Key Points
Lexaptepid modulates the inflammation-induced decrease in serum iron during experimental human endotoxemia.
Hepcidin targeting with the novel compound lexaptepid may be a viable approach to the treatment of anemia of inflammation in humans.
Abstract
Increased hepcidin production is key to the development of anemia of inflammation. We investigated whether lexaptepid, an antihepcidin l-oligoribonucleotide, prevents the decrease in serum iron during experimental human endotoxemia. This randomized, double-blind, placebo-controlled trial was carried out in 24 healthy males. At T = 0 hours, 2 ng/kg Escherichia coli lipopolysaccharide was intravenously administered, followed by an intravenous injection of 1.2 mg/kg lexaptepid or placebo at T = 0.5 hours. The lipopolysaccharide-induced inflammatory response was similar in subjects treated with lexaptepid or placebo regarding clinical and biochemical parameters. At T = 9 hours, serum iron had increased by 15.9 ± 9.8 µmol/L from baseline in lexaptepid-treated subjects compared with a decrease of 8.3 ± 9.0 µmol/L in controls (P < .0001). This study delivers proof of concept that lexaptepid achieves clinically relevant hepcidin inhibition enabling investigations in the treatment of anemia of inflammation. This trial was registered at www.clinicaltrial.gov as #NCT01522794.
Introduction
Anemia of inflammation is the most prevalent form of anemia in a hospital setting,1 occurring in a variety of acute and chronic inflammatory diseases.2 The dysregulation of iron homeostasis is central to the pathogenesis of anemia of inflammation.1,3 A redistribution of iron to the cells of the reticuloendothelial system occurs, with hepcidin as a central mediator.3 Hepcidin induces internalization and degradation of the iron-exporting channel ferroportin on duodenal enterocytes and iron-storing cells,4 thereby preventing the export of iron into the circulation and causing iron-restricted erythropoiesis.5 Hence, hepcidin inhibition may be an attractive therapeutic intervention to improve the utilization of iron from intracellular stores.
Lexaptepid (international nonproprietary name: lexaptepid pegol), a nonnatural mirror-image l-nucleotide modified at its 5′ end with a branched 40 kDa monomethoxy-polyethylene-glycol, inactivates hepcidin in a mode conceptually similar to antibodies. Lexaptepid, as well as other pharmaceutical interventions inhibiting hepcidin, have been shown to prevent ferroportin degradation in vitro and also to prevent and correct anemia of inflammation in animal models.6-13 However, the efficacy in humans remains to be determined. In a first-in-human trial, lexaptepid was well tolerated and effective in elevating serum iron.14
The primary aim was to investigate the effectiveness of lexaptepid in preventing the serum iron decrease observed during systemic inflammation in healthy volunteers. The secondary objective was to assess the effects of lexaptepid on the innate immune response.
Materials and methods
Experimental human endotoxemia
This double-blind, randomized, placebo-controlled trial was approved by the local ethics committee and carried out according to good clinical practice standards and the declaration of Helsinki, including current revisions. The study was registered at clinicaltrial.gov as #NCT01522794.
Experimental human endotoxemia is a generally accepted way of studying systemic inflammation in a controlled manner.15 Following written informed consent and screening, 24 healthy male subjects were prehydrated with 1.5 L NaCl 0.45%/glucose 2.5%.16 At T = 0 hours, 2 ng/kg purified standard reference Escherichia coli O:113 lipopolysaccharide (LPS) (Clinical Center Reference Endotoxin, National Institutes of Health, Bethesda, MD) was administered IV. Lexaptepid (NOXXON Pharma AG, Berlin, Germany) was diluted in glucose 5%, according to the manufacturer’s instructions. At T = 0.5 hours, 1.2 mg/kg lexaptepid or placebo (glucose 5%) was administered IV over 15 minutes in a randomized, double-blind fashion. See supplemental Data File 2 (available at the Blood Web site) for details.
Inflammatory and iron parameters
C-reactive protein, total white blood cell count, and iron parameters were determined by the local laboratory of clinical chemistry (see also supplemental Data File 2 for details). For cytokines (tumor necrosis factor α [TNF-α], interleukin-6 [IL-6], IL-10, and IL-1 receptor antagonist), EDTA-anticoagulated blood was immediately processed and plasma was stored at −80°C until batch-wise analysis (Luminex, Merck Millipore, Billerica, MA). Plasma hepcidin-25 concentrations were measured with time-of-flight mass spectrometry as described in supplemental Data File 2. As it is currently technically impossible to discriminate between lexaptepid-bound and free hepcidin, the total of bound and unbound hepcidin is reported.
Pharmacokinetics
Lexaptepid plasma concentrations were determined using a fully validated sandwich hybridization assay specific for the full-length sequence of the oligonucleotide. See supplemental Data File 2 for details.
Statistical analysis
Data are expressed as arithmetic mean ± standard deviation (SD) or median [25th-75th percentile], depending on their distribution. Differences in group means or medians were tested with the Student t test for parametric data or the Mann-Whitney U test for nonparametric data, as appropriate. The primary end point was the change from baseline in serum iron at T = 9 hours after LPS administration. See supplemental Data File 2 for details.
Results and discussion
As hepcidin is the principal regulator of iron homeostasis, it has become a target for the development of novel therapeutics for iron disorders.17 Lexaptepid has proven effective in counteracting hepcidin’s effects in animal models, including an IL-6–induced anemia model in cynomolgus monkeys.11 In the present study, we extend the concept of hepcidin inhibition to humans for the first time.
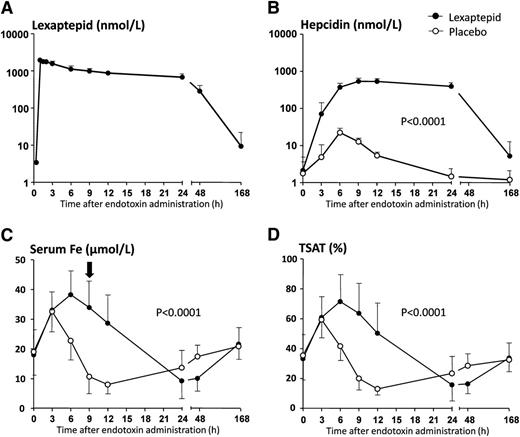
No differences in baseline parameters between the treatment groups were found (supplemental Table 1). Lexaptepid was well tolerated. Maximum lexaptepid plasma concentrations (Cmax) of 1.96 ± 0.19 μmol/L were measured at 0.7 ± 0.4 hours after administration (Figure 1A), after which levels declined in a biphasic manner (initial elimination half-life of 9.7 ± 3.6 hours, mean terminal elimination half-life of 22.5 ± 4.3 hours).
Plasma lexaptepid and iron parameters during experimental human endotoxemia. (A) Drug concentration in lexaptepid-treated subjects only. (B) Concentration of hepcidin in lexaptepid- and placebo-treated subjects. (C) Serum iron concentrations. Black arrow indicates the primary end point: change in serum iron levels at 9 hours after LPS administration. (D) Transferrin saturation (TSAT). Data are expressed as mean ± SD. Changes in all parameters over time were analyzed with 2-way analysis of variance with repeated measures, interaction term. Interaction terms are displayed within each graph. In the lexaptepid-treated subjects, hepcidin levels were still elevated at T = 168 hours (day 8) compared with baseline values (Student paired t test: P = .005).
Plasma lexaptepid and iron parameters during experimental human endotoxemia. (A) Drug concentration in lexaptepid-treated subjects only. (B) Concentration of hepcidin in lexaptepid- and placebo-treated subjects. (C) Serum iron concentrations. Black arrow indicates the primary end point: change in serum iron levels at 9 hours after LPS administration. (D) Transferrin saturation (TSAT). Data are expressed as mean ± SD. Changes in all parameters over time were analyzed with 2-way analysis of variance with repeated measures, interaction term. Interaction terms are displayed within each graph. In the lexaptepid-treated subjects, hepcidin levels were still elevated at T = 168 hours (day 8) compared with baseline values (Student paired t test: P = .005).
In placebo-treated subjects, LPS administration resulted in an increase in plasma hepcidin, peaking at T = 6 hours with maximum concentrations of 23.0 ± 5.2 nmol/L, followed by a gradual decrease reaching baseline levels at T = 24 hours (Figure 1B). In the lexaptepid-treated group, total hepcidin levels increased more rapidly and extensively (maximum levels: 547 ± 117 nmol/L, peak at T = 9 hours). This may largely be attributed to a reduced elimination of the lexaptepid-hepcidin complex through the kidney due to increased size and charge as also reported for an antihepcidin antibody.12 In a previous phase 1 study, constant concentration increases of hepcidin after increasing doses of lexaptepid from 0.3 to 4.8 mg/kg indicated that the administration of lexaptepid did not induce hepcidin production by itself.14 In addition, hepcidin production may have been influenced by the major changes in serum iron as observed throughout the experiment.
There was a marked induction of serum iron in both the lexaptepid-treated and placebo group at 3 and 6 hours after endotoxin administration (rise to 203% ± 13% and 221% ± 22% from baseline respectively in the lexaptepid treated group, versus 181% ± 14% and 146% ± 14% in the placebo (Figure 1C,D and supplemental Figure 1). This immediate increase was previously observed during experimental endotoxemia18 and may be due to an acute inflammation-induced abrogation of erythropoiesis and iron uptake by the bone marrow, whereas the export of iron from iron storing cells persists as long as ferroportin is expressed. In concordance, serum iron levels were comparable in both groups at T = 3 hours and started to decline in the placebo-treated subjects concurrent with the increase of hepcidin concentrations. In contrast, lexaptepid administration resulted in a prolonged increase in serum iron, peaking at T = 6 hours. The transient LPS-induced hypoferremia was effectively prevented by lexaptepid. Accordingly, the predefined primary end point, change in serum iron concentration 9 hours after LPS administration, was met (P < .0001). At this time point, no differences between groups were found in changes in hematologic parameters and cytokine levels (supplemental Table 2). A total of 24 to 48 hours after LPS administration, serum iron was actually lower in the lexaptepid-treated group compared with controls. We suspect that the prolonged and strongly elevated serum iron concentrations may have stimulated hepcidin production to overcome the effect of lexaptepid, eventually resulting in hepcidin-induced hypoferremia. We do not anticipate that treatment of patients with anemia of chronic disease and functional iron deficiency, who typically have serum iron concentrations below 10 µmol/L, would react with similarly high increases in serum iron concentrations when treated with lexaptepid, and we therefore do not consider this observation as predictive for actual patients. Currently, a phase 2 trial (clinicaltrials.gov #NCT01691040) investigates whether lexaptepid is able to elevate hemoglobin in cancer patients, and initial results from a pilot phase are encouraging.19
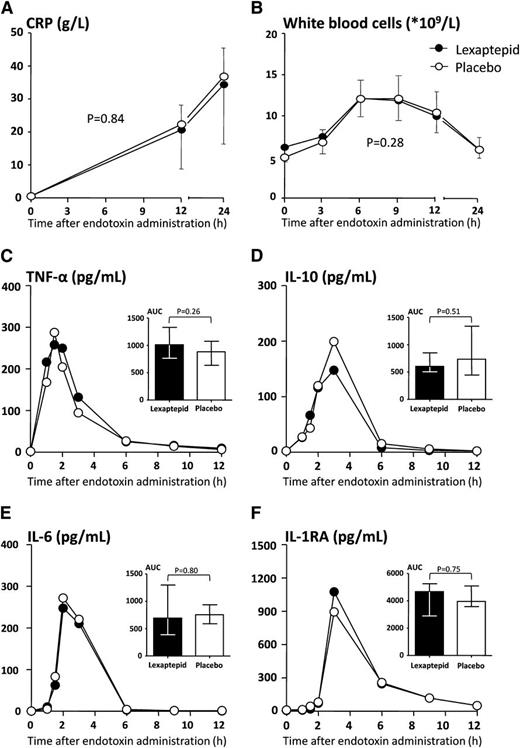
Although an increase over time was noted for both total iron-binding capacity and plasma ferritin concentration after endotoxin administration, no differences between groups were observed (supplemental Figure 2A,B). No effect of lexaptepid was observed on innate immunity. Laboratory parameters of inflammation (Figure 2A-F) and inflammatory signs and symptoms (supplemental Figure 3A,B) caused by LPS were not different between the groups.
Inflammatory parameters. (A) C-reactive protein (CRP) and (B) white blood cell count. CRP peaked at 24 hours and had fully normalized by day 8 in both treatment groups. Values in panels A and B are depicted as mean and SD. Differences over time were analyzed with 2-way analysis of variance with repeated measures. The P value of the interaction terms are depicted in the respective panels. Plasma concentrations of (C) TNF-α, (D) IL-10, (E) IL-6, and IL-1 receptor antagonist (F) (IL-1RA) are displayed for the treatment groups. As cytokine concentrations were not normally distributed, only median cytokine concentrations are depicted. Areas under the concentration-time curve (AUC) were calculated for each individual and compared between groups as depicted in the inserted bar charts. Differences in areas under the curve between groups were tested with the Mann-Whitney U test. No significant differences in cytokine concentrations between the treatment groups were observed.
Inflammatory parameters. (A) C-reactive protein (CRP) and (B) white blood cell count. CRP peaked at 24 hours and had fully normalized by day 8 in both treatment groups. Values in panels A and B are depicted as mean and SD. Differences over time were analyzed with 2-way analysis of variance with repeated measures. The P value of the interaction terms are depicted in the respective panels. Plasma concentrations of (C) TNF-α, (D) IL-10, (E) IL-6, and IL-1 receptor antagonist (F) (IL-1RA) are displayed for the treatment groups. As cytokine concentrations were not normally distributed, only median cytokine concentrations are depicted. Areas under the concentration-time curve (AUC) were calculated for each individual and compared between groups as depicted in the inserted bar charts. Differences in areas under the curve between groups were tested with the Mann-Whitney U test. No significant differences in cytokine concentrations between the treatment groups were observed.
In conclusion, the antihepcidin treatment with lexaptepid was well tolerated and effective in blocking the inflammation-induced reduction in serum iron during systemic inflammation in humans. It therefore represents an interesting compound for the future treatment of anemia of inflammation.
Presented in abstract form at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012.
The online version of the article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from NOXXON Pharma A.G., Berlin, Germany and the German Federal Ministry of Education and Research (BMBF) through a grant for small and midsize companies (KMU-innovativ-7).
Authorship
Contribution: Data were analyzed by L.T.v.E., F.S., S.V., S.Z., M.K., and K.R., and all other authors had access to the primary data; L.T.v.E. codesigned the study, conducted the endotoxemia experiments, analyzed the data, interpreted findings, and wrote the manuscript; A.S.E.J. participated in the execution of the experiments and gathering of data and corrected the manuscript; F.S. contributed to designing the study, interpreting findings, and reviewing the manuscript; L.S. took part in designing the study and gathering and cleaning of the data; S.V. and S.Z. planned, performed, and interpreted the pharmacokinetics, monitored hepcidin measurements, and corrected the manuscript; C.M.L. developed and validated an assay for total plasma hepcidin-25, performed the hepcidin measurements, and corrected the manuscript; M.K. analyzed the study data results and corrected the manuscript; J.G.v.d.H. interpreted findings and edited the manuscript; D.W.S. took part in the design of the study, supervised measurements of hepcidin and development of an assay for total hepcidin, interpreted findings, and edited the manuscript; K.R. took part in designing the study, analyzing the results, and correcting the manuscript; and P.P. was primary investigator, supervised the endotoxemia experiments and the data analyses, and corrected the manuscript.
Conflict-of-interest disclosure: The study was sponsored by NOXXON Pharma AG, Berlin, Germany. F.S., L.S., S.V., S.Z., and K.R. are employees of NOXXON Pharma AG. D.W.S. and C.M.L. are involved in the hepcidinanalysis.com initiative, by which the Radboud University Medical Center offers high-quality hepcidin measurements to the scientific and medical community. The remaining authors declare no competing financial interests.
Correspondence: Peter Pickkers, Department of Intensive Care Medicine (710), Radboud University Medical Center, PO Box 9101, 6500 HB, Nijmegen, The Netherlands; e-mail: peter.pickkers@radboudumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal