Key Points
A cellular vaccine incorporating the glycolipid α-galactosylceramide prevents relapse of acute leukemia following cytarabine chemotherapy.
Abstract
Acute leukemias with adverse prognostic features carry a high relapse rate without allogeneic stem cell transplantation (allo-SCT). Allo-SCT has a high morbidity and is precluded for many patients because of advanced age or comorbidities. Postremission therapies with reduced toxicities are urgently needed. The murine acute leukemia model C1498 was used to study the efficacy of an intravenously administered vaccine consisting of irradiated leukemia cells loaded with the natural killer T (NKT)-cell agonist α-galactosylceramide (α-GalCer). Prophylactically, the vaccine was highly effective at preventing leukemia development through the downstream activities of activated NKT cells, which were dependent on splenic langerin+CD8α+ dendritic cells and which led to stimulation of antileukemia CD4+ and CD8+ T cells. However, hosts with established leukemia received no protective benefit from the vaccine, despite inducing NKT-cell activation. Established leukemia was associated with increases in regulatory T cells and myeloid-derived suppressor cells, and the leukemic cells themselves were highly suppressive in vitro. Although this suppressive environment impaired both effector arms of the immune response, CD4+ T-cell responses were more severely affected. When cytarabine chemotherapy was administered prior to vaccination, all animals in remission posttherapy were protected against rechallenge with viable leukemia cells.
Introduction
Induction chemotherapies for acute leukemias typically induce morphologic remission, but without allogeneic stem cell transplantation (allo-SCT), most patients with high-risk genetic features subsequently relapse.1-5 Allo-SCT has high morbidity and mortality, is costly, and is often precluded by age, comorbidities, or lack of a suitable donor.6-8 There is an unmet need for effective postremission therapies that do not carry the toxicities and cost of allo-SCT.9
Relapse of acute leukemia is mediated by a population of blasts that fall below the threshold used to define morphologic remission but may be detected by using sensitive flow cytometric or molecular assays.10-14 In addition to overexpressing certain self-antigens,15 leukemic blasts harbor numerous mutations,16 resulting in expression of tumor-specific antigens capable of eliciting autologous CD4+ and CD8+ T-cell responses.17 This can potentially be exploited by postremission immunotherapy.18,19
The use of irradiated whole leukemia cells in vaccines for postremission immunotherapy is technically feasible20 and has the potential to elicit immune responses against multiple leukemia-specific antigens without needing to first define leukemia-specific T-cell epitopes or patient tissue type. However, administration of a vaccine without a suitable adjuvant is unlikely to elicit an effective immune response and may lead to tolerance.21,22 The glycolipid α-galactosylceramide (α-GalCer) has recently been shown to be a useful adjuvant for whole tumor cell vaccination by eliciting stimulatory interactions between dendritic cells (DCs) and natural killer T (NKT) cells.23-26 When DCs acquire cellular material from irradiated tumor cells that have been treated with α-GalCer, the protein content is presented as peptides via major histocompatibility complex (MHC) molecules to CD4+ and CD8+ T cells, whereas the α-GalCer is presented via the MHC-like molecule CD1d to NKT cells. Interactions between DCs and NKT cells promote CD40 signaling, leading to DC activation, which increases the capacity of DCs to stimulate peptide-specific T cells.23,24 Significantly, vaccines comprising irradiated tumor cells pulsed with α-GalCer have been shown to be effective in murine models of hematopoietic malignancies, including acute myeloid leukemia (AML) and acute lymphoid leukemia,23,27,28 and in other malignancies.25,27,29
Cancer-associated immunosuppression can present a significant barrier to effective vaccine-based immunotherapy.30 AML generates an immunosuppressive environment,31,32 characterized by impaired DC function33 and increased levels of regulatory T cells (Tregs).35-37 It follows that immunotherapy may be most effectively used during morphologic remission after induction chemotherapy.
Here we investigated the efficacy of a vaccine comprising irradiated leukemia cells pulsed with α-GalCer in a murine acute leukemia model. Although vaccination was capable of eliciting a leukemia-specific T-cell response in mice with established disease, the activity was impaired by leukemia-associated immunosuppression. However, when the vaccine was administered to mice in remission after cytarabine chemotherapy, it protected against rechallenge with an increased dose of viable leukemic blasts. These findings have implications for the design of clinical trials that test immunotherapies for acute leukemias.
Materials and methods
Animal ethics
Inbred C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Also used were lang-EGFPDTR and lang-EGFP mice, which express the human diphtheria toxin (DT) receptor and/or enhanced green fluorescent protein (EGFP) under the langerin promoter,38 and FoxP3-GFP mice that have EGFP inserted into the first coding exon of the Foxp3 gene.39 All animals were bred and housed at the Malaghan Institute of Medical Research Biomedical Research Unit, Wellington, New Zealand. Experiments were approved by the Animal Ethics Committee, Victoria University, Wellington, New Zealand (reference 2012R28M).
Media and reagents
The acute leukemia line C149840 (American Type Culture Collection, Manassas, VA), was cultured in Iscove's Modified Dulbecco's Medium supplemented with 5% fetal bovine serum (SAFC Bioscience, Auckland, New Zealand), 100 U/mL penicillin, 100 g/mL streptomycin, and 50 M 2-mercaptoethanol (all from Invitrogen, Auckland, New Zealand). α-GalCer was manufactured by synthesizing a protected phytosphingosine derivative from phytosphingosine (TCI, P1765), as previously described.41,42
Leukemia challenge treatment with whole tumor vaccines
For leukemia challenge experiments, mice were administered 1 × 105 C1498 cells intravenously via the lateral tail vein, unless otherwise stated. To generate vaccines, C1498 cells were cultured for 24 hours in Iscove modified Dulbecco medium with 200 ng/mL α-GalCer, washed with phosphate-buffered saline, and γ-irradiated to 150 Gy. Vaccines comprising 7.5 × 105 cells were administered intravenously. Mice were monitored for onset of leukemia-associated symptoms, such as weight loss or overt behavioral symptoms (hunching, reduced activity, or reduced grooming), and were euthanized after they developed symptoms. Although weight loss was monitored for all mice in symptom-free survival experiments, the onset of symptoms often preceded weight loss in this model. Experiments were conducted with 5 to 6 animals per treatment group. CD4+ or CD8+ T cells were depleted by injection of anti-CD4 antibodies (GK1.5; 125 μg per mouse), or anti-CD8 antibodies (2.43; 250 μg per mouse), respectively, administered 5, 12, and 19 days after vaccination; depletion methods were sufficient to maintain >95% depletion for GK1.5 and >90% for 2.43 over the course of the experiment (supplemental Figure 1, available on the Blood Web site). Anti-CD25 (clone PC61) was used to deplete Tregs, resulting in >95% reduction of CD4+CD25+ cells (supplemental Figure 2). Langerin+ DCs were depleted from lang-EGFPDTR mice by intraperitoneal administration of 350 ng of DT 2 days before vaccine, resulting in >95% reduction of langerin+CD8α+ DCs for 3 days.43,44 In some experiments, 3 doses of 3 mg of cytarabine (Pfizer, Auckland, New Zealand) were administered 10 hours apart the day after leukemia challenge.
Histology
Femurs were placed in 4% formalin (Sigma-Aldrich, St. Louis, MO), decalcified with 10% formic acid, and processed. Paraffin-embedded sections were stained with hematoxylin and eosin (made in-house), and blood smears were stained with Romanowsky stain variant (Siemens Healthcare, Erlangen, Germany). Slides were examined with an Olympus BX51 microscope (Precision Microscopy Equipment, Wellington, New Zealand) and captured with an Olympus DP70 (Precision Microscopy Equipment) using Cell^F software (Olympus).
