Abstract
Secretion of ligands of the tumor necrosis factor (TNF) superfamily is a conserved response of parenchymal tissues to injury and inflammation that commonly perpetuates elimination of dysfunctional cellular components by apoptosis. The same signals of tissue injury that induce apoptosis in somatic cells activate stem cells and initiate the process of tissue regeneration as a coupling mechanism of injury and recovery. Hematopoietic stem and progenitor cells upregulate the TNF family receptors under stress conditions and are transduced with trophic signals. The progeny gradually acquires sensitivity to receptor-mediated apoptosis along the differentiation process, which becomes the major mechanism of negative regulation of mature proliferating hematopoietic lineages and immune homeostasis. Receptor/ligand interactions of the TNF family are physiological mechanisms transducing the need for repair, which may be harnessed in pathological conditions and transplantation. Because these interactions are physiological mechanisms of injury, neutralization of these pathways has to be carefully considered in disorders that do not involve intrinsic aberrations of excessive susceptibility to apoptosis.
Introduction
The hallmark of stem and progenitor cell activity in the adult is reconstitution of cells, tissues, and organs under stress conditions such as disease and injury. The immense regenerative capacity of the immunohematopoietic system is best emphasized by spontaneous recovery after radiochemotherapy and therapeutic transplantation of reconstituting progenitors in oncology patients, with perspectives of much wider implementation in the treatment of immune disorders, autoimmunity, and correction of congenital disorders. High turnover rates of bone marrow (BM) hematopoiesis, replacing various lineages at frequencies of 1 day to 12 weeks, render this system excessively sensitivity to disease, irradiation, and toxic agents. Efforts to harness the reconstituting capacity of stem cells for regenerative purposes focused interest in the mechanisms of survival and apoptosis. One of the best characterized signaling systems involves receptors and ligands of the tumor necrosis factor (TNF) superfamily involved in survival, differentiation, proliferation, and function of immunohematopoietic cells.1,2 Negative regulation by TNF receptors controls the size of expanding hematopoietic and immune clones in the terminal stages of differentiation to make way to the activity of other hematopoietic stem and progenitor cells (HSPC).3,4 It is possible that this mode of regulation of clonal expansion maintains hematopoietic heterogeneity, responds to frequent changes in demand, and contains mutagenesis associated with the burst of activity of individual precursors.5 In parallel, all immune cell types upregulate TNF family receptors upon activation and become susceptible to physical elimination by apoptosis,6 a functional characteristic that regulates the intensity of immune reactions through activation-induced cell death (AICD). The present discussion of the involvement of the TNF superfamily in hematopoiesis focuses on 3 main receptor/ligand interactions: (1) Fas is the common executioner of apoptosis in the family and requires trimerization by membrane-bound Fas-ligand, (2) TNFα signals through 2 receptors but only TNF-R1 contains a death domain, and (3) TNF-related apoptosis-induced ligand (TRAIL) signals apoptosis through receptors 1 and 2 among 5 cognate receptors.
Suppressive and supportive impact of TNF receptors in hematopoietic progenitors
Several competing hypotheses have emerged from characterization of the mechanisms of negative regulation in the hematopoietic and immune systems. The first concept has adoptively transferred the dominant role of TNF family receptors in negative regulation from the distal to proximal stages of hematopoietic differentiation. Favoring a negative regulatory role of the Fas and the TNF receptors is deterioration of progenitor viability and clonogenic activity in culture,7,8 and physical and functional suppression of donor progenitor activity by residual host cells.9,10 Consequently, cytokine-induced upregulation of Fas was used to demonstrate inhibition of progenitors in cultures supplemented with supporting growth factors and chemokines.11-13 The second concept suggests that TNF family receptors have dual supportive activity in progenitors and mediate apoptosis in differentiated hematopoietic cells.14-17 Hierarchical susceptibility of immunohematopoietic cells to injury is evident from nonmyeloablative radiochemotherapy, which causes depletion of mature lineages, and spontaneous recovery of surviving endogenous HSPC ensures reconstitution.18,19 According to this scenario, the causes of relative insensitivity of progenitors to apoptosis were derived from somatic cells: (1) low levels of TNF family receptors, (2) upregulated expression of individual antiapoptotic factors, and (3) detrimental consequences of TNF family receptor activation.
