Abstract
Myelofibrosis (MF) is a BCR-ABL1–negative myeloproliferative neoplasm characterized by clonal myeloproliferation, dysregulated kinase signaling, and release of abnormal cytokines. In recent years, important progress has been made in the knowledge of the molecular biology and the prognostic assessment of MF. Conventional treatment has limited impact on the patients’ survival; it includes a wait-and-see approach for asymptomatic patients, erythropoiesis-stimulating agents, androgens, or immunomodulatory agents for anemia, cytoreductive drugs such as hydroxyurea for the splenomegaly and constitutional symptoms, and splenectomy or radiotherapy in selected patients. The discovery of the Janus kinase (JAK)2 mutation triggered the development of molecular targeted therapy of MF. The JAK inhibitors are effective in both JAK2-positive and JAK2-negative MF; one of them, ruxolitinib, is the current best available therapy for MF splenomegaly and constitutional symptoms. However, although ruxolitinib has changed the therapeutic scenario of MF, there is no clear indication of a disease-modifying effect. Allogeneic stem cell transplantation remains the only curative therapy of MF, but due to its associated morbidity and mortality, it is usually restricted to eligible high- and intermediate-2–risk MF patients. To improve current therapeutic results, the combination of JAK inhibitors with other agents is currently being tested, and newer drugs are being investigated.
Introduction
Myelofibrosis (MF), formerly known as idiopathic MF, MF with myeloid metaplasia, or agnogeneic myeloid metaplasia, is one of the classical BCR-ABL1-negative chronic myeloproliferative neoplasms (MPNs), a group also including essential thrombocythemia (ET) and polycythemia vera (PV).1 Either appearing de novo (primary MF [PMF]) or following a previous ET or PV (post-ET or post-PV MF),2 the disease is essentially the same. MF is a clonal proliferation of a pluripotent hematopoietic stem cell,3 in which the abnormal cell population releases several cytokines and growth factors in the bone marrow that lead to marrow fibrosis and stroma changes and colonizes extramedullary organs such as the spleen and liver.4 Discovery of the V617F mutation of the Janus kinase (JAK)2 gene in 60% of patients with PMF or post-ET MF and 95% of those with post-PV MF represented an important step in the understanding of the pathogenesis of MF.5-7 Mutations in the thrombopoietin receptor gene (MPL) were subsequently found in 3% to 8% of patients with PMF and post-ET MF,8 whereas mutations in the calreticulin gene (CALR) have been observed in half of patients with PMF and post-ET MF lacking JAK2 and MPL mutations.9,10 Mutations shared by other myeloid neoplasms are found in some patients.11 However, the genetic trigger of MF is unknown.
MF mostly affects elderly people. At present, there is no curative treatment other than allogeneic hemopoietic stem cell transplantation (allo-SCT), which can be applied to a minority of patients. Therefore, treatment remains essentially palliative and aimed at controlling disease symptoms and complications and improving the patients’ quality of life. The therapeutic landscape of MF has changed with the introduction of the JAK inhibitors.
In the present article, I will discuss the treatment options currently available for MF, how I have incorporated the new prognostic information to the clinical decision-making process, and how I have integrated novel therapeutic modalities in my practice. The discussion on the more relevant clinical scenarios of MF will be preceded by a representative case study to illustrate how I decide the treatment strategy for the main clinical situations of this complex disease.
Clinical picture of MF
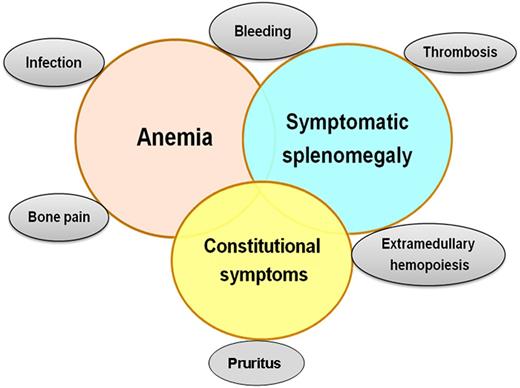
The clinical manifestations of MF are heterogeneous (Figure 1). Up to 30% of patients are initially asymptomatic12 ; most patients present with symptoms from anemia or splenomegaly or constitutional symptoms. As the disease evolves, all patients become symptomatic due to marrow failure, increasing splenomegaly causing abdominal symptoms and early satiety, and constitutional symptoms such as weight loss, night sweats, and low-grade fever. Aquagenic pruritus, bone pain, or thrombosis may be a problem. In the advanced phases, extramedullary hematopoiesis in sites other than the spleen and liver can be seen. Evolution to acute myeloid leukemia can be observed.