Cytokine production assay
Supernatant cytokine levels were measured by cytokine bead array (Biorad Laboratories, Inc, Auckland, New Zealand) following culture with 1 000 000 splenocytes and 10 000 bone marrow (BM)–derived DCs (BM-DCs) loaded with C1498 lysate for 4 hours. BM-DCs were prepared from syngeneic BM cultured in interleukin-4 (IL-4) and granulocyte macrophage colony-stimulating factor for 6 days, followed by 18 hours with 100 ng/mL of lipopolysaccharide; lysate from C1498 was added at a ratio of 1 DC to the equivalent of 6 tumor cells for the last 4 hours.
T-cell suppression assay
Lymph node preparations from naïve C57BL/6 mice were stained with carboxyfluorescein succinimidyl ester (CFSE) and cultured for 72 hours with 2 µg/mL anti-CD3 (clone 2C11) and 2 µg/mL anti-CD28 (clone 37.51) (both prepared in-house) in the presence of purified splenic CD11b+ cells or C1498 cells. CFSE dilution on T cells was analyzed by flow cytometry.
Flow cytometry
Nonspecific Fc receptor binding was blocked with anti-CD16/32 clone 2.4G2 (prepared in-house). Dead cells were excluded by staining with propidium iodide (BD Pharmingen, San Jose, CA), 4′,6,-diamidino-2-phenylindole (Invitrogen) or LIVE/DEAD Fixable Blue (Invitrogen). For intracellular staining, cells were restimulated for 20 hours with anti-CD3 and anti-CD28 antibodies, and 1 µg/mL monensin was added for the last 4 hours of incubation. Flow cytometry was performed by using a FACSCalibur or LSRII flow cytometer (BD Biosciences) and was analyzed by using FlowJo software (TreeStar Inc.). Doublet and dead-cell exclusion was performed. The antibodies used were anti-CD3-fluorescein isothiocyanate (145-2C11), CD3-phycoerythrin (PE)-Cy7 (145-2C11), anti-CD4-A488 (GK1.5), anti-CD8-A700 (53-6.7), anti-CD11b biotin (M170), anti-CD86 PE (GL-1), anti-CD44-peridinin chlorophyll-Cy5.5 (IM7), anti-FoxP3-PE (FJK-16s), and anti-interferon gamma (IFN-γ)-PE-Cy7 (XMG1-2), all from eBioscience (Auckland, New Zealand); anti-CD11c-PE-Cy7 (N418), anti-CD4-allophycocyanin (GK1.5), anti-CD8-fluorescein isothiocyanate (53-6.7), and anti-CD11c-allophycocyanin (N418), all from Biolegend (San Diego, CA); and anti-CD8-Pacific blue (53-6.7), anti-CD40-biotinylated (3/23), and streptavidin-PE-Cy7, all from BD Bioscience. Invariant NKT cells were detected by using α-GalCer-loaded CD1d tetramers (National Institutes of Health Tetramer Core Facility, Atlanta, GA).
Statistical analyses
Bars and error bars in the figures depict the mean and standard deviation of the mean. For comparisons of 1 variable, the Mann-Whitney U test was used for unpaired data, the Wilcoxon matched pairs test was used for paired data, and a 1-way analysis of variance with a Bonferroni posttest was used for experiments comparing more than 2 groups. The log-rank test was used to determine significance between Kaplan-Meier survival curves. Analysis was performed with Prism 5.0 software (GraphPad Software, Inc.); P values <.05 were considered significant.
Results
A vaccine comprising irradiated α-GalCer–pulsed leukemia cells protects against acute leukemia through the activities of CD4+ and CD8+ T cells and langerin+CD8α+ DCs
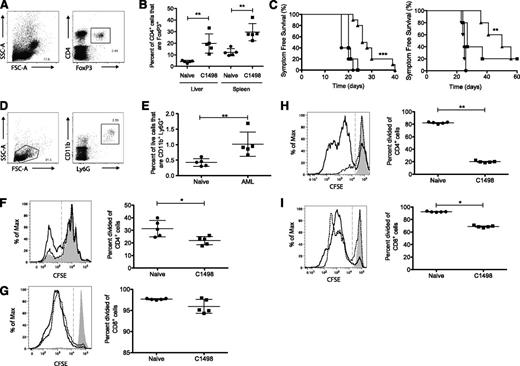
Mice intravenously challenged with the cell line C1498 developed leukemia characterized by replacement of normal BM hematopoiesis and leukocytosis with circulating blasts (Figure 1A-C). However, mice vaccinated with irradiated leukemia cells pulsed with the glycolipid adjuvant α-GalCer (leukemia/α-GalCer) 7 days before C1498 challenge did not develop leukocytosis (Figure 1C), and no blasts were seen in the peripheral blood smear or by histologic examination of BM (Figure 1A-C). Animals vaccinated with the leukemia/α-GalCer vaccine were protected from leukemia development and remained symptom-free for the duration of the experiment (Figure 1D). Vaccination with irradiated leukemia cells without α-GalCer or with α-GalCer alone did not protect hosts from leukemia development (Figure 1D).
An α-GalCer–pulsed whole leukemia cell vaccine protects against acute leukemia and is dependent on CD4+ and CD8+ T cells and langerin+CD8α+ DCs. A vaccine comprising α-GalCer–pulsed irradiated C1498 cells was administered intravenously 7 days before C1498 challenge. (A) BM histology, (B) peripheral blood smear, and (C) peripheral white blood cell counts were performed at symptom onset in unvaccinated animals, 40 days after C1498 challenge in vaccinated animals, and in naïve control mice. (D) Kaplan-Meier plot showing symptom-free survival of vaccinated and unvaccinated mice after leukemia administration on day zero. Statistical analysis compared unvaccinated and vaccinated with α-GalCer–pulsed irradiated leukemia cell groups. (E) Kaplan-Meier plots showing symptom-free survival of mice vaccinated with leukemia/α-GalCer and challenged with C1498 at day zero. (C-E) Symbols represent treatments: unvaccinated (●), nonleukemic and nonvaccinated (◆), vaccinated with α-GalCer–pulsed irradiated leukemia cells (▪), vaccinated with unpulsed irradiated leukemia cells (□), vaccinated with free α-GalCer (△), depletion of CD4+ cells (▲), and depletion of CD8+ cells (▼). Statistical analyses compared the depletion groups to mice vaccinated with α-GalCer–pulsed irradiated leukemia cells. (F) lang-EGFPDTR mice were prophylactically vaccinated, and 1 group was administered DT. Symbols represent treatment groups: unvaccinated (●), prophylactic α-GalCer vaccination (▪), and prophylactic vaccination and DT treatment (▲). Symptom-free survival was analyzed and is graphed. *P < .05 (1-way analysis of variance [ANOVA] with a Bonferroni posttest). (A-C) represent a single experiment, (D) represents 3 experiments, and (E-F) represent 2 experiments; 5 mice per group were used for each experiment. *P < .05; **P < .01; ***P < .001 (Mantel-Cox log-rank test).