Both concepts attribute injury, signaled in part by the TNF family ligands, a potential negative impact on HSPC activity, but they do not account for several features of stress hematopoiesis. A certain degree of damage is rather necessary for functional engraftment in the transplant setting because reconstitution is diminished when progenitor grafting is delayed by several days, a period sufficient for stromal recovery from conditioning-induced injury.20,21 In fact, stem and progenitor cells should be able to fulfill their role as units of regeneration under harsh environments that cause death of somatic cells. Some injury signals such as TNFα and IL-1α are rather protective and enhance recovery of endogenous progenitors from radiation-induced injury.22-24 These features led to the formulation of a third hypothesis, suggesting that insensitivity of the most primitive units of regeneration to signals that cause death of somatic cells is a prerequisite of their reconstituting activity.
Hematopoietic progenitors are responsive to TNF receptor signaling
The majority of grafted progenitors upregulate the TNF family receptors early after transplantation10,25-27 and under virtually all conditions of stress hematopoiesis. This is a conserved response of progenitors to stress similar to activation of mature immune cells, which may be related to transcriptional programs of gene expression induced by the nuclear factor of activated T cells (NFAT) and nuclear factor-κB (NF-κB).28,29 Acute upregulation of the receptors is most characteristic of progenitors that home successfully to the bone marrow and is affected by donor-host antigen disparity and the nature of hosting parenchymal stroma.25 Furthermore, the possibility of autocrine signaling arises from co-expression of the cognate ligands,25 which is easier to determine for Fas and membrane-bound Fas-ligand (FasL) but is also valid for soluble TNFα.30 This is an apparent paradoxic phenomenon that renders donor cells susceptible to functional suppression9 and physical elimination,10 because the most significant transplant-related early event is protection of HSPC viability by interaction with the marrow stroma.31 Most intriguing is the coordinated ubiquitous upregulation of the TNF family receptors in most primitive murine hematopoietic precursors characterized by absent lineage markers and co-expression of c-Kit and SCA-1 (KLS), as well as the small-sized subset of long-term reconstituting cells isolated by elutriation,25,32 suggesting physiological involvement of the TNF family receptors in the engraftment process. These considerations refute the contention that HSPC are unresponsive to TNF signaling: reconstituting units are highly responsive to these signals under stress conditions such as radiochemotherapy, transplantation, infection, and bleeding.
Hematopoietic progenitors are inherently resistant to apoptosis
Donor lineage–negative, KLS, and elutriated murine HSPC harvested from the BM of recipients are remarkably insensitive to an apoptotic challenge despite ubiquitous expression of death receptors.25-27,32 Likewise, human CD34+ progenitors derived from umbilical cord blood (UCB), bone marrow (BMC), and mobilized peripheral blood (MPB) are resistant to apoptosis triggered by FasL, TNFα, and TRAIL.26,29,33,34 Profiling of human progenitors derived from the 3 main sources—UCB, BMC, and MPB—emphasized robust transcription of components of receptor-related and mitochondrial apoptotic signaling pathways.29,35-38 Functional mitochondrial apoptotic pathway might be anticipated from the extreme metabolic stress experienced during burst in progenitor activity at the onset of differentiation and proliferation, with suicidal consequences in case of energetic compromise. Survival of progenitors under stress conditions of ex vivo culture has been attributed to a series of antiapoptotic factors, which are found at higher levels in more primitive hematopoietic precursors. In most cases, equilibrium between pro- and antiapoptotic factors favors survival of human UCB progenitors, such as high Bcl-2/Bax protein ratios39,40 that sustain viability in the presence of functional Fas signaling.41,42 Likewise, high FLIP/caspase-8 protein ratios are considered to preserve progenitor viability,43,44 along with increased transcriptional and translational upregulation of survivin, a member of the inhibitor of apoptosis (IAP) family.45 Expression of these factors is regulated by NF-κB, a pathway associated with preservation of progenitor viability under stress conditions.38,46,47 Although these factors may be significant in preservation of progenitor viability, transcriptional profiles demonstrate ample and developed mechanisms of protection from apoptosis. For example, similar to death induced by the topoisomerase II inhibitor etoposide,35 apoptosis triggered by Fas crosslinking was associated with low Bcl-2/Bax ratios, primarily as a result of transcriptional upregulation of Bax and, to a lesser extent, downregulation of Bcl-2.29 Progenitors resistant to Fas crosslinking also display low transcriptional FLIP/caspase-8 ratios along high and stable members of the IAP family, questioning whether these are the dominant regulators of cell viability.29
Survival of Fas crosslinking was specifically associated with an inherent transcriptional pattern unfavorable to the transduction of apoptotic signals, including marked upregulation of constituents of the NF-κB pathways. Both canonical (adaptive) and noncanonical (developmental) pathways are upregulated from the level of the triggering receptors to the end products, corresponding to dimers of the NF-κB1 and 2 translocation factors and the partially homologous RelA and RelB transactivation domains.29,38 The NF-κB pathways are involved primarily in sustained clonogenic activity48 and, to a lesser extent, in progenitor commitment to differentiation49 ; thus their earliest role is to sustain cell viability before engagement in developmental programs. The reason for transcriptional upregulation of a significant number of target genes of NF-κB in mitotically quiescent and inactive progenitors is intermittent shuttling of the NF-κB1/RelA signaling unit conjugated to its physiological inhibitor IκB to the nucleus.50 Rather vast patterns of transcription29,38 and translation46,47 may be a wasteful mode of regulation of apoptosis and survival; however, they endow stem and progenitor cells with flexible capacity in adjusting viability by the processing of multiple receptor–mediated environmental cues and integration of signals of energetic sufficiency. A burst in stem and progenitor cell function is preempted by inherent dual transcriptional upregulation of pro- and antiapoptotic pathways, along significant buffering capacity for deterministic triggers of cell death and products of the NF-κB gene expression programs. It is tempting to speculate that the transcriptomes and proteasomes of mitotically and functionally quiescent HSPC affect the nature of response: the default mechanism of stem cells to extreme stress is differentiation, whereas somatic cells (mature progeny) are induced into apoptosis. The capacity to regulate survival is particularly significant under conditions that deviate from the optional linear differentiation, including progenitor dedifferentiation and transient engagement of stem cells in various differentiation traits.51-53
Dual signaling mediated by the TNF family receptors
Continuous reconstitution of developmental systems from progenitors involves elaborate mechanisms of activation, differentiation, proliferation, and incorporation in the injured tissue. Toxic chemokines and inflammatory cytokines are released by the injured BM stroma and hematopoietic cells, activating extrinsic pathways of programed cell death to ensure deletion of defective cellular components that might have survived injury. Dysfunctional cellular elements should be eliminated and replaced to resume proper tissue function through activation of progenitors. Some of the factors released by dead cells are unknown, such as those that suppress clonogenic activity in culture.29,54 Among the identified factors that contribute to compensatory regeneration are TNF family receptor/ligand interactions, which enhance reconstitution by activation of stem and progenitor cells. It is therefore not surprising that hematopoietic engraftment depends to a certain extent on injury18,19 that signals the need to terminate the state of stem cell dormancy,55 questioning the consequences of TNF family receptor signaling in modulation of such acute responses. Trophic activities of TNF family receptors have been recognized in several vital organs, for example neural and glial cells are resistant to apoptosis triggered by Fas and TRAIL-R2 and are sensitive to apoptosis induced by TNF-R1,56-58 but these receptors also induce neural progenitor growth.59,60 This functional duality raises debate over the potential therapeutic efficiency of neutralization of these receptors to reduce cell death in neurodegenerative disorders, which concomitantly may block their trophic activities.57 Likewise, hepatocytes are excessively sensitive to Fas-mediated apoptosis, yet liver regeneration depends on functional Fas signaling.61 The murine and human hematopoietic system displays similar differential responses to TNF family receptors: trophic signaling in stem and progenitor cells and induction of apoptosis in the differentiated and mature progeny.26,27,33,34,54 As growth factors, the TNF family members couple injury with mechanisms of cellular repair.
The TNF family receptors do not induce particular traits of differentiation, but rather they activate HSPC and synergize with other growth factors in initiating clonogenic activity. TNF-R1 and TRAIL-R1 synergize with granulocyte-macrophage colony stimulating factor (GM-CSF) and erythropoietin (EPO) and substantially increase the number of human colonies by ∼50%.27,33,54 In addition to support of committed human and murine progenitors in semisolid cultures, myeloid differentiation is also enhanced in xenochimeric mice after pretransplant exposure of human progenitors to TNFα, affirmed by secondary clonogenic assays and transplants.27,29,33,34,54 This surrogate assay evaluates more primitive hematopoietic precursors, possibly associated with the human reconstituting cells, emphasizing that acute TNF receptor activation is memorized during the very long period of human cell development under conditions of partial interspecies chemokine compatibility. Trophic signaling of the TNFα and TRAIL receptors may be mediated by termination of the state of dormancy and/or diversion of signaling toward growth, which might represent an autocrine mechanism of activation because TNFα induces both secretion of growth factors, such as IL-3 and GM-CSF, and concomitantly upregulates the cognate receptors.62 The molecular mechanisms of trophic signaling by the TNF family receptors remain elusive. TNF-R1 and TRAIL-R1 potentiate recruitment and initiation of the activity of colony-forming units in cultures stimulated by SCF, IL-3, and GM-CSF, whereas Fas indirectly suppresses clonogenic activity through apoptosis of sensitive cells. A distinct checkpoint is involvement of caspase-8 in trophic activity of TNF-R1, which is not required for signaling by Fas and TRAIL-R1, whereas effector caspase-3 is dispensable in trophic activities of all receptors.26,29,54,63 Susceptibility to receptor-mediated apoptotic signaling develops gradually along the differentiation process, resulting in decreased size of colonies mediated predominantly by Fas and TNF-R1.25-27,29,54
Receptor specificity and species differences
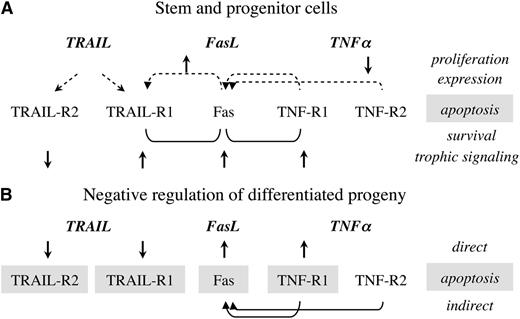
Trophic activities are not equally distributed among members of the TNF superfamily. TRAIL sustains viability of human HSPC, enhances engraftment in xenogeneic chimeras after extended ex vivo culture, and is the most potent stimulant of clonogenic activity (Figure 1).26,64 The ligand induces expression of both cognate receptors without affecting the proliferation of human progenitors. The TRAIL receptors operate in a compensatory mode, with TRAIL-R1 supporting activation and TRAIL-R2 suppressing HSPC activity, and concurrent signaling of both receptors converges to induce a marked increase in the size of myeloid colonies.26 The only murine TRAIL-2 receptor expressed in one-third of fresh BM progenitors is upregulated within several days after transplantation, including universal expression in KLS cells and assumes both apoptotic and trophic activities, resulting in a substantial 50% increase in the number of colonies in semisolid cultures.26 Human and murine progenitor stimulation requires high concentrations of TRAIL, usually one order of magnitude higher than other ligands within the TNF family.
Interactions between TNF family receptors. (A) Stem and progenitor cells; (B) the differentiated progeny. The impact of receptor ligation by the cognate ligands and crosstalk are presented in reference to proliferation, induced expression, apoptosis, survival, and trophic signaling. Joint activation of Fas/TNF-R1 and Fas/TRAIL-R1 results in superior survival, with dominant influence of the TNFα and TRAIL receptors.
Interactions between TNF family receptors. (A) Stem and progenitor cells; (B) the differentiated progeny. The impact of receptor ligation by the cognate ligands and crosstalk are presented in reference to proliferation, induced expression, apoptosis, survival, and trophic signaling. Joint activation of Fas/TNF-R1 and Fas/TRAIL-R1 results in superior survival, with dominant influence of the TNFα and TRAIL receptors.