Because current therapies other than allo-SCT are not able to control all clinical manifestations of MF, treatment choice is mainly guided by the main symptom or feature. However, the situation is often more complex, because patients may have several symptoms, and a therapy instituted for one can worsen another, as it is the case of anemia, frequently accentuated by the therapy for splenomegaly.
How I treat anemia
Case study 1
A 51-year-old woman was diagnosed with ET after the incidental discovery of thrombocytosis, with the marrow biopsy being normocellular and showing proliferation of megakaryocytes, most of them with hyperlobulated nuclei, with absent fibrosis. The patient remained asymptomatic without treatment for 8 years, when spontaneous platelet normalization was noted, with hemoglobin (Hb) decrease, dacryocytes in blood, slight leukocytosis with leukoerythroblastosis, and increased serum lactate dehydrogenase. The spleen was not palpable. The V617F JAK2 mutation was negative, and the mutation W515L of the MPL gene was found. Bone marrow biopsy confirmed post-ET MF. Serum ferritin, vitamin B12, and folate were normal. Because anemia was mild and well tolerated, no treatment was instituted. However, 1 year later, the patient started complaining of fatigue. A spleen tip was palpable. Hb was 9.4 g/dL, without other changes in the blood. I had to decide on the most appropriate treatment of the patient.
Fatigue is a common symptom in MF.13 Its main cause is anemia, but it can also be due to the disease activity itself, especially in patients with constitutional symptoms and signs of hyperproliferation. A role for the abnormal expression of several proinflammatory cytokines in the fatigue of MF patients has been pointed out.14 This patient did not have any of the abovementioned features, and fatigue appeared coincidently with the accentuation of anemia. Therefore, she needed treatment of the anemia.
Anemia is the more frequent manifestation of MF, being due to decreased bone marrow production, ineffective erythropoiesis, hypersplenism, and occasional bleeding. Dilution by the increased plasma volume secondary to splenomegaly can contribute. Sometimes, iron, vitamin B12, or folate deficiency is found, whereas autoimmune hemolysis is rare.15 The anemia is often accentuated by the cytoreductive agents or the JAK inhibitors.
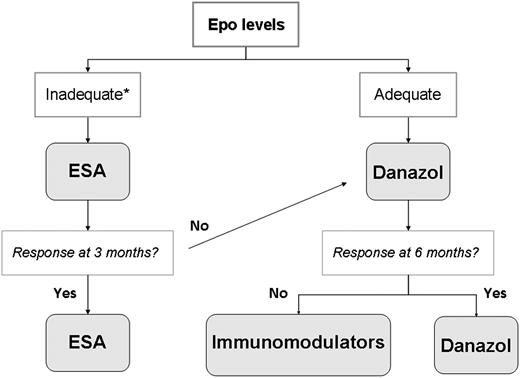
I always start studying the anemia of MF by excluding treatable causes, such as iron, folate, or vitamin B12 deficiency, which are infrequent. When there is marked hemolysis, I perform a Coombs’ test because, if positive, corticosteroids are the therapy of choice, but this is exceedingly rare. Most patients require an anemia-treating agent. Hb <10 g/dL is the threshold usually triggering treatment institution, but there are individual variations. Thus, an elderly person with cardiac failure may need treatment with an Hb of 10.5 g/dL, whereas a younger patient may tolerate values of 9 to 10 g/dL. Therapeutic options include erythropoiesis-stimulating agents (ESAs), androgens, immunomodulators, splenectomy, and prednisone. Figure 2 shows my therapeutic algorithm for the anemia of MF, in which the initial choice between ESA and danazol is based on the patient’s serum erythropoietin (Epo) levels.
ESAs
With recombinant human Epo or darbepoetin-α, anemia responses (transfusion independence with normal Hb, transfusion decrease >50%, or sustained Hb increase >2 g/dL in non–transfusion-dependent patients) are achieved in 23% to 60% of patients.16-18 Median duration of responses is 12 months, and half are long lasting; they are usually restricted to patients with inadequate Epo levels (<125 mU/mL),16-18 being less frequent in those with large spleens or transfusion dependence.16-19 I start with a weekly dose of 20 000 U of recombinant human Epo or 150 μg of darbepoetin-α, and I double the dose if no response is observed after 4 weeks. If there is no response at 3 months, therapy should be stopped. A spleen increase can be seen with ESA treatment.
Androgens
Nandrolone, fluoxymesterone, methandrostenolone, and oxymetholone improve anemia in 30% to 60% of patients.20,21 Factors associated with a favorable response are female gender, previous splenectomy or lack of huge splenomegaly, and normal karyotype. Similar results, with less toxicity, are obtained with danazol, a semisynthetic attenuated androgen that can also correct thrombocytopenia.22 Responses approach 40%.22 Before starting danazol, men must be asked for prostatic symptoms, and serum prostate-specific antigen levels must be determined to rule out prostate cancer. Dose must be sufficient (600 mg daily) and should be maintained for ≥6 months, because most responses are seen between 3 and 6 months. Then, it must be progressively reduced to the minimum necessary dose to maintain response, usually 200 mg/day. Hepatic alterations are the main toxicity; they appear in <20% of patients and are generally moderate, requiring dose reduction but rarely definitive discontinuation of the drug. Patient’s liver function must be monitored at every visit, and ultrasound imaging must be performed annually to exclude appearance of liver tumors; men must be periodically screened for prostate cancer.