An α-GalCer–pulsed whole leukemia cell vaccine protects against acute leukemia and is dependent on CD4+ and CD8+ T cells and langerin+CD8α+ DCs. A vaccine comprising α-GalCer–pulsed irradiated C1498 cells was administered intravenously 7 days before C1498 challenge. (A) BM histology, (B) peripheral blood smear, and (C) peripheral white blood cell counts were performed at symptom onset in unvaccinated animals, 40 days after C1498 challenge in vaccinated animals, and in naïve control mice. (D) Kaplan-Meier plot showing symptom-free survival of vaccinated and unvaccinated mice after leukemia administration on day zero. Statistical analysis compared unvaccinated and vaccinated with α-GalCer–pulsed irradiated leukemia cell groups. (E) Kaplan-Meier plots showing symptom-free survival of mice vaccinated with leukemia/α-GalCer and challenged with C1498 at day zero. (C-E) Symbols represent treatments: unvaccinated (●), nonleukemic and nonvaccinated (◆), vaccinated with α-GalCer–pulsed irradiated leukemia cells (▪), vaccinated with unpulsed irradiated leukemia cells (□), vaccinated with free α-GalCer (△), depletion of CD4+ cells (▲), and depletion of CD8+ cells (▼). Statistical analyses compared the depletion groups to mice vaccinated with α-GalCer–pulsed irradiated leukemia cells. (F) lang-EGFPDTR mice were prophylactically vaccinated, and 1 group was administered DT. Symbols represent treatment groups: unvaccinated (●), prophylactic α-GalCer vaccination (▪), and prophylactic vaccination and DT treatment (▲). Symptom-free survival was analyzed and is graphed. *P < .05 (1-way analysis of variance [ANOVA] with a Bonferroni posttest). (A-C) represent a single experiment, (D) represents 3 experiments, and (E-F) represent 2 experiments; 5 mice per group were used for each experiment. *P < .05; **P < .01; ***P < .001 (Mantel-Cox log-rank test).
To determine the effector cells responsible for vaccine-induced protection against development of leukemia, mice were vaccinated and depleted of CD4+ or CD8+ cells 2 days before C1498 challenge to ensure that the depletion would not interfere with cells potentially important for immune priming. Vaccine-induced protection was reduced following depletion of either CD4+ or CD8+ cells, suggesting that both CD4+ and CD8+ T cells mediated the protection afforded by the vaccine (Figure 1E).
In the spleen, CD8α+ DCs are thought to be responsible for phagocytosing circulating apoptotic cells,21 and a subpopulation of these cells in the marginal zone that express CD103 and langerin have been shown to induce robust cytotoxic T-cell responses through efficient CD8+ T-cell cross-priming.44-46 To establish whether langerin+ DCs were involved in the protection afforded by the leukemia/α-GalCer vaccine, lang-EGFPDTR hosts were depleted of langerin-expressing cells by DT treatment before vaccination, as previously described.44 This led to complete abrogation of the protective effect of leukemia/α-GalCer (Figure 1F), indicating that langerin+ DCs are essential for vaccine efficacy.
Vaccination is ineffective in the presence of established leukemia despite retaining capacity to stimulate NKT cells and DCs
Having shown that prophylactic leukemia/α-GalCer vaccination can elicit an antitumor effect, we next determined whether the vaccine could prolong survival in mice with established leukemia. Mice inoculated with C1498 1 week before vaccination with leukemia/α-GalCer received no therapeutic benefit, and the onset of symptoms was comparable to that in nonvaccinated controls (Figure 2A).
Leukemia/α-GalCer vaccination is ineffective in the presence of established acute leukemia despite NKT-cell and DC activation. Mice were challenged with C1498 cells intravenously 1 week before vaccination with α-GalCer–pulsed or unpulsed irradiated leukemia cells. (A) Kaplan-Meier graph showing survival of mice. Symbols represent treatment groups: unvaccinated (●), therapeutic α-GalCer vaccination (▪), and treatment with unpulsed irradiated leukemia cells (▲). This graph represents 3 experiments, each with 5 mice per group. (B-E) Mice were inoculated with C1498 cells 7 or 14 days before vaccination with irradiated α-GalCer–pulsed leukemia cells. (B) Representative flow cytometry plots showing identification of splenic NKT cells (CD3+ α-GalCer–loaded CD1d tetramer+ [tet]). (C) Frequency of splenic NKT cells after vaccination in mice with and without established acute leukemia. (D) Serum IL-4 levels and (E) serum IFN-γ levels 2 hours after vaccination. (F-G) The splenic langerin+CD8α+ DC population in lang-EGFP mice was analyzed 24 hours after vaccination. (F) Representative flow cytometry plots showing identification of splenic langerin+CD8α+ DCs (CD11c+GFP+). (G) Frequency of splenic langerin+CD8α+ DCs. (H-I) The expression of CD40 and CD86 on langerin+CD8α+ DCs, respectively. (J-K) The CD8α+ DC population in C57BL/6 mice was analyzed 24 hours after vaccination. The expression of CD40 and CD86 on CD8α+ DCs, respectively. (L) Serum IL-12p70 was quantified 5 hours after vaccination. These results are indicative of 2 independent experiments, each with 5 mice per group. **P < .01; ***P < .001 (1-way ANOVA with a Bonferroni posttest). FSC-A, forward scatter area; MFI, mean fluorescent intensity; SSC, side scatter.
Leukemia/α-GalCer vaccination is ineffective in the presence of established acute leukemia despite NKT-cell and DC activation. Mice were challenged with C1498 cells intravenously 1 week before vaccination with α-GalCer–pulsed or unpulsed irradiated leukemia cells. (A) Kaplan-Meier graph showing survival of mice. Symbols represent treatment groups: unvaccinated (●), therapeutic α-GalCer vaccination (▪), and treatment with unpulsed irradiated leukemia cells (▲). This graph represents 3 experiments, each with 5 mice per group. (B-E) Mice were inoculated with C1498 cells 7 or 14 days before vaccination with irradiated α-GalCer–pulsed leukemia cells. (B) Representative flow cytometry plots showing identification of splenic NKT cells (CD3+ α-GalCer–loaded CD1d tetramer+ [tet]). (C) Frequency of splenic NKT cells after vaccination in mice with and without established acute leukemia. (D) Serum IL-4 levels and (E) serum IFN-γ levels 2 hours after vaccination. (F-G) The splenic langerin+CD8α+ DC population in lang-EGFP mice was analyzed 24 hours after vaccination. (F) Representative flow cytometry plots showing identification of splenic langerin+CD8α+ DCs (CD11c+GFP+). (G) Frequency of splenic langerin+CD8α+ DCs. (H-I) The expression of CD40 and CD86 on langerin+CD8α+ DCs, respectively. (J-K) The CD8α+ DC population in C57BL/6 mice was analyzed 24 hours after vaccination. The expression of CD40 and CD86 on CD8α+ DCs, respectively. (L) Serum IL-12p70 was quantified 5 hours after vaccination. These results are indicative of 2 independent experiments, each with 5 mice per group. **P < .01; ***P < .001 (1-way ANOVA with a Bonferroni posttest). FSC-A, forward scatter area; MFI, mean fluorescent intensity; SSC, side scatter.
To explore why therapeutic vaccination of mice with established leukemia was ineffective, we investigated the cascade of immune activation involved in α-GalCer-adjuvanted vaccination. Because the adjuvant effect of α-GalCer involves reciprocal interaction and activation of NKT cells and DCs,47-50 we first assessed the level of NKT-cell activation by analyzing NKT-cell number and function in animals vaccinated 7 or 14 days after C1498 challenge. A substantial increase in the number of splenic NKT cells was observed in all vaccinated animals compared with unvaccinated controls, although there was a trend toward reduced accumulation of NKT cells in leukemia-bearing animals (Figure 2B-C). A notable feature of activated NKT cells is the ability to rapidly produce high levels of the cytokines IL-4 and IFN-γ, which can be detected in serum.47 Both cytokines were detected in host serum after vaccination, and levels were similar in mice with and without established leukemia (Figure 2D-E). Together these data suggest that the capacity of the vaccine to stimulate NK T cells in established leukemia is largely intact.