Fas is the common executioner of apoptosis within the TNF family, unique in its requirement of receptor trimerization by oligomers of Fas-ligand, usually bound to cell membrane,28 whereas the soluble isoform blocks receptor trimerization and neutralizes this mechanism.65,66 Both human and murine progenitors are insensitive to Fas-induced apoptosis, whereas the differentiating progeny acquires sensitivity to negative regulation (Figure 1). Trophic activities seen in semisolid cultures of murine BM progenitors and in vivo32 are less evident in human progenitors, although engraftment is enhanced in xenogeneic chimeras.29
The functions of TNF receptors are conserved in human and murine progenitors, with dual apoptotic and trophic activity of TNF-R1 and no detectable effect of TNF-R2 on viability and differentiation.27,54 TNF-R1 supports murine and human progenitor viability, augments clonogenic activity, enhances hematopoietic reconstitution, and fosters early myeloid recovery after pretransplant exposure to TNFα. The only significant impact of TNF-R2 in unstimulated UCB cells is suppression of proliferation (Figure 1)64 of CD34+TNF-R2+ progenitors that cycle at faster rates than progenitors expressing TNF-R1.54,67 However, impaired competitiveness and durable reconstitution of progenitors deficient in either one of the TNF receptors attribute TNF-R2, a significant, yet unidentified role in hematopoiesis.27 It is possible that some of the features of TNF-R2 are evaluated in suboptimal surrogate assays, because it is preferentially activated by membrane-bound TNFα, whereas TNF-R1 is activated by the soluble ligand.68,69
Crosstalk and interactions between TNF receptors
Interpretation of the activities of TNF family receptors has to consider inductive crosstalk, such as the classical inductive effect of TNFα on Fas,7,11,12 which is mediated by both TNF receptors.64 In contrast to the association of Fas crosslinking with impaired function of chemokine-stimulated progenitors, we found that TNF-R1 is dominant over Fas and TNF-R2 in protecting survival of progenitors in the absence of supporting chemokines.33,64 Fas indirectly suppresses clonogenic activities of progenitors by induction of apoptosis in sensitive cells, as demonstrated by the restrictive impact of dead cells in semisolid cultures.29,54 This phenomenon might bias the interpretation of in vitro assays as direct negative regulation of progenitor viability and function, and suggests that chemokine supplements sensitize to Fas-induced apoptosis and abolish the dominant protective effect of TNF-R1.70,71
Additional inductive interactions between receptors include Fas-mediated upregulation of TRAIL-R1 and reversal of slow proliferation of CD34+TRAIL-R1+ progenitors,26 which result in decreased fractional apoptosis.64 These patterns of crosstalk are interesting because both TRAIL receptors and Fas are common targets of gene transcription induced by NF-κB, yet only TRAIL-R1 is transcribed and translated, whereas transcription of TRAIL-R2 is not induced by Fas crosslinking.64 Furthermore, TRAIL is the only TNF family member that upregulates both cognate receptors 1 and 2 in hematopoietic progenitors (Figure 1). If upregulated expression of the cognate receptors by TRAIL and of Fas by TNFα was mediated solely by NF-κB translocation, it is unexplained why the transcription program is only partially performed: TRAIL does not induce Fas and TNFα does not induce the TRAIL receptors.
Physiological implications of progenitor survival
The best characterized model of hematopoietic progenitor activation is the acute response to infection and inflammation,72 mediated by a series of receptors to cytokines and chemokines expressed by HSPC.29,35,38 The influence of growth factors has been long recognized,73 with subsequent identification of the involvement of toll-like receptors74 and cytokines such as interferons α and γ, which suppress and induce hematopoietic progenitor function upon tonic and phasic exposure, respectively.75,76 Similar differential effects are observed for short and sustained TNF receptor activation,77,78 which induces transcription of different sets of genes controlled by NF-κB and has distinct consequences under various experimental conditions.7-17,70,72 Identification of multiple functions of TNF superfamily receptor/ligand interactions in hematopoietic cells questions the physiological significance. Engraftment of murine lineage–negative progenitors deficient in Fas, TNF-R1, and TNF-R2 is markedly deficient, with transient early support of hematopoiesis but reduced competitive durable reconstitution.27,79 The activity of these receptors is therefore essential rather than complementary in the process of hematopoietic reconstitution from stem cells. An apparent confounding observation is superior competitive engraftment of donor cells deficient in either one or both TNF receptors after transplantation of whole BM cells.80 The higher levels of chimerism observed in mice reconstituted with cells deficient in TNF receptors might be caused by extended survival of the progeny in the periphery, rather than the reduced engraftment caused by sensitivity to TNFα.81 Several physiological mechanisms have been identified thus far with direct and indirect influences on immunogenic and nonimmunogenic events in the course of hematopoietic reconstitution.