Immunomodulating drugs
Thalidomide, at 100 to 200 mg/day, is associated with high withdrawal due to side effects such as constipation, fatigue, paresthesiae, sedation, hematologic toxicity, and myeloproliferative acceleration23 ; anemia response is 29%. To minimize toxicity, lower doses (50 mg daily) are given in combination with oral prednisone (0.5 mg/kg daily for 3 months and then taper), resulting in less withdrawal and similar response rates.24 The efficacy of this combination has been ascribed to prednisone, because following its discontinuation, many responses are lost.25
Lenalidomide produces 22% anemia responses and 10% to 42% responses in splenomegaly.26-28 Dose is 5 to 10 mg daily (depending on platelet count) for 3 weeks, every 4 weeks. Withdrawal is high because of side effects, mainly hematologic toxicity.26 To reduce toxicity, lenalidomide has been combined with a low-dose prednisone taper; in 1 study, anemia response increased to 30%,27 but in another, it was 19%.28 Lenalidomide is the choice treatment of the rare cases of MF with 5q deletion.29
Splenectomy
Splenectomy can be considered in patients with transfusion-dependent anemia refractory to drug therapy, but the procedure involves substantial risk. Indeed, in a large series, perioperative morbidity was 31% and mortality was 9%.32 The main complications are bleeding (especially hemoperitoneum), infections, and thrombosis, primarily in the splanchnic veins.33 Massive hepatomegaly due to compensatory myeloid metaplasia develops in 16% to 24% of patients, some of whom may die of liver failure.34 Durable responses in transfusion-dependent anemia are 23%.33 The decision on splenectomy should be taken carefully, balancing the risks against the possible benefits. Splenectomy is the choice treatment of MF-associated immune hemolysis unresponsive to corticosteroids.
Corticosteroids
I use prednisone for palliation of anemia if the abovementioned drugs fail and the patient is not a good candidate for allo-SCT or splenectomy. I start with 30 mg daily, which I reduce to 15 to 20 mg after a few weeks. Hb increases of 1 to 2 g/L are often seen, which can be sufficient to improve patient well-being.
Iron chelation?
A substantial proportion of MF patients require red blood cell transfusions, but there are no data supporting the value of iron chelation therapy.35 Given the short survival associated with transfusion dependency, I restrict iron chelation to potential candidates for transplantation.
How I treat splenomegaly
Case study 2
A 64-year-old woman was diagnosed with PMF after several months of abdominal discomfort. The spleen was palpable at 12 cm below costal margin. Hb was 11.2 g/dL, leukocyte count was 14 × 109/L, with leukoerythroblastosis and no blasts, and platelet level was 486 × 109/L. Bone marrow cytogenetic study was normal, and marrow biopsy showed clusters of dysplastic megakaryocytes with marked reticulin fibrosis, corresponding to MF-2 of the World Health Organization histological grading. The JAK2 and MPL mutations were negative, and the patient was recently found to have a 52-bp deletion of CALR. Hydroxyurea was instituted, resulting in marked spleen reduction and disappearance of symptoms. However, progressive splenomegaly was observed over time despite increasing hydroxyurea dose. The patient refused splenectomy, and splenic radiation was performed, but it had to be interrupted due to pancytopenia. When hematologic values recovered, the patient went back to hydroxyurea, with poor control of the splenomegaly and symptoms. Once it was available, I started ruxolitinib.
Splenomegaly is a characteristic feature of MF, being due to extramedullary hematopoiesis36 that frequently also affects the liver. Despite its clinical relevance, it has not been a prognostic factor in most studies, because it is usually associated with other poor prognosis factors.12 Symptoms from splenomegaly correlate with spleen size. Thus, moderate splenomegaly may not produce symptoms, but, as spleen increases, it causes important abdominal symptoms, often with constitutional symptoms, accentuation of the cytopenias, and signs of portal hypertension.
It is generally agreed that a wait-and-see approach is a reasonable option in patients with moderate and asymptomatic splenomegaly, with therapy being delayed until appearance of symptoms, especially considering that treatment may worsen the cytopenias. This is my approach. However, some authors have called for earlier treatment in asymptomatic patients, mainly with interferon-α, which has been shown to achieve disease stability or improvement and occasional fibrosis reversal.37-39 Nevertheless, sooner or later, all patients will require therapy. Conventional modalities include cytoreductive drugs, splenectomy, and splenic radiation. The JAK inhibitors have changed this scenario.
Cytoreductive drugs
Hydroxyurea was for many years the choice therapy for MF splenomegaly. My starting daily dose is 500 mg, which I escalate depending on response and tolerability. With increasing doses, accentuation of anemia, requiring anemia-alleviating drugs, is often seen. Oral or leg ulcers are the most characteristic nonhematologic toxicity of hydroxyurea, which is usually associated with prolonged administration and high doses. The overall response is 40%, and median duration is 13.2 months.40 Therefore, at roughly 1 year of hydroxyurea start, 80% of patients require an alternative therapy. Moreover, the responses are not comparable with those of JAK inhibitors. However, hydroxyurea can be an option for moderately symptomatic splenomegaly, especially in the setting of difficult access to JAK inhibitors. Other drugs such as busulfan or melphalan are rarely used.41
Splenectomy
Splenectomy may be indicated for large and painful splenomegaly refractory to drug therapy. As previously mentioned, the decision must be individualized due to the associated risks. Although the availability of JAK2 inhibitors has reduced the use of splenectomy in MF, it can be considered in eligible patients resistant to these drugs.
Radiation therapy
Splenic radiation, on a fractioned basis, at a daily dose of 0.4 to 1 Gy, with weekly evaluation of spleen size and hematologic values until therapeutic effect is achieved or hematological toxicity develops, can be applied to patients that are refractory to JAK2 inhibitors and poor candidates to surgery. In one series, median total dose per course was 9.8 Gy (range, 0.6-30.05 Gy).42 However, its benefit is transient, whereas, due to the effect on circulating progenitors,43 it involves the risk of severe and prolonged cytopenias, developing in one-third of patients.44 Therefore, I do not recommend routine use of splenic radiation; JAK inhibitors have further reduced the use of this therapy.
Ruxolitinib and other JAK2 inhibitors
The discovery of the JAK2 mutation triggered development of molecular targeted therapies for MF. These agents mainly inhibit dysregulated JAK-STAT signaling, present in all patients irrespective of JAK2 mutational status.45 All have overlapping activity against other members of the JAK family (including JAK1, JAK2, JAK3, and Tyk2) and sometimes against other tyrosine kinases. Unlike the BCR-ABL1 inhibitors, the JAK inhibitors are not selective for mutated JAK2,46 which explains their efficacy in JAK2-positive and JAK2-negative MF and their hematologic toxicity, given the importance of the JAK-STAT pathway in hematopoiesis. Table 1 shows a summary of the JAK2 inhibitors tested in MF.
JAK inhibitors tested in clinical trials in MF
| Agent . | Other targets . | Phase . |
|---|---|---|
| Ruxolitinib | JAK1 | 3 (approved) |
| Fedratinib (SAR302503) | FLT3, RET | 3 (withdrawn) |
| Pacritinib (SB1518) | FLT3 | 3 |
| Momelotinib (CYT387) | JAK1, JNK1, TYK2, CDK2, RICJ2 | 3 |
| Lestaurtinib (CEP-701) | FLT3, TRKA | 2 (withdrawn) |
| AZD1480 | JAK1, JAK3, FLT4, FGFR1, TRKA | 2 |
| Gandotinib (LY2784544) | — | 1 (withdrawn) |
| XL019 | — | 1 (withdrawn) |
| NS-018 | SRC, FLT3, ABL | 1/2 |
| BMS-911543 | — | 1/2 (withdrawn) |
| Agent . | Other targets . | Phase . |
|---|---|---|
| Ruxolitinib | JAK1 | 3 (approved) |
| Fedratinib (SAR302503) | FLT3, RET | 3 (withdrawn) |
| Pacritinib (SB1518) | FLT3 | 3 |
| Momelotinib (CYT387) | JAK1, JNK1, TYK2, CDK2, RICJ2 | 3 |
| Lestaurtinib (CEP-701) | FLT3, TRKA | 2 (withdrawn) |
| AZD1480 | JAK1, JAK3, FLT4, FGFR1, TRKA | 2 |
| Gandotinib (LY2784544) | — | 1 (withdrawn) |
| XL019 | — | 1 (withdrawn) |
| NS-018 | SRC, FLT3, ABL | 1/2 |
| BMS-911543 | — | 1/2 (withdrawn) |
Ruxolitinib, an oral JAK1/JAK2 inhibitor, is the first agent approved for the treatment of MF. In the phase 1-2 trial, it was well tolerated, with thrombocytopenia as the dose-limiting toxicity.47 Spleen reduction and control of symptoms were usually dramatic but also drug and dose dependent, because drug discontinuation or reduction was followed by rapid spleen increase and reappearance of symptoms. Ruxolitinib is also effective in MF-associated hepatomegaly. Normalization of several proinflammatory cytokines correlates with symptomatic improvement, with this being ascribed to the drug anti-JAK1 activity. Sudden ruxolitinib withdrawal has been reported to provoke a shock-like syndrome due to reemergence of the suppressed cytokines.48 Although this complication was not observed in the phase 3 studies, abrupt interruption of ruxolitinib must be avoided, and the drug must be tapered, combined with a prednisone taper. In most patients, accentuation of anemia is observed, especially during the first months of treatment.
Two phase 3 studies, in patients with intermediate-2 and high-risk MF, compared ruxolitinib with placebo (COMFORT-I)49 or best-available therapy (COMFORT-II).50 In both studies, ruxolitinib achieved the primary end point of >35% reduction in spleen size, by imaging techniques, at 24 or 48 weeks of treatment, respectively. Based on these results, ruxolitinib was approved in the United States for patients with high- or intermediate-risk MF and in Europe for splenomegaly and/or constitutional symptoms, irrespective of the risk group, which makes more sense. Response was independent of the JAK2 mutational status, without differences between PMF and post-ET/PV MF. With longer follow-up, the effect on JAK2V617F allele burden has been modest.51 Concerning the effect on marrow fibrosis, a patient has been reported in whom complete resolution of fibrosis was observed after 3 years of treatment.52 In the phase 1 and 2 ruxolitinib trials, at 5 years, improvement in fibrosis was seen in 36% of patients, whereas in the majority, no significant histological changes were observed.53 Longer follow-up will allow determination of whether prolonged ruxolitinib administration could increase the proportion of patients achieving fibrosis improvement or resolution. Historical comparison of ruxolitinib-treated patients with matched MF populations has shown a survival advantage for the former.54,55 Moreover, despite the crossover, extended follow-up of the COMFORT studies indicates a survival advantage for patients assigned to ruxolitinib.51,55,56
An increase in urinary tract infections has been noted under ruxolitinib.51 Occasional reactivation of tuberculosis and other opportunistic infections has also been reported,57-59 being attributed to continuous suppression of T lymphocytes. However, this complication is anecdotal among the many patients currently receiving the drug worldwide.
In the phase 3 studies, ruxolitinib dose was 15 or 20 mg twice daily, depending on platelet counts (100-200 × 109/L or >200 × 109/L). To minimize the negative effect on anemia, I start with 10 mg twice daily in patients without massive splenomegaly and with moderate symptoms, and I escalate the dose depending on the response achieved. A recent study in patients with moderate thrombocytopenia (from 50 × 109/L to 100 × 109/L) has shown the feasibility of starting with 5 mg twice daily and then escalating to 10 mg (and occasionally to 15 mg) twice daily, without causing severe thrombocytopenia.60 Treatment should be stopped if platelets fall below 50 × 109/L. In case of renal or liver function impairment, I start with a dose of 10 mg twice daily. With regard to treatment duration, I definitively stop therapy if there is no clinically meaningful response after 6 months. In this sense, although no specific criteria for ruxolitinib failure are available, I consider the response unsatisfactory when spleen reduction is inferior to 25% of the baseline value (by palpation) and constitutional symptoms persist. The recently developed scale for symptom assessment in patients with MPNs can help in evaluating the impact of therapy on MF-associated symptoms.13 With certain frequency, ruxolitinib is unable to control leukocytosis or thrombocytosis; if there is clear benefit in spleen and symptoms, I consider adding hydroxyurea.
Despite its dramatic symptomatic improvement and the suggestion of survival prolongation, there is no clear indication of a disease-modifying effect of ruxolitinib. Besides, at 3 years, 60% of patients are off treatment.51 Therefore, further efforts are necessary to improve ruxolitinib results. This is currently being attempted by combination with anemia-alleviating drugs or with agents aimed at achieving deeper disease-modifying effect (Table 2). Other JAK inhibitors that selectively suppress the clonal cells and restore normal hematopoiesis need to be developed.
Drugs tested in combination with the JAK inhibitors in MF
| Aimed at improving anemia . | Aimed at a deeper effect . |
|---|---|
| ESA | Pegylated interferon |
| Danazol | Histone deacetylase inhibitors |
| Lenalidomide | Hypomethylating agents |
| Pomalidomide | Hedgehog inhibitors |
| mTOR inhibitors | |
| PI3K inhibitors | |
| HSP90 inhibitors | |
| PIM inhibitors | |
| pERK 1/2 inhibitors | |
| CDK 4/6 inhibitors |
| Aimed at improving anemia . | Aimed at a deeper effect . |
|---|---|
| ESA | Pegylated interferon |
| Danazol | Histone deacetylase inhibitors |
| Lenalidomide | Hypomethylating agents |
| Pomalidomide | Hedgehog inhibitors |
| mTOR inhibitors | |
| PI3K inhibitors | |
| HSP90 inhibitors | |
| PIM inhibitors | |
| pERK 1/2 inhibitors | |
| CDK 4/6 inhibitors |
Fedratinib (SAR302503), a preferential JAK2 inhibitor, has recently been withdrawn due to occurrence of Wernicke’s encephalopathy in some patients.61
Momelotinib (CYT3879), a JAK1/JAK2 inhibitor, produced 45% spleen responses, with frequent improvement in constitutional symptoms.62 Among patients evaluable for anemia response, 50% responded, including 58% with transfusion dependence. Grade 3-4 thrombocytopenia appeared in 25% of patients; hyperlipasemia and headache were the most characteristic nonhematologic adverse effects. A phase 3 study comparing momelotinib with ruxolitinib is in progress.
Pacritinib (SB1518), a selective JAK2 inhibitor, reduced splenomegaly in 57% of patients, with scarce myelosuppression and some gastrointestinal side effects.63 A phase 3 study is also ongoing.
The approval of ruxolitinib has led to its incorporation into MF treatment algorithms as the best available therapy for splenomegaly and/or constitutional symptoms. Quite likely, other JAK2 inhibitors will follow. The trade-offs between clinical activity and toxicity will help choosing the right drug. Longer follow-up is required to establish the definitive role of JAK inhibitors in MF; information on nonhematologic long-term effects is also needed.
How I decide on transplantation
Case study 3
A 57-year-old woman was diagnosed with JAK2-positive PMF after several months of weight loss, night sweats, fatigue, and abdominal pain. Spleen and liver were palpable at 14 and 6 cm below the costal margin. Hb was 9.6 g/dL, WBC count was 28 × 109/L, with leukoerythroblastosis and 5% blasts, platelet count was 520 × 109/L, and lactate dehydrogenase level was 1834 U/L. Trisomy 8 and marked fibrosis were found in the bone marrow. Hydroxyurea was started, with a rapid reduction of splenomegaly and leukocytosis, disappearance of symptoms, and improvement in the general condition. The patient had a compatible brother and, because she had high-risk PMF by both the International Prognostic Scoring System (IPSS) and dynamic IPSS (DIPSS)-plus (that considers also the unfavorable karyotype),12,64 I decided to proceed to allo-SCT.
Median survival of PMF currently approaches 7 years.65 Survival is heterogeneous, with some patients living for >20 years and others dying within 1 or 2 years. Prognosis at presentation is assessed using the IPSS, based on 5 poor prognostic factors: age >65 years, constitutional symptoms, Hb <10 g/dL, leukocytes >25 × 109/L, and blood blasts ≥ 1%. They allow recognizing 4 prognostic groups (low, intermediate-1, intermediate-2, and high risk), with median survival around 11, 8, 4, and 2 years, respectively.12 The IPSS has been complemented by DIPSS, based on the same factors but giving higher weight to anemia, for its use during the evolution.66 Of note, sometimes the DIPSS is not used correctly, when applied at presentation. The IPSS was derived from 1054 patients and, although Hb <10 g/dL was the main prognostic factor, its prognostic weight was slightly higher than that of the other factors; therefore, 1 point was assigned to the 5 factors. The DIPSS was derived from the IPSS series but using half of patients; therefore, these results cannot substitute those obtained from the whole series. Acquiring an Hb <10 g/dL during the evolution had double prognostic impact than acquisition of the other factors; because of this, 2 points were assigned to anemia. In summary, the IPSS must be applied at presentation and the DIPSS during the evolution. The DIPSS has been refined into a DIPSS-plus model, also including thrombocytopenia, transfusion need, and karyotype.64 Table 3 summarizes current PMF prognostic models. Although the 3 models were derived from PMF patients, they are also applied to post-ET and post-PV MF. However, recent data in this setting indicate that, although the IPSS would be useful to identify high-risk patients, it would not accurately discriminate between the other risk categories.67 The lack of prognostic significance of some of the IPSS variables (notably, the leukocyte count) in these patients might be ascribed to the effect of the cytoreductive therapy that many were receiving for ET or PV at time of myelofibrotic transformation.
Current prognostic models for MF
| Variable . | IPSS . | DIPSS . | DIPSS-plus . |
|---|---|---|---|
| Age > 65 years | + | + | + |
| Constitutional symptoms | + | + | + |
| Hb < 10 g/dL | + | + | + |
| Leukocytes > 25 × 109/L | + | + | + |
| Blood blasts ≥ 1% | + | + | + |
| Platelets < 100 × 109/L | + | ||
| Red blood cell transfusion need | + | ||
| Unfavorable karyotype: +8, −7/7q-, −5/5q-, i17q, 12p-, 11q23 rearrangement | + | ||
| Score: | 1 point each | 1 point each (Hb: 2 points) | The sum of the patient’s DIPSS score (int-1: 1 point; int-2: 2 points; high: 3 points) plus 1 additional point for each of the following: platelets < 100 × 109/L; unfavorable karyotype; transfusion need |
| Variable . | IPSS . | DIPSS . | DIPSS-plus . |
|---|---|---|---|
| Age > 65 years | + | + | + |
| Constitutional symptoms | + | + | + |
| Hb < 10 g/dL | + | + | + |
| Leukocytes > 25 × 109/L | + | + | + |
| Blood blasts ≥ 1% | + | + | + |
| Platelets < 100 × 109/L | + | ||
| Red blood cell transfusion need | + | ||
| Unfavorable karyotype: +8, −7/7q-, −5/5q-, i17q, 12p-, 11q23 rearrangement | + | ||
| Score: | 1 point each | 1 point each (Hb: 2 points) | The sum of the patient’s DIPSS score (int-1: 1 point; int-2: 2 points; high: 3 points) plus 1 additional point for each of the following: platelets < 100 × 109/L; unfavorable karyotype; transfusion need |
IPSS: low risk, 0 points; intermediate-1 risk, 1 point; intermediate-2 risk, 2 points; high risk, 3 to 5 points; DIPPS: low risk, 0 points; intermediate-1 risk, 1 to 2 points; intermediate-2 risk, 3 to 4 points; high risk, 5 to 6 points; DIPPS-plus: low risk, 0 points; intermediate-1 risk, 1 point; intermediate-2 risk, 2 to 3 points; high risk, 4 to 6 points.
Recently, advances have been made in molecular prognostication of PMF. Concerning MPN phenotype driver mutations, there is no agreement on the prognostic value of the JAK2 mutation or its allelic burden,68,69 whereas MPL mutations do not seem to be prognostically relevant.70 On the contrary, CALR mutations seem to be associated with better prognosis.71,72 Of note, triple-negative patients (ie, without JAK2, MPL, or CALR mutations) have a particularly poor outcome.71,72 Quite likely, this information will soon be incorporated into MF prognostic assessment.
With regard to other molecular abnormalities, mutations in ASXL1, EZH2, IDH1/2, and SFSF2 are associated with poorer outcome,69 having been proposed for molecular prognostication of PMF.73 Of note, most patients with these mutations belonged to the intermediate-2 and high-risk groups, therefore having poor prognosis; however, roughly 20% of patients in the low and intermediate-1 groups also displayed some of these mutations. For now, presence of these alterations in the absence of other poor prognostic features is not sufficient to support the indication of intensive therapies such as allo-SCT. They can be considered as a warning, recommending the patient’s closer monitoring to detect early changes indicating the need for a different therapeutic strategy. In summary, this new information will require validation and consolidation before its routine incorporation into decision-making.
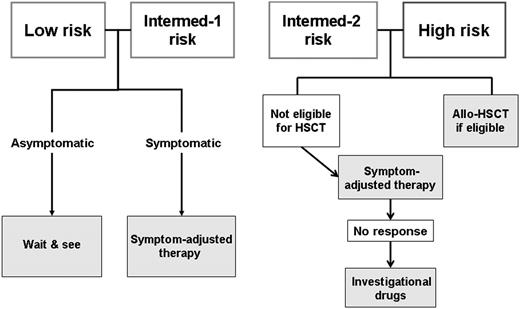
The main utility of prognostication in MF is to help decide on allo-SCT. Some authors suggest transplantation in all eligible patients, irrespective of the risk group.74,75 However, given the associated mortality and morbidity,76 there is wide consensus on indicating allo-SCT in high-risk patients and not in low- and intermediate-1–risk MF (Figure 3). Transplantation is also indicated for intermediate-2–risk patients. In this group, my personal approach is to perform allo-SCT in patients 60 years old or younger; over this age, given the procedure’s high mortality, I try drug therapy first and I use transplantation in the case of unsatisfactory response. Under 45 to 50 years of age, I use myeloablative regimens with targeted busulfan and cyclophosphamide,77 whereas in older patients, I use reduced-intensity conditioning with fludarabine and busulfan.78 In case of massive splenomegaly, I no longer perform splenectomy before transplantation; instead, I currently give JAK inhibitors to reduce tumor burden and to improve the general status.79-81 In good candidates for allo-SCT who improve under JAK inhibitors, I take advantage to proceed to transplantation in better general conditions and I do not postpone the procedure until response is lost, because this could jeopardize the success of transplantation.
How I treat special situations
Portal hypertension can develop in MF as a consequence of increased splenic blood flow, myeloid metaplasia of the liver, and splanchnic vein thrombosis. In addition to the general measures for portal hypertension, splenectomy can be considered in selected patients. Favorable results have been reported with the use of ruxolitinib in this setting.82
Low-dose radiation is the choice treatment of symptomatic extramedullary hematopoiesis in sites other than spleen and liver83 and for MF-associated pulmonary hypertension.84
Thrombosis is a complication of MF85,86 appearing in ∼7% of patients; predisposing factors are older age, presence of JAK2 mutation, and leukocytosis.86 Given the competing risk of death from other complications, unlike in PV and ET, thrombosis is not a major issue in MF. Because of this, the use of antiplatelet therapy to prevent thrombosis is not clearly recommended. In my practice, as long as platelets remain high, I maintain low-dose aspirin in patients with post-ET or post-PV MF who were receiving such therapy for ET or PV. In PMF, I prescribe antiplatelet treatment only in patients with a history of ischemic events.
Blast phase of MF has an especially poor prognosis, with a median survival of ∼2 months.87 In subjects <70 years of age, I administer acute myeloid leukemia-like chemotherapy with the aim of achieving a favorable response that allows transplantation. With such an approach, 20% to 45% of selected patients were finally transplanted, and half remained alive at 2 years.88 However, the majority of patients are candidates for palliative therapy only, based in my practice on transfusion support and oral mercaptopurine. New options, such as the JAK inhibitors and the hypomethylating agents, are being investigated.
Conclusions and future directions
Despite recent advances, for most MF patients, treatment remains unsatisfactory. Newer drugs are being tested (Table 4), but, because of space constraints, they have not been discussed here. Some will be withdrawn, but others will be incorporated into the MF therapeutic armamentarium. Therefore, my management of MF patients also includes enrollment into clinical trials in the hope that this will lead to the availability of drugs able to modify the disease natural history.
New drugs other than the JAK inhibitors for MF
| Agent . | Drug class . | Phase . |
|---|---|---|
| Pomalidomide | Immunomodulator | 3 (withdrawn) |
| Azacitidine | Hypomethylating agent | 2 |
| Decitabine | Hypomethylating agent | 1-2 |
| Givinostat | Histone deacetylase inhibitor | 2 |
| Panobinostat | Histone deacetylase inhibitor | 2 |
| Everolimus | m-TOR inhibitor | 2 |
| Obatoclax mesylate | Bcl-2 inhibitor | 2 |
| AUY-922 | HSP90 inhibitor | 2 |
| PF-04449913 | Hedgehog inhibitor | 2 |
| PRM-151 | Anti-fibrosis agent | 2 |
| GC-1008 | Anti-fibrosis agent | 1 |
| GS-6624 | Anti-fibrosis agent | 1 |
| Imetelstat | Telomerase inhibitor | 1-2 |
| IPI-926 | Hedgehog inhibitor | 1 |
| LDE-225 | Hedgehog inhibitor | 1 |
| BKM-120 | PI3K/AKT inhibitor | 1 |
| MK-2206 | PI3K/AKT inhibitor | Preclinical |
| ABT-737 | BCL-XL inhibitor | Preclinical |
| PU-H71 | HSP90 inhibitor | Preclinical |
| Agent . | Drug class . | Phase . |
|---|---|---|
| Pomalidomide | Immunomodulator | 3 (withdrawn) |
| Azacitidine | Hypomethylating agent | 2 |
| Decitabine | Hypomethylating agent | 1-2 |
| Givinostat | Histone deacetylase inhibitor | 2 |
| Panobinostat | Histone deacetylase inhibitor | 2 |
| Everolimus | m-TOR inhibitor | 2 |
| Obatoclax mesylate | Bcl-2 inhibitor | 2 |
| AUY-922 | HSP90 inhibitor | 2 |
| PF-04449913 | Hedgehog inhibitor | 2 |
| PRM-151 | Anti-fibrosis agent | 2 |
| GC-1008 | Anti-fibrosis agent | 1 |
| GS-6624 | Anti-fibrosis agent | 1 |
| Imetelstat | Telomerase inhibitor | 1-2 |
| IPI-926 | Hedgehog inhibitor | 1 |
| LDE-225 | Hedgehog inhibitor | 1 |
| BKM-120 | PI3K/AKT inhibitor | 1 |
| MK-2206 | PI3K/AKT inhibitor | Preclinical |
| ABT-737 | BCL-XL inhibitor | Preclinical |
| PU-H71 | HSP90 inhibitor | Preclinical |
Acknowledgment
This study was supported in part by Instituto de Salud Carlos III, Spanish Ministry of Health grant RD012/0036/0004.
Authorship
Contribution: F.C. wrote the paper.
Conflict-of-interest disclosure: F.C. is a consultant for Novartis, Sanofi-Aventis, Gilead, and CTI-Baxter.
Correspondence: Francisco Cervantes, Hematology Department, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain; e-mail: fcervan@clinic.ub.es.