We next determined whether the presence of established leukemia impaired the ability of the vaccine to activate DCs. lang-EGFP hosts were used to identify langerin+CD8α+ DCs by flow cytometry. A decrease in the proportion of the langerin+CD8α+ subset of DCs was found in the spleens of all vaccinated mice regardless of the presence of established leukemia, which is consistent with previous reports showing that these DCs are depleted in response to NKT-cell stimulation (Figure 2F-G).21,45,51 The remaining langerin+CD8α+ DCs upregulated CD40 and CD86 after vaccination, irrespective of the presence of established leukemia (Figure 2H-I), although the expression of CD86 on langerin+CD8α+ DCs was reduced in mice with established leukemia. The expression of CD40 and CD86 was similarly upregulated in CD8α+ DCs (Figure 2J-K).
The interaction between DCs and NKT cells involves CD40-CD40 ligand interaction that contributes to significant production of IL-12, primarily from langerin+CD8α+ DCs.44 Serum IL-12 was analyzed 5 hours after vaccine administration in the presence or absence of established leukemia, and similar levels were observed (Figure 2L). Together, these results indicate that the inefficacy of the vaccine was not attributed to a failure to activate NKT cells or DCs.
Vaccine-induced activation of CD4+ T cells is suppressed in the presence of established leukemia
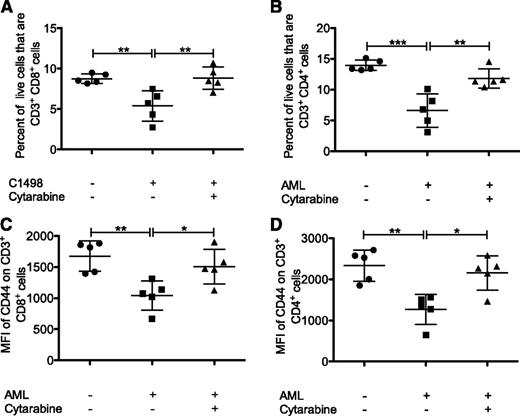
To establish whether leukemia suppressed effector T cells, the vaccine-induced T-cell response was examined. Splenocytes from animals vaccinated in the presence or absence of established leukemia were cultured with BM-DCs loaded with C1498 lysate, and levels of IFN-γ in the supernatant were quantified. Splenocytes from vaccinated animals had elevated supernatant IFN-γ following restimulation, suggesting a leukemia antigen–specific immune response had been induced, although overall levels from leukemia-bearing animals were not significantly different from those in animals without leukemia (Figure 3A). Flow cytometric analysis of splenic CD8+ T cells 7 days after vaccination showed that upregulation of the activation marker CD44 and intracellular IFN-γ production were not significantly impaired by the presence of established leukemia (Figure 3C-D). However, established leukemia prevented vaccine-induced CD44 and IFN-γ expression on CD4+ T cells (Figure 3E-F). Therefore, in the established leukemia setting, the induction of leukemia-specific CD4+ T cells was impaired.
Established leukemia disrupts leukemia/α-GalCer vaccine–mediated CD4+ T-cell function. Mice were challenged with C1498 intravenously 7 days before vaccination, and responses were analyzed 1 week later. (A) Splenocytes were cultured for 24 hours with (○) or without (●) DCs loaded with C1498 lysate. Supernatant IFN-γ was quantified. (B-F) The splenic CD4+ and CD8+ T-cell populations were analyzed by flow cytometry. (B) Representative flow cytometry plots showing identification of CD4+ and CD8+ T cells from mouse spleens. IFN-γ+ cells were determined by comparison with an isotype control antibody (lower left). MFI of CD44 expressed on (C) CD8+ and (E) CD4+ T cells. The proportion of (D) CD8+ and (F) CD4+ cells that produced IFN-γ. Graph in (A) represents 3 experiments. Statistical analysis compared experiments performed in the presence of DCs only. (B-F) Graphs represent 2 experiments, each with 5 mice per group. *P < .05, **P < .01, ***P < .001 (1-way ANOVA with a Bonferroni posttest for [A,C-F]). FSC-H, foward scatter height.
Established leukemia disrupts leukemia/α-GalCer vaccine–mediated CD4+ T-cell function. Mice were challenged with C1498 intravenously 7 days before vaccination, and responses were analyzed 1 week later. (A) Splenocytes were cultured for 24 hours with (○) or without (●) DCs loaded with C1498 lysate. Supernatant IFN-γ was quantified. (B-F) The splenic CD4+ and CD8+ T-cell populations were analyzed by flow cytometry. (B) Representative flow cytometry plots showing identification of CD4+ and CD8+ T cells from mouse spleens. IFN-γ+ cells were determined by comparison with an isotype control antibody (lower left). MFI of CD44 expressed on (C) CD8+ and (E) CD4+ T cells. The proportion of (D) CD8+ and (F) CD4+ cells that produced IFN-γ. Graph in (A) represents 3 experiments. Statistical analysis compared experiments performed in the presence of DCs only. (B-F) Graphs represent 2 experiments, each with 5 mice per group. *P < .05, **P < .01, ***P < .001 (1-way ANOVA with a Bonferroni posttest for [A,C-F]). FSC-H, foward scatter height.
Established leukemia is associated with numerous suppressive mechanisms
Increased numbers of Tregs are found in patients with acute leukemias, such as AML and acute lymphoid leukemia.34,52-56 To determine the involvement of regulatory cells in C1498, the percentage of CD4+FoxP3+ Tregs was determined by flow cytometry. Mice with established leukemia had a significantly increased percentage of CD4+FoxP3+ cells in both the liver and spleen (Figure 4A-B). To determine whether alleviation of Treg-related immunosuppression could enhance the therapeutic effect of the leukemia/α-GalCer vaccine, an anti-CD25 antibody was used to deplete Tregs from leukemia-bearing mice before vaccination.57 Mice depleted of Tregs prior to vaccination had increased survival, although all animals ultimately succumbed to leukemia outgrowth (Figure 4C). This was dependent on the vaccine because Treg depletion alone did not delay symptom onset. These results indicate that Tregs are partially responsible for the inefficacy of therapeutic leukemia/α-GalCer vaccination.
Elevated immune suppression in hosts with established acute leukemia. (A-B) Mice were challenged with C1498 intravenously, and the immune response in the liver and spleen was analyzed 20 days later. (A) Flow cytometric identification of Tregs. (B) Percentage of Tregs in the spleens and livers of naïve and leukemia-challenged mice. (C) Symptom-free survival of mice challenged with C1498 and vaccinated 7 days later. One group was depleted of Tregs the day after leukemia challenge. Symbols represent treatment groups: unvaccinated (●), therapeutic α-GalCer vaccination (▪), therapeutic α-GalCer vaccination plus PC61 (▲), and irradiated C1498 cells plus PC61 (▼). Statistical analysis compared therapeutic α-GalCer vaccination plus PC61 to therapeutic α-GalCer vaccination. (D-G) Mice were challenged with C1498 intravenously and euthanized 20 days later. (D) Flow cytometric identification of splenic CD11b+Ly6G+ MDSCs. (E) The proportion of MDSCs within live splenocytes. (F-G) Splenic CD11b+ cells were isolated from naïve or C1498-challenged mice and cultured with CFSE-labeled lymphocytes and anti-CD3 and anti-CD28. A representative histogram of CFSE dilution of (F) CD4+ cells and (G) CD8+ cells incubated with CD11b cells from naïve mice (black line) or C1498-challenged mice (dotted line); unstimulated (shaded) and graph of reduced CFSE. (H-I) CFSE-labeled lymph node cells stimulated with anti-CD3 and anti-CD28 were cultured with naïve splenocytes or C1498 cells for 72 hours. A representative histogram of CFSE dilution of (H) CD4+ and (I) CD8+ T cells cultured with naïve splenocytes (black line) or C1498 cells (dotted line); unstimulated (shaded). The percent divided of (H) CD4+ cells or (I) CD8+ cells. This figure represents 3 experiments, each with 5 mice per group. (B,E-H) *P < .05, **P < .01 (Student t test with Mann-Whitney U test). (C) *P < .05 (log-rank Mantel-Cox test).