The first mechanism involves trophic signals that support HSPC activity and foster repopulation of the immune-hematopoietic system, mediated by autocrine and/or paracrine signaling in progenitors displaying joint upregulation of TNF family receptors and ligands under stress conditions. Autocrine signaling was evident from the markedly improved engraftment when ectopic metalloproteinase-resistant FasL protein was adsorbed onto the surface of murine BM progenitors.25,32 Paracrine signaling was evident from impaired engraftment under conditions of incompetent Fas/FasL interaction caused by both the absence of receptors in donor cells (lpr) and the ligand in recipients (gld).79 In both cases, trophic signaling was mediated by functional Fas trimerization because neutralizing antibodies and the soluble FasL isoform reversed the beneficial effects on progenitor activity in culture.32,65 Furthermore, human progenitors responsive to TNFα displayed enhanced clonogenic activity and myeloid differentiation in culture54,82 and myelomonocytic reconstitution in xenogeneic chimeras,33,54,83 possibly mediated by activation of the NF-κB pathway.29,48 TNFα is abundantly secreted as a consequence of tissue injury and is also produced by hematopoietic progenitors and immune cells.30,83,84
The second mechanism involves self-defense of the hematopoietic progenitors from host versus graft rejection in nonmyeloablative transplants. Physiological and pharmacologic overexpression of FasL improve the chances of hematopoietic progenitors to engraft in the host BM.25,85 FasL is inherently expressed in approximately one-third of most primitive hematopoietic stem cells79 endowed with long-term reconstituting potential and nonhematopoietic differentiation capacity,86,87 conferring immune privilege to progenitors in hostile environments. Furthermore, murine KLS progenitors ubiquitously upregulate FasL after transplantation, as a mechanism of immune privilege and defense from residual recipient immunity.25 A similar veto effect is imposed by TNFα to ensure survival of human progenitors and protect from rejection.88,89 In the transplant setting, polarized insensitivity of stem cells and sensitivity of committed and more differentiated progenitors to apoptosis endows the more primitive precursors with a competitive advantage in homing to and seeding in the marrow niches.25 This prioritization mechanism favors engraftment of units of regeneration over cells with more limited reconstituting capacity.
The third mechanism involves bidirectional interactions of hematopoietic progenitors with the niches, which govern and direct the activity of progenitors at distinct sites of residence within the marrow space through inductive and restrictive signals emanating from the stroma, osteoblasts, and extracellular matrix.90 An interesting mechanism is the activation of stromal Fas by the cognate ligand expressed in hematopoietic progenitors.79 Impaired engraftment of wild-type progenitors in Fas-deficient recipients (lpr) and of FasL-deficient progenitors (gld) in wild-type recipients was reversed in the latter case by overexpression of ectopic FasL protein, demonstrating functional signaling in the process of engraftment. Reduced engraftment in the absence of competent stromal Fas signaling was validated in chimeras expressing Fas only in the BM, removing the trophic consequences of Fas signaling in the engrafting progenitors. Likewise, myeloablative transplants showed that the mechanism of stromal signaling is independent of the immune privilege conferred to the grafted progenitors by FasL, particularly because cell-stroma interactions in the BM are not restricted by antigenic barriers.91 Reverse stromal signaling through constitutively expressed Fas92 may be involved in potentiation of the BM to accept additional cells after initial colonization by donor progenitors, resulting in a virtually unlimited seeding space.91
Therapeutic implementation of progenitor resistance to apoptosis
Resistance to apoptotic signaling is a fundamental characteristic of hematopoietic stem and progenitor cells regulated at the transcriptional level, which has several potential therapeutic implications. Pretransplant exposure of the hematopoietic cell graft to apoptotic ligands accomplishes concomitant independent mechanisms that converge to improve the outcome of transplants. First, an apoptotic challenge is a simple physiological tool for collection of larger numbers of viable HSPC unrestricted by particular phenotypic identifiers, because cellular grafts can be safely processed without impairing the viability of the units of reconstitution.26,29,33,34,54 Receptor-mediated apoptosis can also be used to deplete differentiated progeny under the influence of growth factors in extended cultures of UCB expansion.33 Second, pretransplant exposure of human progenitors to FasL, TNFα, and TRAIL trigger trophic signals that improve subsequent myeloid reconstitution in vivo.26,29,54 Third, equally important is the nature of cells depleted by the apoptotic challenge, including donor T cells that mediate potentially lethal graft-versus-host disease (GVHD). Functional depletion and inactivation of donor T cells may be a better approach to GVHD prophylaxis93 in view of the limited success of phenotypic deletion, because this immune reaction is mediated by redundant effector activity of numerous T-cell subsets instructed by antigen-presenting cells of donor and host origin.94 Depletion of T cells sensitive to apoptosis without host-specific sensitization reduces significantly graft-versus-host reactivity in murine and human grafts, while preserving facilitation of progenitor engraftment and graft-vs-tumor reactivity.33,95 Fourth, some types of malignant cells display excessive sensitivity to apoptosis and can be effectively purged by short graft processing before autologous hematopoietic reconstitution after aggressive radiochemotherapy in oncology patients.96,97 An intriguing possibility is malignant transformation that occurs in more primitive hematopoietic precursors, where inherited resistance to apoptosis might cause aggressive behavior and poor response to therapy.98-100
Differential trophic responses elicited by the individual receptors allow flexible implementation to modulate several transplant-related parameters. For example, an algorithm of the limitations of various types of transplants can be applied to consider donor-host antigen disparity, the source of HSPC, conditioning, and the anticipated complications in individual patients. Autologous reconstitution in oncology patients requires prompt purging of residual tumor cells using FasL, TNFα, and TRAIL, whereas UCB transplants are limited by the low numbers of cells and delayed engraftment, which may be improved by negative selection and simultaneous trophic signaling by TNFα and TRAIL. Allogeneic and haplo-identical transplants using MPB grafts containing significant numbers of donor T cells present high risk of severe GVHD, which may be ameliorated by exposure to FasL and TNFα. Transplants are frequently performed in heavily pretreated patients with dormant and subclinical infections that might flare in the presence of immunosuppression; thus the use of additional cytotoxic agents to suppress GVHD is unwarranted.
The multifaceted involvement of TNF family receptors in hematopoietic stem and progenitor cell function calls for extreme care in neutralization of these signaling pathways. Apoptotic activities of these receptor/ligand interactions are in fact a mechanism of defense from lymphoproliferation, immunodeficiency, and malignancy, conditions triggered by prolonged administration of immunosuppressive agents. Although members of the TNF family act as cytotoxic pathways in severe GVHD reactions,93 and initial clinical studies suggested that early deletion of TNF-R1+ T cells may be beneficial depending on conditioning,101 randomized studies showed little benefit of this approach to treat severe GVHD.102 Likewise, individual members of the TNF family might participate in particular aspects of dysfunctional hematopoiesis in aplastic anemia and myelodysplastic syndromes.103-106 In perspective of the trophic activities of the TNF superfamily in hematopoiesis, receptors and ligands are expected to increase as a feature of the effort to recover from hypoplasia; thus therapeutic neutralization has to be carefully considered. It remains to be determined which types of dysfunctional hematopoiesis are caused by inherent hypersensitivity of hematopoietic progenitors to apoptosis mediated by specific receptors and/or aberrant responses to certain environments, and whether neutralization or supplementation are of therapeutic potential.103-107
Authorship
Contribution: K.M. and N.A. prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nadir Askenasy, Frankel Laboratory, Center for Stem Cell Research, Schneider Children’s Medical Center of Israel, 14 Kaplan St, Petach Tikva, Israel 49202; e-mail: anadir@012.net.il.