In this issue of Blood, the findings of Chakraborty et al and Emile et al support a model in which the mitogen-activated protein kinase (MAPK) and PI3K/AKT pathways are critical in the pathogenesis of 2 of the most common histiocytoses—Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD)—whereas their respective mutational profiles demonstrate important similarities and differences.1,2
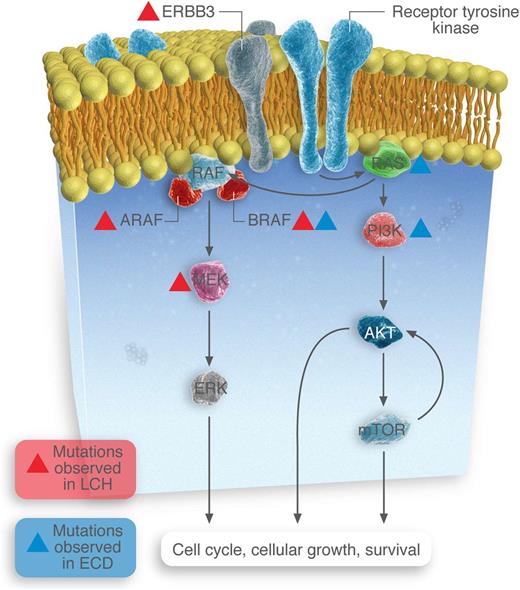
Somatic mutations identified in MAPK and PI3K-AKT-mTOR pathway members in LCH and ECD patients.
Somatic mutations identified in MAPK and PI3K-AKT-mTOR pathway members in LCH and ECD patients.
The discovery in 2002 of the BRAFV600E mutation in patients with melanoma focused attention on the extracellular signal–regulated kinase (ERK) pathway as one of major clinical relevance in human malignancy. BRAF is a member of the RAF family of protein kinases and functions downstream of RAS in the MAPK signaling pathway, which also includes MAPKs 1 and 2 (MEK1 and -2), which activate ERKs 1 and 2 (see figure). Moreover, the successful treatment of melanoma patients with BRAF inhibitors such as vemurafenib and dabrafenib has exemplified the potential of individualized mutational analysis as a diagnostic and therapeutic tool in the management of malignancy.
BRAF mutations have now been recognized with varying frequency in a variety of other solid tumors, including gliomas and thyroid, colon, ovarian, and hepatobiliary cancers. For the hematologist, interest in this pathway was recently brought to the fore with the recognition that BRAFV600E mutations are universally detected in hairy cell leukemia.3 Recently, the histocytoses have been in the spotlight; BRAF mutations were recognized in approximately 50% of patients with either LCH or ECD.4-7 Indeed, like melanoma patients with the BRAFV600E mutations, such patients have been successfully treated with BRAF inhibitors.8
Two articles in this edition of Blood further explore gene mutations in LCH and ECD. Chakraborty et al used whole-exome sequencing (WES) to examine the extent and range of somatic mutations that underlie LCH pathogenesis.1 When they flow-sorted CD207+ cells, they found that 20 of 41 had single BRAFV600E mutations. Importantly, other mutations were seen in wild-type BRAF tumors, of which mutations in MAP2K1 (which encodes MEK1 protein) were the most common (7/21). This is the first time that non-BRAFV600E mutations have been reported in more than one patient with LCH. The authors also identified mutations in the ARAF and ERBB3 genes in individual tumors (see figure). Of note, 23 other somatic mutations were identified but did not include genes that either were members of the MAPK pathway or could affect that pathway (such as members of the PI3K/AKT/mTOR pathway).
The manuscript by Emile et al focuses on ECD and provides findings that have important similarities and differences from those observed in LCH.2 This group did not use WES but searched for mutations in specific genes using pyrosequencing, Sequenom mass spectrometry–based genotyping assays, next-generation targeted sequencing, and Illumina MiSeq for regions in BRAF, N/KRAS (MAPK pathways), and PIK3CA and AKT1 (PI3K/AKT/mTOR pathway). Like LCH, they detected BRAFV600E mutations in >50% of samples (46/80). Unlike LCH, they detected mutations in NRAS and PIK3CA. Notably, they did not specifically test for MAP2K1 mutations. In addition, the Chakraborty group, using WES only, analyzed only 1 patient with ECD (and found no mutations), so the incidence of mutations outside the RAS-RAF-MEF-ERK and RAS-PI3K-AKT-mTOR pathways in patients with ECD remains unknown. Another important difference in ECD is that additional mutations are present not infrequently in patients with BRAFV600E mutations (not just wild-type BRAFV600E); 4 of 46 BRAFV600E mutants had coexistent PIK3CA mutations.
Of note, both of these studies provide evidence that these are activating mutations and generally result in phosphorylation of ERK. Thus, they are very likely to be clinically relevant in the pathogenesis of their respective diseases. Supportive of these mutations being pathologically important “driving mutations” is the finding by the authors that there is a remarkably low frequency of somatic mutations in LCH lesions, with a median of 1 mutation per sample. Of note, with respect to LCH, it seems that ERK activation is a universal end point in LCH from the various pathologic activation of upstream signaling proteins. At this time, the same cannot necessarily be said for ECD. Only further studies will determine whether mutations outside the ERK axis have the same clinical and treatment-response implications as those within the MAPK-ERK pathway (ie, BRAFV600E).
Taken together, targeting these various mutations beyond the BRAFV600E is no doubt a worthwhile objective. We have already found this with the BRAF inhibitors and it is hoped that, given the relatively low incidence of multiple mutations, resistance to therapy may be relatively less common than that observed in melanoma. Indeed, recent consensus guidelines confirm the need for routine mutational analysis to guide the treatment of ECD.9 What, then, about targeting other mutations? The in vitro studies by Chakraborty et al support the premise that MEK inhibition may be therapeutically useful, but more studies are needed. Finally, both studies demonstrated that in a significant minority of patients, no mutations are identified. In part this may be caused by the relative impurity of the sample with respect to the clonal cell. Indeed, Emile et al and others have demonstrated that both BRAFV600E and NRAS mutations can be found in monocytes in the peripheral blood.5,10 Thus, with analysis of samples that are of higher purity and enriched for the clonal cells, we may in the future be able to identify mutations in the vast majority of patients with ECD or LCH. Moreover, such “mutational profiling” may align with different clinical patterns of disease; we already recognize that the clinical presentation of LCH and ECD is very heterogeneous, and mutational analysis may help subclassify clinical patterns of disease/response (although the authors were unable to find such associations with their small sample size).
These 2 articles confirm that every patient with LCH or ECD should have mutational analysis for at least the BRAFV600E mutation, and the mutational panel will continue to grow as we explore for other mutations in the MAPK and PI3K-AKT pathways.
Conflict-of-interest disclosure: The author declares no competing financial interests.