Elevated immune suppression in hosts with established acute leukemia. (A-B) Mice were challenged with C1498 intravenously, and the immune response in the liver and spleen was analyzed 20 days later. (A) Flow cytometric identification of Tregs. (B) Percentage of Tregs in the spleens and livers of naïve and leukemia-challenged mice. (C) Symptom-free survival of mice challenged with C1498 and vaccinated 7 days later. One group was depleted of Tregs the day after leukemia challenge. Symbols represent treatment groups: unvaccinated (●), therapeutic α-GalCer vaccination (▪), therapeutic α-GalCer vaccination plus PC61 (▲), and irradiated C1498 cells plus PC61 (▼). Statistical analysis compared therapeutic α-GalCer vaccination plus PC61 to therapeutic α-GalCer vaccination. (D-G) Mice were challenged with C1498 intravenously and euthanized 20 days later. (D) Flow cytometric identification of splenic CD11b+Ly6G+ MDSCs. (E) The proportion of MDSCs within live splenocytes. (F-G) Splenic CD11b+ cells were isolated from naïve or C1498-challenged mice and cultured with CFSE-labeled lymphocytes and anti-CD3 and anti-CD28. A representative histogram of CFSE dilution of (F) CD4+ cells and (G) CD8+ cells incubated with CD11b cells from naïve mice (black line) or C1498-challenged mice (dotted line); unstimulated (shaded) and graph of reduced CFSE. (H-I) CFSE-labeled lymph node cells stimulated with anti-CD3 and anti-CD28 were cultured with naïve splenocytes or C1498 cells for 72 hours. A representative histogram of CFSE dilution of (H) CD4+ and (I) CD8+ T cells cultured with naïve splenocytes (black line) or C1498 cells (dotted line); unstimulated (shaded). The percent divided of (H) CD4+ cells or (I) CD8+ cells. This figure represents 3 experiments, each with 5 mice per group. (B,E-H) *P < .05, **P < .01 (Student t test with Mann-Whitney U test). (C) *P < .05 (log-rank Mantel-Cox test).
Because myeloid-derived suppressor cells (MDSCs) can also potentially contribute to immunosuppression in patients with acute leukemias,34,52-55 the proportion of MDSCs was assessed in the spleens of leukemic animals. A significantly increased percentage of CD11b+Ly6G+ cells was observed in animals with established leukemia (Figure 4D-E). These cells could be distinguished from leukemic blasts by CD11b, which is not expressed in C1498 (J.D.G., unpublished data, January 14, 2013). When splenic CD11b+ cells were isolated 20 days after leukemia challenge and cultured with CFSE-labeled lymphocytes, the proliferation of CD4+ T cells was significantly reduced relative to cultures containing CD11b+ cells from control mice, suggesting greater suppressive activity on a per cell basis (Figure 4F). Although a trend toward reduced CD8+ T-cell proliferation was also observed, this failed to reach significance in all experiments (Figure 4G) (J.D.G., unpublished data, January 14, 2013).
To analyze whether the C1498 line itself had immunosuppressive capabilities, C1498 cells were cultured with CFSE-labeled lymphocytes, and T-cell proliferation was compared with cultures containing naïve CD11b+ splenocytes. Proliferation of CD4+ and CD8+ T cells was severely impaired by coculture with C1498 cells, although suppression of CD4+ T-cell proliferation was more pronounced (Figure 4H-I). Overall, these data indicate that there may be several immunosuppressive activities at play in the context of established leukemia.
Cytarabine pretreatment does not suppress vaccine-induced responses
Although induction chemotherapy can drastically reduce the tumor cell burden in leukemic patients and potentially provide an opportunity for immunotherapeutic intervention, it can also induce lymphopenia and an expansion of Tregs.59 To determine whether cytarabine chemotherapy could be used successfully in combination with immunotherapy, the effect of cytarabine treatment on T cells was analyzed in mice with acute leukemia. Although the percentage of T cells was reduced in the spleens of mice with untreated leukemia, mice treated with cytarabine after leukemia challenge had similar proportions of CD4+ and CD8+ T cells compared with naïve controls (Figure 5A-B). Moreover, although expression of the activation marker CD44 on CD8+ and CD4+ T cells was reduced in animals with leukemia, in cytarabine-treated animals, expression was similar to that seen in naïve healthy controls (Figure 5C-D), surprisingly suggesting that rather than suppressing endogenous immune responses, cytarabine may instead be restoring the T-cell compartment.
Chemotherapy restores the T-cell compartment in leukemic mice. Mice were challenged with C1498 cells, and 24 hours later three 3-mg doses of cytarabine were administered intraperitoneally 10 hours apart. (A-D) The T-cell populations in the spleen were analyzed and identified as CD3+ expressing either CD8 or CD4. The proportion of live cells expressing (A) CD3 and CD8 or (B) CD3 and CD4. The MFI of CD44 (C) CD8+ cells and (D) CD4+ cells. (A-D) Represent 3 experiments, with 5 mice per group. *P < .05; **P < .01; ***P < .001 (1-way ANOVA with a Bonferroni posttest).
Chemotherapy restores the T-cell compartment in leukemic mice. Mice were challenged with C1498 cells, and 24 hours later three 3-mg doses of cytarabine were administered intraperitoneally 10 hours apart. (A-D) The T-cell populations in the spleen were analyzed and identified as CD3+ expressing either CD8 or CD4. The proportion of live cells expressing (A) CD3 and CD8 or (B) CD3 and CD4. The MFI of CD44 (C) CD8+ cells and (D) CD4+ cells. (A-D) Represent 3 experiments, with 5 mice per group. *P < .05; **P < .01; ***P < .001 (1-way ANOVA with a Bonferroni posttest).
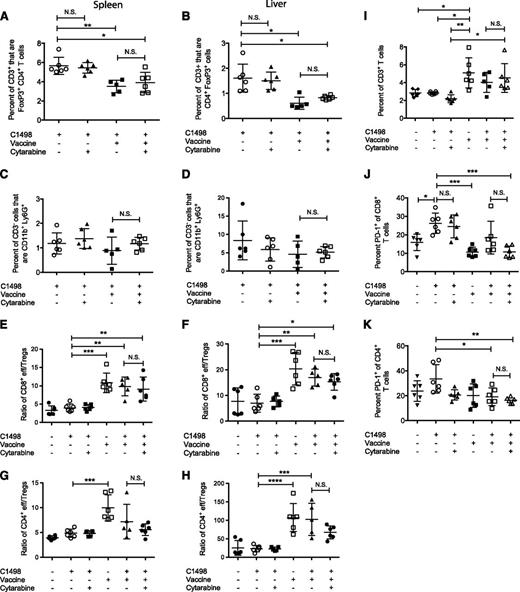
We next wanted to determine the effect of cytarabine pretreatment on the vaccine-induced response. C1498-challenged mice were treated with cytarabine, and 20 days later, they were administered the leukemia/α-GalCer vaccine or left unvaccinated, and the proportions of Tregs and MDSCs were assessed 1 week after vaccination. Although we observed a reduction in the percentages of Tregs in both the spleen and liver of vaccinated animals compared with untreated leukemic mice, the addition of cytarabine to the treatment regimen had no effect (Figure 6A-B). Similarly, there were no changes in the proportions of MDSCs (Figure 6C-D). The ratio of effector CD8+ T cells (CD44hi) to Tregs was increased in both the spleen and liver of vaccinated mice relative to that in untreated animals and was also unaffected by cytarabine pretreatment. We observed a similar trend in the ratio of effector CD4+ T cells (CD44hi) to Tregs, again establishing that the addition of cytarabine did not have an impact on the vaccine-induced response in leukemic mice (Figure 6E-H).
Cytarabine pretreatment does not suppress vaccine-induced immune responses. Mice were challenged with C1498 cells, and 24 hours later, three 3-mg doses of cytarabine were administered intraperitoneally 10 hours apart. On day 23, 1 group of chemotherapy-treated mice was vaccinated with leukemia/α-GalCer, and responses were analyzed 1 week later. The percentage of CD4+FoxP3+ cells of CD3+ in (A) the spleen and (B) the liver. The percentage of CD11b+Ly6G+ of CD3– in the (C) spleen and (D) liver. The ratio of CD44hi CD8+ effector T cells to CD4+FoxP3+ Tregs and ratio of CD44hi CD4+ effector (eff) T cells to CD4+FoxP3+ Tregs in (E,G) the spleen and (F,H) the liver, respectively. (I) The percentage of CD3+ T cells in the BM. The percentage of (J) PD-1+CD8+ and (K) PD-1+CD4+ T cells in the BM. Graphs represent 1 experiment, with 5 to 6 mice per group. *P < .05; **P < .01; ***P < .001; ****P < .0001 (1-way ANOVA with a Bonferroni posttest).
Cytarabine pretreatment does not suppress vaccine-induced immune responses. Mice were challenged with C1498 cells, and 24 hours later, three 3-mg doses of cytarabine were administered intraperitoneally 10 hours apart. On day 23, 1 group of chemotherapy-treated mice was vaccinated with leukemia/α-GalCer, and responses were analyzed 1 week later. The percentage of CD4+FoxP3+ cells of CD3+ in (A) the spleen and (B) the liver. The percentage of CD11b+Ly6G+ of CD3– in the (C) spleen and (D) liver. The ratio of CD44hi CD8+ effector T cells to CD4+FoxP3+ Tregs and ratio of CD44hi CD4+ effector (eff) T cells to CD4+FoxP3+ Tregs in (E,G) the spleen and (F,H) the liver, respectively. (I) The percentage of CD3+ T cells in the BM. The percentage of (J) PD-1+CD8+ and (K) PD-1+CD4+ T cells in the BM. Graphs represent 1 experiment, with 5 to 6 mice per group. *P < .05; **P < .01; ***P < .001; ****P < .0001 (1-way ANOVA with a Bonferroni posttest).
The different treatments were associated with significant changes in proportions of T cells in the BM, the major site of leukemia cell accumulation. Vaccinated animals had an increased proportion of CD3+ T cells compared with unvaccinated mice that was unaffected by cytarabine (Figure 6I). Interestingly, leukemia challenge was associated with an increase in the percentage of CD8+ T cells expressing the T-cell exhaustion marker programmed-death 1 (PD-1) in the BM, which was unchanged following cytarabine treatment alone. However, treatment with cytarabine followed by vaccination resulted in reduced percentages of PD-1–expressing CD8+ T cells compared with untreated C1498-challenged animals, similar to that observed in vaccinated nonleukemic mice (Figure 6J). Similarly, the lowest percentage of PD-1–expressing CD4+ T cells was observed in mice that received the combination therapy (Figure 6K). These results demonstrate that cytarabine pretreatment does not suppress vaccine-induced immune responses; rather, it suggests that the therapies may be successfully combined. Importantly, chemotherapy to induce minimal residual disease for acute leukemia may provide a window for immunotherapy.
Vaccination following cytarabine treatment protects against leukemia rechallenge
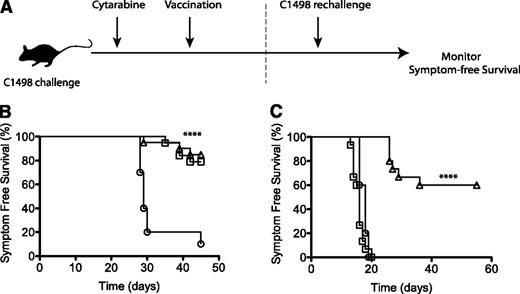
Because cytarbine pretreatment did not negatively impact vaccine-induced immune responses, we next assessed the efficacy of the leukemia/α-GalCer vaccine as a postremission therapy (Figure 7A). Mice were challenged with C1498 and then treated with cytarabine. After 20 days, 1 group was vaccinated with the leukemia/α-GalCer vaccine. Cytarabine treatment prolonged survival in both groups after C1498 challenge (Figure 7B) as previously reported,60,61 although all animals eventually relapsed (supplemental Figure 3). Surviving cytarabine-treated animals, as well as additional naïve controls, were challenged with an elevated dose of viable C1498 cells to determine whether they had developed protective immunity to acute leukemia. Strikingly, animals that received vaccination after cytarabine chemotherapy had superior protection from C1498 rechallenge, whereas all animals that received chemotherapy alone developed symptoms associated with leukemia progression within 20 days (Figure 7B). Therefore, vaccination provided durable protection against leukemia when administered during remission following cytarabine chemotherapy, suggesting that an α-GalCer–pulsed irradiated leukemia cell vaccine may be a promising postremission immunotherapy for acute leukemia.
Vaccination following chemotherapy protects against leukemic rechallenge. (A-B) Mice were challenged with C1498 cells, and 24 hours later, three 3-mg doses of cytarabine were administered intraperitoneally 10 hours apart. On day 23, 1 group of chemotherapy-treated mice were administered the leukemia/α-GalCer vaccine, and symptom-free survival was monitored. (A,C) Surviving mice were rechallenged on day 45 with a fivefold increased dose of 5 × 105 viable C1498 cells, and symptom-free survival was again followed. Symbols represent treatment groups: leukemia only (○), n = 10; cytarabine treatment (□), n = 19; and cytarabine plus α-GalCer vaccination (△), n = 20. (B-C) Represent 2 experiments. ****P < .0001 (log-rank Mantel-Cox test).
Vaccination following chemotherapy protects against leukemic rechallenge. (A-B) Mice were challenged with C1498 cells, and 24 hours later, three 3-mg doses of cytarabine were administered intraperitoneally 10 hours apart. On day 23, 1 group of chemotherapy-treated mice were administered the leukemia/α-GalCer vaccine, and symptom-free survival was monitored. (A,C) Surviving mice were rechallenged on day 45 with a fivefold increased dose of 5 × 105 viable C1498 cells, and symptom-free survival was again followed. Symbols represent treatment groups: leukemia only (○), n = 10; cytarabine treatment (□), n = 19; and cytarabine plus α-GalCer vaccination (△), n = 20. (B-C) Represent 2 experiments. ****P < .0001 (log-rank Mantel-Cox test).
Discussion
There is an unmet need for effective postremission therapies for acute leukemia that have reduced toxicity and cost compared with allo-SCT. By using the aggressive acute leukemia cell line C1498,62 we show that a simple vaccine comprising whole irradiated leukemia cells pulsed with the glycolipid adjuvant α-GalCer protected against the development of leukemia in vivo. Despite displaying immunologic activity, the vaccine did not delay disease progression in mice with established disease, at least in part because of leukemia-related immunosuppressive activities. However, in animals that were in remission after cytarabine chemotherapy, the vaccine protected against leukemia rechallenge.
The glycolipid α-GalCer acts as an adjuvant via a third-party mechanism by binding to CD1d on DCs and recruiting NKT cells to create a stimulatory environment that leads to enhanced peptide-specific responses by conventional CD4+ and CD8+ effector T cells.23,47,63 Although CD1d is weakly expressed on C1498 cells and has been identified in other acute leukemic cell lines, including AML-ETO9a,23 EL4, and WEHI-3B (L.R.A, unpublished data, June 14, 2014), we have previously demonstrated that a CD1d-negative α-GalCer–pulsed glioma vaccine can provide protection against glioma challenge.25 We have also shown that CD1d-deficient DCs can transfer α-GalCer to host resident CD1d-expressing antigen-presenting cells in vivo to induce potent invariant NKT-cell activation, which likely reflects transfer of α-GalCer embedded within membranes of the injected cells.46 It is therefore not necessary for NKT cells to interact directly via CD1d on the cells of the vaccine to provide adjuvant activity. Rather, we favor the hypothesis that host DCs acquire α-GalCer from the leukemia cells of the vaccine; these DCs then become licensed by presenting the α-GalCer via CD1d to NKT cells, in turn leading to enhanced induction of peptide-specific CD4+ and CD8+ effector T cells. Consistent with this mechanism, the activity of the leukemia/α-GalCer vaccine required langerin-expressing DCs and involved CD4+ and CD8+ T cells for full efficacy.
Therapeutic administration of the leukemia/α-GalCer vaccine was unable to prolong survival in mice with established leukemia, despite stimulating NKT cells, and leading to activation of DCs. We identified several mechanisms by which C1498 cells could suppress immune responses, including increased proportions of Tregs and MDSCs, and a direct suppressive effect of the leukemia cells on T-cell proliferation. It is notable that acute leukemias have been reported to produce arginase64 and indolamine 2,3-dioxygenase,65 which may be responsible for this direct effect. Our results suggest that leukemia-specific CD4+ T cells play a significant role in the efficacy of the leukemia/α-GalCer vaccine, and depletion experiments indicated that CD4+ T cells were of importance similar to that of CD8+ T cells for vaccine efficacy. In leukemia-bearing mice, IFN-γ production by CD4+, but not CD8+ T cells, was impaired. Interestingly, MDSCs from leukemic mice induced a more pronounced suppression of CD4+ T cells compared with those harvested from nonleukemic animals, although we were unable to show a similar reproducible effect on CD8+ T cells. In addition, the potent suppression of T-cell proliferation by the leukemia cells themselves appeared to be greater on CD4+ T cells than on CD8+ T cells. It is possible therefore that the leukemic environment induces broad CD4+ T-cell dysfunction, perhaps including the inability to provide T-cell help to CD8+ T cells. In this context, it is notable that vaccine efficacy in the prophylactic setting was dependent on langerin+CD8α+ DCs, which have been shown to have a potent capacity for stimulating cytotoxic T cells; the vaccine may ultimately depend on CD4+ T-cell help to mobilize effective cytotoxic T cells through these DCs. However, given that some antitumor activity was seen in the absence of CD8+ T cells, other CD4+ T-cell functions must be involved, perhaps as effectors in their own right,66 or through interactions with other MHC class II–expressing cells.
Other groups have also shown that α-GalCer–pulsed acute leukemia cells can be used as a prophylactic vaccine. By using the AML cell line AML-ETO9a, Mattarollo et al23 showed that irradiated α-GalCer–pulsed AML cells prevented the development of AML in the prophylactic setting but only delayed progression when administered therapeutically, and Shimizu et al29 demonstrated effective prophylactic vaccination by using the myelomonocytic WEHI-3B model. Our experiments extend these findings by elucidating immunosuppressive activities in established leukemia and indicating that protection against acute leukemia can also be invoked during remission after cytarabine chemotherapy. Because this chemotherapeutic agent is in routine clinical use for leukemia induction and consolidation,67,68 these results are of particular relevance clinically.27,29 Our results support a previous in vivo study with C1498 in which mice treated with a granulocyte macrophage colony-stimulating factor–secreting irradiated cell vaccine 5 or 7 days after cytarabine treatment were protected against the development of leukemia, despite transient development of severe neutropenia and lymphopenia following chemotherapy.59
We have demonstrated successful combination of immunotherapy after chemotherapy pretreatment of leukemic mice and have shown that cytarabine alone did not induce changes to the immune environment that could be detected by day 20. Given the suppressive effects of C1498, we expected that cytarabine pretreatment would have alleviated some of the leukemia-induced suppression and, consequently, more robust immune responses would be detected in mice that received the combined treatment. In contrast to this, we observed little improvement in vaccine-mediated responses after cytarabine. We expect that although the leukemic burden was certainly reduced in cytarabine-treated mice, the number of circulating tumor cells may have been substantially increased by the time of vaccination, which was delayed until nearly 3 weeks after chemotherapy. Evidence for this is reported in a study by Lin et al, in which aggressive C1498 progression was demonstrated by in vivo imaging, and a 10 000-fold increase in whole body photon counts of leukemic cells was observed within 2 weeks of tumor challenge.60 Comparable with our findings, relapse also occurred in mice treated with the same dose of cytarabine we used in this study, despite using half the dose of viable C1498 cells for leukemic challenge. It may be possible that earlier administration of the vaccine after treatment with cytarabine could generate more robust immune responses in this group. Because we found little evidence to support a role for cytarabine in modulating proportions of Tregs and MDSCs, the efficacy against rechallenge we observe when cytarabine is combined with vaccination may simply reflect the capacity of this drug to drastically reduce the burden of leukemia cells and the preexisting immunity afforded by the vaccine before rechallenge.
In comparison with vaccination with defined antigens, an α-GalCer–loaded autologous whole cell vaccine for acute leukemia has the advantages of stimulating both innate and daptive immunity and covering a broad range of leukemia-associated antigens without being limited by HLA phenotype. Because leukemic blasts are readily obtained from the blood or BM of patients at diagnosis69 and because α-GalCer has been used safely in early-phase clinical trials,70-72 vaccination with α-GalCer–pulsed irradiated leukemia cells may represent a feasible postremission immunotherapy. We are aware that other approaches to adjuvanting autologous tumor cell vaccines have been described for hematologic malignancies, including toll-like receptor ligands.73 Although we have not directly compared α-GalCer and toll-like receptor ligands in this model, the two approaches are not mutually exclusive, and it is possible that these adjuvants may synergize to provide enhanced therapy.74,75
In summary, an α-GalCer–adjuvanted whole leukemia cell vaccine that is effective at preventing leukemia development in naïve animals is ineffective in the presence of established leukemia because of the suppressive activities of Tregs, MDSCs, and the leukemia cells themselves. However, in the setting of remission after cytarabine treatment, the vaccination leads to durable protection against subsequent leukemia rechallenge. We suggest that postinduction immunotherapy with an autologous irradiated leukemia cell vaccine adjuvanted with α-GalCer may prove to be a useful strategy for prevention of high-risk acute leukemias.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the National Institutes of Health Tetramer Core Facility (contract HHSN272201300006C) for provision of CD1d tetramers, and thank Chingwen Tang and Taryn Osmond for their technical assistance.
This work was supported by grant no. C08X0808 from the Marsden Fund Council (government funding) administered by the Royal Society of New Zealand, and by the New Zealand Ministry of Science and Innovation.
Authorship
Contribution: J.D.G., L.R.A., R.W., T.R.P., and I.F.H. designed the research; J.D.G., L.R.A., R.W., and I.F.H. analyzed and interpreted the data and wrote the manuscript; J.D.G. and L.R.A. conducted the experiments; and G.F.P. and B.J.C. synthesized α-GalCer.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ian F. Hermans, Malaghan Institute of Medical Research, PO Box 7060, Wellington 6242, New Zealand; e-mail: ihermans@malaghan.org.nz.

![Figure 1. An α-GalCer–pulsed whole leukemia cell vaccine protects against acute leukemia and is dependent on CD4+ and CD8+ T cells and langerin+CD8α+ DCs. A vaccine comprising α-GalCer–pulsed irradiated C1498 cells was administered intravenously 7 days before C1498 challenge. (A) BM histology, (B) peripheral blood smear, and (C) peripheral white blood cell counts were performed at symptom onset in unvaccinated animals, 40 days after C1498 challenge in vaccinated animals, and in naïve control mice. (D) Kaplan-Meier plot showing symptom-free survival of vaccinated and unvaccinated mice after leukemia administration on day zero. Statistical analysis compared unvaccinated and vaccinated with α-GalCer–pulsed irradiated leukemia cell groups. (E) Kaplan-Meier plots showing symptom-free survival of mice vaccinated with leukemia/α-GalCer and challenged with C1498 at day zero. (C-E) Symbols represent treatments: unvaccinated (●), nonleukemic and nonvaccinated (◆), vaccinated with α-GalCer–pulsed irradiated leukemia cells (▪), vaccinated with unpulsed irradiated leukemia cells (□), vaccinated with free α-GalCer (△), depletion of CD4+ cells (▲), and depletion of CD8+ cells (▼). Statistical analyses compared the depletion groups to mice vaccinated with α-GalCer–pulsed irradiated leukemia cells. (F) lang-EGFPDTR mice were prophylactically vaccinated, and 1 group was administered DT. Symbols represent treatment groups: unvaccinated (●), prophylactic α-GalCer vaccination (▪), and prophylactic vaccination and DT treatment (▲). Symptom-free survival was analyzed and is graphed. *P < .05 (1-way analysis of variance [ANOVA] with a Bonferroni posttest). (A-C) represent a single experiment, (D) represents 3 experiments, and (E-F) represent 2 experiments; 5 mice per group were used for each experiment. *P < .05; **P < .01; ***P < .001 (Mantel-Cox log-rank test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/19/10.1182_blood-2014-04-568956/4/m_2953f1.jpeg?Expires=1769813470&Signature=KhukBG3DExMCrZiNpVobtXouqQTF2k2tyA0jYfLJjGMZ3YwKc7JnWwz~VXJOSvokoKMpVCtzlVTGietti-qQAO683-3QI2DpqskBhB7IfmpAbkRlqtnjkq4f0vamVTxF0VTKibOQ6fOCyQF6R14pBEfUL5tN6WpQXtPDc4Jq9tWq-Zp9ltEolDLI1E3gE7wOH5mOGuP4HJgcJd9r6vBrp41kR0oP4oMToiQZbv8irJEA2-a0QEA5N2XG8hXkLBtVKMVa48iBN3od28IfO-rPkdXSZTykNPGFe5C53OqmHCt31NB3SxaTHyuqh1gRgFBX8V0efH~88wUh9RBjBK~KQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Leukemia/α-GalCer vaccination is ineffective in the presence of established acute leukemia despite NKT-cell and DC activation. Mice were challenged with C1498 cells intravenously 1 week before vaccination with α-GalCer–pulsed or unpulsed irradiated leukemia cells. (A) Kaplan-Meier graph showing survival of mice. Symbols represent treatment groups: unvaccinated (●), therapeutic α-GalCer vaccination (▪), and treatment with unpulsed irradiated leukemia cells (▲). This graph represents 3 experiments, each with 5 mice per group. (B-E) Mice were inoculated with C1498 cells 7 or 14 days before vaccination with irradiated α-GalCer–pulsed leukemia cells. (B) Representative flow cytometry plots showing identification of splenic NKT cells (CD3+ α-GalCer–loaded CD1d tetramer+ [tet]). (C) Frequency of splenic NKT cells after vaccination in mice with and without established acute leukemia. (D) Serum IL-4 levels and (E) serum IFN-γ levels 2 hours after vaccination. (F-G) The splenic langerin+CD8α+ DC population in lang-EGFP mice was analyzed 24 hours after vaccination. (F) Representative flow cytometry plots showing identification of splenic langerin+CD8α+ DCs (CD11c+GFP+). (G) Frequency of splenic langerin+CD8α+ DCs. (H-I) The expression of CD40 and CD86 on langerin+CD8α+ DCs, respectively. (J-K) The CD8α+ DC population in C57BL/6 mice was analyzed 24 hours after vaccination. The expression of CD40 and CD86 on CD8α+ DCs, respectively. (L) Serum IL-12p70 was quantified 5 hours after vaccination. These results are indicative of 2 independent experiments, each with 5 mice per group. **P < .01; ***P < .001 (1-way ANOVA with a Bonferroni posttest). FSC-A, forward scatter area; MFI, mean fluorescent intensity; SSC, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/19/10.1182_blood-2014-04-568956/4/m_2953f2.jpeg?Expires=1769813470&Signature=YJj0pHxOVst7XYYaZFA-mpzNg8GbmK-R5E5ElF-VXKRAAqBuwpGUkjDTtMPXCS38Yn-IYRxS7Ywkg9aU2hlE89AbfA0wjXPWMsylsv-uPpnG8DVtPUMxTF6EGbIZY5bTs82~28NoOJRfgTCjNPZoi6zmoJ3U3cXOeWM4smSli5CdEBpvSiTdSXctCJf4hcVGWDgOha2THyd04Dnh4oGULDEzbPDCDsXxrQm5kriVbC2Pg5B7nkarLw3jjQE3VhSA2BHTANT-OXI~B0VRN4GOY5RwEJ-lyPfNmtKtmrQvD3CzVyozWpCYiLLZ7EGhholbOgbBjZOZpo8CnK2ioj53Hg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Established leukemia disrupts leukemia/α-GalCer vaccine–mediated CD4+ T-cell function. Mice were challenged with C1498 intravenously 7 days before vaccination, and responses were analyzed 1 week later. (A) Splenocytes were cultured for 24 hours with (○) or without (●) DCs loaded with C1498 lysate. Supernatant IFN-γ was quantified. (B-F) The splenic CD4+ and CD8+ T-cell populations were analyzed by flow cytometry. (B) Representative flow cytometry plots showing identification of CD4+ and CD8+ T cells from mouse spleens. IFN-γ+ cells were determined by comparison with an isotype control antibody (lower left). MFI of CD44 expressed on (C) CD8+ and (E) CD4+ T cells. The proportion of (D) CD8+ and (F) CD4+ cells that produced IFN-γ. Graph in (A) represents 3 experiments. Statistical analysis compared experiments performed in the presence of DCs only. (B-F) Graphs represent 2 experiments, each with 5 mice per group. *P < .05, **P < .01, ***P < .001 (1-way ANOVA with a Bonferroni posttest for [A,C-F]). FSC-H, foward scatter height.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/19/10.1182_blood-2014-04-568956/4/m_2953f3.jpeg?Expires=1769813470&Signature=Z8Axr6mGzrYmnm37Pmhu9GEIaXZx4Blu5oJ-aEXvA9P2ociFft~v96dZRCvDNHzV97BxfSDqTsaKijulUjhowx0vSzplgVgTB-WhUFKw1DYLaf3E3SaHFvtZ6efF4ew2JxFI~1ydtBJU~7chQ3M6996E64mfJGPGE4CrhlqyFThpAgYPmf2uThwMR4rtlfGglHSAHWpi55Oq--eQzVXDHBu~1vkNVB5wZK9SKEUig3YBOx0GhE6BorPMKXgasBLwEiQsfOVNFbUhSYdPNsU7lbvB-RIXlr3P5wAK69EVaRapmvzWqpzeU4uEzvx8wVdS-qlxVCnaVvUt2BbS6TbpGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)