (P = .006, mva) | 31 |
| 317 | 400 000 | 64 (20.2) | Prospective | Time to progression: (P = .2, uva) | 32 |
| Chemotherapy response: 32.8% vs 56% (P = .001) | |||||
| 611 | 400 000 | 98 (16) | Retrospective | OS: HR, 1.29 (P = .0348, mva) | 33 |
| 1115 | 400 000 | 358 (32.1) | Retrospective | OS: HR, 4.24 (P < .001, mva) | 34 |
| Mesothelioma | |||||
| 336 | 400 000 | 184 (54.8) | Retrospective | OS: HR, 1.57 (P < .001, mva) | 35 |
| Breast | |||||
| 4300 | 400 000 | 161 (3.7) | Retrospective | OS: HR, 1.73 (P = .0064, mva) | 36 |
| Gynecologic | |||||
| 578 | 450 000 | 129 (22.3) | Retrospective | DFS: HR, 1.38 (P = .02, mva) | 37 |
| OS: HR, 1.45 (P = .003, mva) | |||||
| Stage I/II (n = 127); OS: HR, 5.06 (P = .008, mva) | |||||
| 3490 | Various | 709 (20.3) | Meta-analysis | OS: RR, 1.62 (P < .0001, mva) | 38 |
| Colorectal | |||||
| 453 | 300 000 | 226 (49.9) | Retrospective | OS: HR, 1.64 (P = .039, mva) | 39 |
| 636 | 370 000 | 77 (12.1) | Retrospective | OS: HR, 3.04 (P < .001, mva) | 40 |
| DFS: HR, 2.54 (P < .001, mva) | |||||
| Gastric | |||||
| 369 | 400 000 | 42 (11.4) | Retrospective | OS: HR, 2.48 (P = .015, mva) | 41 |
| Renal | |||||
| 804 | 450 000 | 63 (7.8) | Retrospective | OS: OR, 1.8 (P < .0001, mva) | 42 |
DFS, disease-free survival; HR, hazard ratio; mva, multivariate analysis; OS, overall survival; RR, risk ratio; uva, univariate analysis.
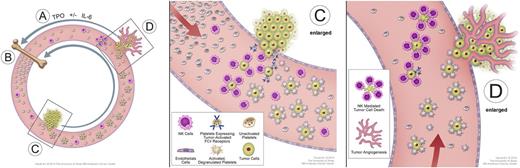
In a landmark study of paraneoplastic thrombocytosis in patients with epithelial ovarian cancer, Stone et al conducted parallel clinical and laboratory analyses of 619 women with newly diagnosed disease.26 More than 90% of patients had high-grade serous or other epithelioid histology and stage 3 to 4 disease, and 192 patients (31%) had a platelet count above normal (>450 000/mm3). The authors found that patients with thrombocytosis had significantly higher levels of IL-6 and TPO than those without thrombocytosis, and they also had significantly shorter progression-free survival (P < .001) and OS (2.62 vs 4.65 years; P < .001). Blocking IL-6 and TPO production with small interfering RNA led to normalization of platelet counts in an animal model of ovarian cancer, as did the clinical use of the anti-IL-6 antibody siltuximab in patients with ovarian cancer.26 On the basis of these studies, we propose a paracrine circuit in which tumors produce cytokines such as IL-6 that induce thrombocytosis, and in turn, the increased number of platelets promotes tumor growth and distant metastasis (Figure 1).
Model of a “vicious cycle” of cooperation between platelets and tumors. (A) Tumors stimulate TPO production, usually indirectly by their elaboration of IL-6 or other cytokines; (B) bone marrow responds to TPO stimulation by increasing platelet production and release into the circulation; (C) circulating platelets are activated either by direct contact with tumor cells via tumor-activated Fcγ receptors or indirectly through the generation of the potent platelet agonist thrombin in the tumor microenvironment (not shown), and in turn, activated platelets that are attracted to the site of tumor stick to and provide a “protective cloak” for circulating tumor cells, shielding them from immune destruction by natural killer cells (C enlarged); and (D) platelets further facilitate metastasis by augmenting circulating tumor cell extravasation. Platelets can also promote metastatic tumor growth by releasing proangiogenic proteins, including vascular endothelial growth factor (not shown), thereby stimulating tumor angiogenesis. The positive-feedback loop is completed as the platelet-assisted growing tumors secrete more TPO-stimulatory cytokines (D enlarged).
Model of a “vicious cycle” of cooperation between platelets and tumors. (A) Tumors stimulate TPO production, usually indirectly by their elaboration of IL-6 or other cytokines; (B) bone marrow responds to TPO stimulation by increasing platelet production and release into the circulation; (C) circulating platelets are activated either by direct contact with tumor cells via tumor-activated Fcγ receptors or indirectly through the generation of the potent platelet agonist thrombin in the tumor microenvironment (not shown), and in turn, activated platelets that are attracted to the site of tumor stick to and provide a “protective cloak” for circulating tumor cells, shielding them from immune destruction by natural killer cells (C enlarged); and (D) platelets further facilitate metastasis by augmenting circulating tumor cell extravasation. Platelets can also promote metastatic tumor growth by releasing proangiogenic proteins, including vascular endothelial growth factor (not shown), thereby stimulating tumor angiogenesis. The positive-feedback loop is completed as the platelet-assisted growing tumors secrete more TPO-stimulatory cytokines (D enlarged).
Targeting paraneoplastic thrombocytosis in anticancer therapy
Given the correlation between paraneoplastic thrombocytosis and reduced survival, as well as documented roles of platelets in tumor growth and metastasis, strategies aimed at blocking various steps in platelet-facilitated tumorigenesis have been investigated in tumor cells and patients. A fundamental unanswered question, however, is whether an increase in platelet count is enough to fuel cancer progression. If this is the case, and the relationship between platelets and tumors is simply a quantitative one, strategies to reduce the platelet count safely and specifically might be salutary. However, it is also possible that malignant tumors can selectively stimulate either the production of platelet populations that are selectively enriched in tumor-promoting properties (eg, with a disproportionally high granule content of proangiogenic proteins) or the induction of specific platelet properties conducive to tumor metastasis (eg, by upregulation of platelet and endothelial adhesion molecules). These qualitative relationships would make the simple strategy of platelet cytoreduction less effective.
One approach is to reduce the production of IL-6 using the anti-IL-6 antibody siltuximab, which not only decreases platelet counts in patients but also depletes levels of the growth factors produced by malignant tumors and platelets.26,27 In a mouse model of ovarian cancer, treatment with paclitaxel and siltuximab reduced tumor growth by more than 90% compared with controls, which is significantly more than either agent alone.26 In a phase II trial of patients with recurrent, platinum-resistant ovarian cancer, single-agent siltuximab inhibited tumor growth in 8 of 18 patients.27 Similar clinical studies in renal cell carcinoma and castration-resistant prostate cancer also demonstrated a beneficial effect.28,29 Another approach is to inhibit platelet function to control tumor growth and metastasis. Clinical data evaluating the effect of antiplatelet agents including aspirin on cancer survival have begun to emerge.5 A recent post hoc analysis of large, randomized cardiovascular prevention trials provided evidence for a reduced risk for cancer metastasis in patients taking aspirin at doses sufficient to inhibit platelet function.43 However, despite the evidence for a role of platelets in tumor metastasis, randomized clinical trials to prospectively test this hypothesis have been rare, mainly because of concerns that antiplatelet drugs may affect normal platelet function, leading to bleeding complications.8,9 In addition, it has been recognized that unfractionated heparins and low-molecular-weight heparins can inhibit the interaction of selectins with their natural ligands.44 This leads to statistically significant improvements in the OS of cancer patients, especially those without metastatic disease who receive heparinoids.45 Interestingly, recent evidence suggests that these anticoagulants can also selectively inhibit platelet release of proangiogenic proteins and diminish platelet-mediated angiogenic response.21 Finally, blockade of platelet Fcγ receptor IIa and αIIbβ3 receptors would also be logical approaches,7 in addition to blockade of the P2Y2 receptor on endothelial cells or its downstream signaling pathway.20 However, these too will require rigorous preclinical testing, especially as prasugrel, a potent P2Y12 receptor blocker used in the treatment of cardiovascular disease, was found to be associated with a higher rate of colonic malignancy in a large study.46
Conclusions
Significant progress has been made in unraveling the complex, reciprocal relationship between tumor progression and platelet function. It is likely that thrombocytosis is not simply an epiphenomenon of malignancy but, rather, a true paraneoplastic abnormality. In fact, paraneoplastic thrombocytosis appears to involve a “positive feedback loop,” in which malignant tumors produce cytokines such as IL-6 that stimulate thrombocytosis, while at the same time, tumor cells themselves directly or indirectly activate platelets. In turn, increased numbers of activated platelets promote further tumor growth and metastasis, which leads to yet greater stimulation of platelet numbers and levels of activity (Figure 1). Interrupting this paracrine circuit with antiplatelet agents, heparinoids, or pharmacologic inhibition of IL-6 might prove beneficial for patients with solid tumors and thrombocytosis. However, it remains unclear whether the worse prognosis in these patients is a result of thrombocytosis induced by IL-6 or caused by IL-6 itself. This is particularly important, as IL-6 has pleotropic effects in many tumors independent of thrombopoiesis.47 Combining antiplatelet therapy with conventional antitumor therapy should be considered. The effect of existing antiplatelet drugs as adjuvants to conventional chemotherapeutics and hormonal therapies has been understudied. Lessons learned from the use of antiplatelet therapy in the treatment of cardiovascular disease, such as the concept of antiplatelet drug resistance, might have implications in their use in cancer treatment. In addition, more targeted antiplatelet therapy could be designed, based on our current understanding of how tumor cells specifically activate platelets and thereby recruit them to facilitate tumor progression and metastasis. Although not yet unequivocally proven, it is becoming increasingly evident that the activation of platelets, as well as their increased numbers (thrombocytosis), that are associated with a variety of solid tumors are not merely epiphenomena but, rather, are intricately involved in the process of tumor progression.
Acknowledgment
We thank Silva Sergenian for administrative assistance and manuscript editing and Jordan Pietz for graphic illustration. This work was supported by the National Institutes of Health, National Cancer Institute (R01CA177909 to V.A.-K.) and an Ovarian Cancer Research Fund Program Project Development grant.
Authorship
Contribution: R.J.L., V.A.-K., and A.I.S. made comparable contributions to planning, initial writing, editing, and figure design.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew I. Schafer, Division of Hematology/Medical Oncology, Department of Medicine, Weill Cornell Medical College, 1305 York Ave, Box 403, New York, NY, 10021; e-mail: ais2007@med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal