Key Points
Afuresertib has a favorable safety profile with manageable side effects and demonstrates single-agent activity against hematologic malignancies.
Inhibition of AKT with afuresertib may provide a novel therapeutic strategy for hematologic malignancies, especially for multiple myeloma.
Abstract
The PI3K/AKT pathway is constitutively active in hematologic malignancies, providing proliferative and antiapoptotic signals and possibly contributing to drug resistance. We conducted an open-label phase 1 study to evaluate the maximum tolerated dose (MTD), safety, pharmacokinetics, and clinical activity of afuresertib—an oral AKT inhibitor—in patients with advanced hematologic malignancies. Seventy-three patients were treated at doses ranging from 25 to 150 mg per day. The MTD was established at 125 mg per day because of 2 dose-limiting toxicities in the 150-mg cohort (liver function test abnormalities). The most frequent adverse events were nausea (35.6%), diarrhea (32.9%), and dyspepsia (24.7%). Maximum plasma concentrations and area under the plasma concentration-time curves from time 0 to 24 hours were generally dose proportional at >75-mg doses; the median time to peak plasma concentrations was 1.5 to 2.5 hours post dose, with a half-life of approximately 1.7 days. Three multiple myeloma patients attained partial responses; an additional 3 attained minimal responses. Clinical activity was also observed in non-Hodgkin lymphoma, Langerhan's cell histiocytosis, and Hodgkin disease. Single-agent afuresertib showed a favorable safety profile and demonstrated clinical activity against hematologic malignancies, including multiple myeloma. This trial was registered at www.clinicaltrials.gov as #NCT00881946.
Introduction
Multiple myeloma (MM) represents approximately 1% of all cancers (13% of hematologic malignancies) and, despite progress in treatment, remains incurable.1-3 Two drugs recently approved in the United States (carfilzomib and pomalidomide, the latter also approved in Europe) show significant activity in heavily pretreated patients. Neither of these drugs constitutes a novel class4,5 ; they both represent improved versions of known active classes: proteasome inhibitors (carfilzomib) and immunomodulators (pomalidomide). Given the genetic complexity of the disease, its multiclonal character, and the relatively short median progression-free survival of 3.7 and 4.2 months observed for carfilzomib and pomalidomide, respectively,6,7 it seems unlikely that treatment with any single agent will be sufficient to produce long-term remissions or eliminate the diseased clones.8 Therefore, new drugs with novel mechanisms of action and favorable safety profiles are needed to augment currently used combinations and to avoid cross-resistance.
The serine/threonine kinase AKT is a central node leading to the convergence of multiple signal pathways aberrant in cancer. It plays an important role in phosphatidylinositide 3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) signaling, in which activation dependent on PI3K leads to increased cell proliferation, survival, growth, and metabolism.9 Although AKT is rarely activated by intrinsic mutations, the PI3K/AKT pathway is broadly activated in nearly all tumors, including MM and other cancers.9,10 The PI3K/AKT pathway activation in MM is mostly related to signaling from major growth factor receptors such as insulin-like growth factor-1 or interleukin-6.11-13 This dependence on the PI3K/AKT pathway is suggested by the observation that inhibition of the pathway with PI3K inhibitors and/or mTOR inhibitors has been shown to induce apoptosis in myeloma cell lines and in tumor cells derived from patients.11,14
Afuresertib is a reversible, ATP-competitive, oral, low-nanomolar, pan-AKT kinase inhibitor. Oral administration to mice delays the growth of various human tumor xenografts in a dose-dependent manner. Afuresertib potently inhibits cell proliferation of various cell lines derived from hematologic malignancies, as tested in a 3-day proliferation assay. The frequency of sensitivity was particularly high in T-cell acute lymphoblastic leukemia (T-ALL; 19 of 20), B-cell ALL (B-ALL; 9 of 13), chronic lymphocytic leukemia (CLL; 6 of 7), and non-Hodgkin lymphoma (NHL; 8 of 11) cell lines (with median effective concentration <1 µM). Two T-ALL cell lines were tested for downstream effects of AKT inhibition with afuresertib. Both cell lines (A3 and I9.2) demonstrated induction of apoptosis, as evidenced by the accumulation of a sub-G1 population and increase in caspase-3 and/or caspase-7 activity in a dose-dependent manner.15
This first-time-in-human study (PKB112835) was designed to evaluate the safety, pharmacokinetics (PK), and initial clinical activity of once-per-day oral administration of afuresertib capsules in patients with relapsed or refractory hematologic malignancies.
Patients and methods
Patients
Independent ethics committee approval was obtained at each participating institution, and informed consent was provided by each study candidate in accordance with the Declaration of Helsinki. Adult patients with hematologic malignancies who relapsed or were refractory to standard therapy were enrolled. Patients with malignancies associated with HIV infection or solid organ transplant were excluded. Patients with acute leukemias, chronic myeloid leukemia (CML) in blast crisis, myelodysplastic syndromes, and myelofibrosis were excluded from part 1 (dose escalation). There was no limit to the number of prior therapies for patients enrolled in part 1. To be eligible for part 2, patients were required to have one of the following: CLL, CML, B-ALL, or T-ALL, acute myelogenous leukemia (AML), MM, or NHL. Patients enrolled in part 2 were also restricted to no more than 4 prior cytotoxic chemotherapy regimens; targeted agents such as rituximab and alemtuzumab were not considered cytotoxic chemotherapy for the purposes of this study. Further details on exclusion criteria are included in the supplemental Methods (available at the Blood Web site).
Study design and treatment administration
Part 1 began with enrollment of one sentinel patient given a single dose of afuresertib capsule followed by PK assessment, which was designed to facilitate intrapatient dose adjustment if the predicted exposures were significantly different from those predicted from animal data. Upon completion of this PK analysis, a second patient was permitted to enroll at a dose based on the outcome from the sentinel patient. Subsequent cohorts enrolled a minimum number of patients to ensure that at least 1 patient had completed 1 cycle of therapy prior to dose escalation (accelerated dose titration). Accelerated dose titration was terminated, and a traditional 3 + 3 dose escalation process was implemented when one of the following criteria were met: occurrence of one grade 2 toxicity in two patients or the first occurrence of a dose-limiting toxicity (DLT) during the first cycle.16 Escalation continued until the maximum tolerated dose (MTD) was defined. Part 2 was an expansion cohort of patients with selected hematologic malignancies who were treated at the MTD of afuresertib, as defined in part 1. Further details on study drug administration can be found in the supplemental Methods.
Study assessments
Disease assessments included physical examinations, laboratory tests, bone marrow aspirates and/or biopsies, and imaging as appropriate for disease type and location and according to published guidelines. To ensure the comparability between baseline and subsequent assessments, the same method of assessment and the same technique were used when assessing response.17 Disease assessment in patients with MM was performed according to response criteria reported by Durie et al.18 Assessments for other hematologic malignancies were performed for CLL,17 CML,19 malignant lymphoma,20 AML, and ALL.21
Serial fasting glucose sampling and insulin monitoring in different dose cohorts were planned. To minimize the patient burden from intensive blood sampling, serial serum glucose and plasma insulin monitoring were to be initiated if serum drug concentrations reached predefined levels corresponding to ≥25% of the concentrations observed to cause changes in insulin concentrations in preclinical animal models.
Safety was assessed once per week during the first cycle of therapy and every 21 days in subsequent cycles. Further details on safety assessments can be found in the supplemental Methods.
PK assessment
Blood samples for PK analysis were obtained predose and at 0.5, 1, 2, 3, 4, 6, 8, and 24 hours after a single dose (cycle 0) and at repeat dosing (cycle 1, day 8). Additional samples were obtained at 48 and 72 hours after a single dose and predose (cycle 1 day 15). Plasma was stored at –20°C until analysis for afuresertib concentration by an approved analytical methodology. Noncompartmental analysis using WinNonlin Professional version 5.2 software (Pharsight) was used to determine PK parameters.
Results
Patients
The study was conducted from 2009 to 2012 and enrolled a total of 73 patients with the following diseases: AML (9), ALL (1), CLL (7), Hodgkin disease (HD; 8), NHL (13), Langerhan’s cell histiocytosis (LCH; 1), and MM (34). Twenty-six patients participated in the dose escalation (part 1), and 47 participated in the expansion cohort (part 2). The early signals of clinical activity seen in MM patients during part 1 led to preferential enrollment of those patients in part 2. The results presented below reflect the data at the time of study closure (March 2012), when eight continuing patients (MM [4], LCH [1], CLL [1], and HD [2]) were given continued access to afuresertib under a different protocol. Table 1 shows patients’ diagnosis and dose assignment on the study. The median age was 63 years (range, 18 to 82 years), approximately half the patients were male (50.7%), and the most common diagnosis was MM (n = 34). The racial distribution of patients was 67.1% white, 1.4% black, and 31.5% Asian. The baseline Eastern Cooperative Oncology Group performance status was 1 in 48 patients (65.8%), 0 in 17 (23.3%) patients, 2 in 7 (9.6%) patients, and was not assessed in 1 (1.4%) of the patients. All patients were pretreated with chemotherapy (n = 73); some also had prior radiotherapy (n = 25) or surgery (n = 8) as part of their anticancer treatment. Patients with MM were heavily pretreated, with a median of 5.5 lines of prior therapies (range, 2 to 10 prior therapies). The type of prior therapy received by MM patients included chemotherapy (100%), immunomodulators (97.1%), proteasome inhibitors (88.2%), and stem cell transplant (76%).
Patient’s diagnosis and dose-level assignment
| Tumor diagnosis . | No. of patients at dose level (mg) . | All patients . | |||||
|---|---|---|---|---|---|---|---|
| 25 . | 75 . | 100 . | 125 . | 150 . | No. . | % . | |
| AML | 0 | 0 | 0 | 9 | 0 | 9 | 12.3 |
| ALL | 0 | 0 | 0 | 1 | 0 | 1 | 1.4 |
| CLL | 1 | 2 | 1 | 2 | 1 | 7 | 9.6 |
| HD | 1 | 0 | 2 | 4 | 1 | 8 | 11 |
| NHL | 0 | 0 | 3 | 7 | 3 | 13 | 17.8 |
| LCH | 0 | 0 | 0 | 0 | 1 | 1 | 1.4 |
| MM | 0 | 0 | 2 | 32 | 0 | 34 | 46.6 |
| Tumor diagnosis . | No. of patients at dose level (mg) . | All patients . | |||||
|---|---|---|---|---|---|---|---|
| 25 . | 75 . | 100 . | 125 . | 150 . | No. . | % . | |
| AML | 0 | 0 | 0 | 9 | 0 | 9 | 12.3 |
| ALL | 0 | 0 | 0 | 1 | 0 | 1 | 1.4 |
| CLL | 1 | 2 | 1 | 2 | 1 | 7 | 9.6 |
| HD | 1 | 0 | 2 | 4 | 1 | 8 | 11 |
| NHL | 0 | 0 | 3 | 7 | 3 | 13 | 17.8 |
| LCH | 0 | 0 | 0 | 0 | 1 | 1 | 1.4 |
| MM | 0 | 0 | 2 | 32 | 0 | 34 | 46.6 |
PK
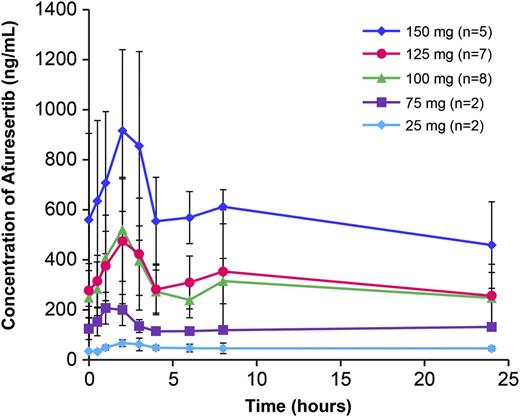
Mean (± standard deviation [SD]) day 8 plasma concentration profiles at each dose are shown in Figure 1. The median time to peak for afuresertib plasma concentrations was 1.5 to 2.5 hours postdose across dose levels after single-dose (cycle 0) and repeat-dose (cycle 1, day 8) afuresertib. Mean accumulation following repeat doses was approximately threefold for all dose levels. This level of accumulation was consistent with a long effective half-life of approximately 1.7 days. Single- and repeat-dose PK parameters at each dose are summarized in Table 2. Plasma concentrations generally increased with increasing dose, although variability was high. Concentrations were similar at 100- and 125-mg doses, whereas concentrations at the 150-mg dose were approximately twofold higher than at the 100-mg dose.
Mean afuresertib plasma concentration-time profiles following repeat dose (day 8). Mean (± standard deviation) day 8 plasma concentration profiles at each dose are shown. Error bars shown represent standard deviation.
Mean afuresertib plasma concentration-time profiles following repeat dose (day 8). Mean (± standard deviation) day 8 plasma concentration profiles at each dose are shown. Error bars shown represent standard deviation.
Pharmacokinetic parameters of afuresertib
| Dose (mg) . | No. of patients . | Cmax (ng/mL) . | AUC0-24 (ng•h/mL) . | Accumulation ratio . | C0 (ng/mL) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Single dose . | Repeat dose . | Single dose . | Repeat dose . | Single dose . | Repeat dose . | Day 8 . | Day 15 . | ||
| 25 | 2 | 26.5, 39.7 | 57.3, 79.8 | 294, 413 | 891, 1393 | — | 3.0, 3.4 | 30.5, 36.7 | 42.3, 48.0 |
| 75 | 2 | 101, 112 | 244, 252 | 1003, 1139 | 3022, 3193 | — | 3.2, 2.7 | 93.8, 155 | 96.9, 131 |
| 100 | 8 | 244 ± 158 | 554 ± 198 | 2366 ± 992 | 6782 ± 2004 | — | 3.3 ± 1.6 | 249 ± 107 | 261 ± 162 |
| 125 | 8 | 175 ± 82.7 | 531 ± 288 | 2378 ± 1004 | 7405 ± 3584 | — | 2.9 ± 0.7 | 259 ± 105 | 342 ± 172 |
| 150 | 6 | 350 ± 162 | 949 ± 348 | 4379 ± 1288 | 13425 ± 3953 | — | 3.0 ± 1.3 | 624 ± 345 | 822 ± 576 |
| Dose (mg) . | No. of patients . | Cmax (ng/mL) . | AUC0-24 (ng•h/mL) . | Accumulation ratio . | C0 (ng/mL) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Single dose . | Repeat dose . | Single dose . | Repeat dose . | Single dose . | Repeat dose . | Day 8 . | Day 15 . | ||
| 25 | 2 | 26.5, 39.7 | 57.3, 79.8 | 294, 413 | 891, 1393 | — | 3.0, 3.4 | 30.5, 36.7 | 42.3, 48.0 |
| 75 | 2 | 101, 112 | 244, 252 | 1003, 1139 | 3022, 3193 | — | 3.2, 2.7 | 93.8, 155 | 96.9, 131 |
| 100 | 8 | 244 ± 158 | 554 ± 198 | 2366 ± 992 | 6782 ± 2004 | — | 3.3 ± 1.6 | 249 ± 107 | 261 ± 162 |
| 125 | 8 | 175 ± 82.7 | 531 ± 288 | 2378 ± 1004 | 7405 ± 3584 | — | 2.9 ± 0.7 | 259 ± 105 | 342 ± 172 |
| 150 | 6 | 350 ± 162 | 949 ± 348 | 4379 ± 1288 | 13425 ± 3953 | — | 3.0 ± 1.3 | 624 ± 345 | 822 ± 576 |
Single- and repeat-dose data are presented as mean ± standard deviation except for 25- and 75-mg doses, which are presented as individual values; the number of patients with repeat doses is the same as that for single dose, except for 125 mg (n = 6) and 150 mg (n = 5).
AUC0-24, area under the plasma concentration-time curve from time 0 to 24 hours; Cmax, maximum plasma concentration; C0, predose concentration on day 8 and day 15.
Safety and DLTs
Dose-escalation cohorts for 25 mg (n = 2), 75 mg (n = 2), and 150 mg (n = 6) were evaluated. Two DLTs were observed at the 150-mg dose, which resulted in exploration of the intervening doses of 100 mg (n = 8) and 125 mg (n = 8). No DLTs were observed at either 100 or 125 mg; therefore, 125 mg was chosen as the dose to be evaluated in part 2. The two DLTs observed in 2 patients in the 150-mg cohort were both grade 3 liver function abnormalities: 1 in a patient with mantle cell lymphoma and 1 in a patient with follicular lymphoma. The first patient was a 67-year-old man with mantle cell lymphoma who developed liver function test (LFT) abnormalities (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and gamma-glutamyltransferase elevation accompanied by elevation of bilirubin and eosinophilia) after 14 days of dosing. Afuresertib was interrupted, and the LFTs normalized within 33 days after the last dose of study drug. This patient was never rechallenged with the study drug and was withdrawn from the trial as a result of this toxicity. The second patient, a 59-year-old male with follicular lymphoma, received a single dose of afuresertib and initiated repeat dosing 4 days later. At this time, the patient presented with elevated LFTs (alanine aminotransferase and aspartate aminotransferase). The patient received afuresertib for 6 more consecutive days, at which time study treatment was interrupted and the patient was withdrawn from the trial. Initially, the LFT abnormalities were considered to be related to study drug; however, further follow-up showed extensive liver involvement by lymphoma infiltrates, and the patient subsequently died of disease progression.
All 73 patients enrolled in the study experienced at least one adverse event (AE), and a majority (83.6%) of them were considered related or possibly related to study treatment. The most frequent (>10% of patients) treatment-related AEs were nausea (23.3%), diarrhea (20.5%), dyspepsia (19.2%), fatigue (16.4%), gastrointestinal reflux disease (15.1%), and anorexia (13.7%). Grade 3 or higher treatment-related AEs (in >1 patient) included grade 3 neutropenia (6.8%), grade 3 rash (4.1%), grade 3 odynophagia (2.7%), grade 3 fatigue (2.7%), grade 3 abnormal LFTs (2.7%), grade 3 thrombocytopenia (1.4%), and grade 3 asthenia (1.4%). No grade 4 or 5 AEs were observed. There was no obvious relationship between the type of AEs and malignancy type. Three deaths were reported during the study (disease progression, n = 2; septic shock, n = 1 [unrelated to study drug]). Table 3 provides a summary of all AEs that occurred in >10% of patients by dose level.
AEs occurring in >10% of patients by dose level (part 1 and part 2)
| AE (preferred term) . | 25 mg (n = 2) . | 75 mg (n = 2) . | 100 mg (n = 8) . | 125 mg (n = 55) . | 150 mg (n = 6) . | Overall (n = 73) . | Related . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 73) . | MM (n = 34) . | |||||||||||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Nausea | 0 | 1 | 50 | 3 | 37.5 | 18 | 32.7 | 4 | 66.7 | 26 | 35.6 | 17 | 23.3 | 7 | 20.6 | |
| Diarrhea | 1 | 50 | 0 | 2 | 25 | 17 | 30.9 | 4 | 66.7 | 24 | 32.9 | 15 | 20.5 | 9 | 26.5 | |
| Dyspepsia | 1 | 50 | 0 | 3 | 37.5 | 14 | 25.5 | 0 | 18 | 24.7 | 14 | 19.2 | 7 | 20.6 | ||
| Fatigue | 1 | 50 | 1 | 50 | 0 | 12 | 21.8 | 1 | 16.7 | 15 | 20.5 | 12 | 16.4 | 7 | 20.6 | |
| Anorexia | 0 | 0 | 1 | 12.5 | 12 | 21.8 | 1 | 16.7 | 14 | 19.2 | 10 | 13.7 | 5 | 14.7 | ||
| Gastrointestinal reflux | 0 | 1 | 50 | 2 | 25 | 10 | 18.2 | 1 | 16.7 | 14 | 19.2 | 11 | 15.1 | 3 | 8.8 | |
| Vomiting | 1 | 50 | 1 | 50 | 4 | 50 | 6 | 10.9 | 2 | 33.3 | 14 | 19.2 | 7 | 9.6 | 2 | 5.9 |
| Anemia | 0 | 1 | 50 | 3 | 37.5 | 7 | 12.7 | 0 | 11 | 15.1 | 2 | 2.7 | 2 | 5.9 | ||
| Asthenia | 0 | 0 | 1 | 12.5 | 8 | 14.5 | 1 | 16.7 | 10 | 13.7 | 2 | 2.7 | 0 | |||
| Upper respiratory tract infection | 0 | 0 | 2 | 25 | 7 | 12.7 | 1 | 16.7 | 10 | 13.7 | 0 | 0 | ||||
| Back pain | 1 | 50 | 0 | 2 | 25 | 5 | 9.1 | 1 | 16.7 | 9 | 12.3 | 0 | 0 | |||
| Insomnia | 2 | 100 | 0 | 0 | 7 | 12.7 | 0 | 9 | 12.3 | 3 | 4.1 | 1 | 2.9 | |||
| Rash | 0 | 0 | 0 | 7 | 12.7 | 2 | 33.3 | 9 | 12.3 | 5 | 6.8 | 2 | 5.9 | |||
| Constipation | 0 | 0 | 1 | 12.5 | 7 | 12.7 | 0 | 8 | 11 | 0 | 0 | |||||
| Cough | 0 | 0 | 3 | 37.5 | 5 | 9.1 | 0 | 8 | 11 | 1 | 1.4 | 0 | ||||
| Odynophagia | 0 | 0 | 0 | 7 | 12.7 | 1 | 16.7 | 8 | 11 | 6 | 8.2 | 0 | ||||
| Headache | 0 | 1 | 50 | 0 | 6 | 10.9 | 0 | 7 | 9.6 | 4 | 5.5 | 2 | 5.9 | |||
| Neutropenia | 1 | 50 | 0 | 2 | 25 | 3 | 5.5 | 1 | 16.7 | 7 | 9.6 | 5 | 6.8 | 2 | 5.9 | |
| Pyrexia | 0 | 0 | 2 | 25 | 5 | 9.1 | 0 | 7 | 9.6 | 0 | 0 | |||||
| AE (preferred term) . | 25 mg (n = 2) . | 75 mg (n = 2) . | 100 mg (n = 8) . | 125 mg (n = 55) . | 150 mg (n = 6) . | Overall (n = 73) . | Related . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 73) . | MM (n = 34) . | |||||||||||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Nausea | 0 | 1 | 50 | 3 | 37.5 | 18 | 32.7 | 4 | 66.7 | 26 | 35.6 | 17 | 23.3 | 7 | 20.6 | |
| Diarrhea | 1 | 50 | 0 | 2 | 25 | 17 | 30.9 | 4 | 66.7 | 24 | 32.9 | 15 | 20.5 | 9 | 26.5 | |
| Dyspepsia | 1 | 50 | 0 | 3 | 37.5 | 14 | 25.5 | 0 | 18 | 24.7 | 14 | 19.2 | 7 | 20.6 | ||
| Fatigue | 1 | 50 | 1 | 50 | 0 | 12 | 21.8 | 1 | 16.7 | 15 | 20.5 | 12 | 16.4 | 7 | 20.6 | |
| Anorexia | 0 | 0 | 1 | 12.5 | 12 | 21.8 | 1 | 16.7 | 14 | 19.2 | 10 | 13.7 | 5 | 14.7 | ||
| Gastrointestinal reflux | 0 | 1 | 50 | 2 | 25 | 10 | 18.2 | 1 | 16.7 | 14 | 19.2 | 11 | 15.1 | 3 | 8.8 | |
| Vomiting | 1 | 50 | 1 | 50 | 4 | 50 | 6 | 10.9 | 2 | 33.3 | 14 | 19.2 | 7 | 9.6 | 2 | 5.9 |
| Anemia | 0 | 1 | 50 | 3 | 37.5 | 7 | 12.7 | 0 | 11 | 15.1 | 2 | 2.7 | 2 | 5.9 | ||
| Asthenia | 0 | 0 | 1 | 12.5 | 8 | 14.5 | 1 | 16.7 | 10 | 13.7 | 2 | 2.7 | 0 | |||
| Upper respiratory tract infection | 0 | 0 | 2 | 25 | 7 | 12.7 | 1 | 16.7 | 10 | 13.7 | 0 | 0 | ||||
| Back pain | 1 | 50 | 0 | 2 | 25 | 5 | 9.1 | 1 | 16.7 | 9 | 12.3 | 0 | 0 | |||
| Insomnia | 2 | 100 | 0 | 0 | 7 | 12.7 | 0 | 9 | 12.3 | 3 | 4.1 | 1 | 2.9 | |||
| Rash | 0 | 0 | 0 | 7 | 12.7 | 2 | 33.3 | 9 | 12.3 | 5 | 6.8 | 2 | 5.9 | |||
| Constipation | 0 | 0 | 1 | 12.5 | 7 | 12.7 | 0 | 8 | 11 | 0 | 0 | |||||
| Cough | 0 | 0 | 3 | 37.5 | 5 | 9.1 | 0 | 8 | 11 | 1 | 1.4 | 0 | ||||
| Odynophagia | 0 | 0 | 0 | 7 | 12.7 | 1 | 16.7 | 8 | 11 | 6 | 8.2 | 0 | ||||
| Headache | 0 | 1 | 50 | 0 | 6 | 10.9 | 0 | 7 | 9.6 | 4 | 5.5 | 2 | 5.9 | |||
| Neutropenia | 1 | 50 | 0 | 2 | 25 | 3 | 5.5 | 1 | 16.7 | 7 | 9.6 | 5 | 6.8 | 2 | 5.9 | |
| Pyrexia | 0 | 0 | 2 | 25 | 5 | 9.1 | 0 | 7 | 9.6 | 0 | 0 | |||||
Response
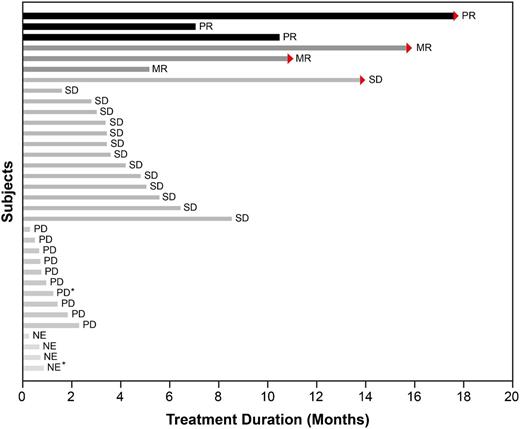
Of the 34 patients with MM, 3 patients at the 125-mg dose showed a confirmed partial response (PR) with 50% to 70% reduction in M-protein levels. Median time on study for those patients was 319 days (range, 215 to 541 days). Three additional patients at the 125-mg dose, who were classified as having stable disease according to the Durie et al response criteria,18 had minimal responses (MRs). The classification of patients as having an MR according to the International Myeloma Working Group criteria was based on a post hoc analysis of changes in M-protein levels. The overall response rate for MM patients (PR or better) was 8.8%, and the clinical benefit rate (PR + MR) was 17.6% (6 of 34). An additional 14 patients with MM had prolonged stabilization of their disease for a median 118.5 days (range, 48 to 426 days). There were no responses noted in MM patients at doses of <125 mg per day. The maximum changes in M-protein values for patients with measurable serum M-protein and the duration of study for MM patients are depicted in Figure 2. Supplemental Figure 1 shows dramatic but incomplete [18F]-fluorodeoxyglucose-positron emission tomography response in an MM patient.
Duration of study treatment of 34 patients with MM. Arrows indicate patients who were still ongoing at the time of study closure. Those patients are continuing treatment with afuresertib on a continuation protocol. Two patients were enrolled in dose escalation (part 1) and were administered 100 mg per day (indicated by *); the remaining patients were administered 125 mg per day. NE, not evaluable; PD, progressive disease; SD, stable disease.
Duration of study treatment of 34 patients with MM. Arrows indicate patients who were still ongoing at the time of study closure. Those patients are continuing treatment with afuresertib on a continuation protocol. Two patients were enrolled in dose escalation (part 1) and were administered 100 mg per day (indicated by *); the remaining patients were administered 125 mg per day. NE, not evaluable; PD, progressive disease; SD, stable disease.
Clinical activity has also been observed in patients with lymphomas, as evidenced by tumor volume reduction on computed tomography scans and/or improvement on positron emission tomography imaging. Of the 13 patients with NHL, 1 patient with diffuse large B-cell lymphoma at the 100-mg dose had a confirmed complete response on [18F]-fluorodeoxyglucose-positron emission tomography imaging, and 2 patients (1 with marginal zone B-cell lymphoma and another with diffuse large B-cell lymphoma) attained a PR (unconfirmed at 125 mg and confirmed at 100 mg, respectively); these responders continued receiving afuresertib for 747, 662, and 106 days, respectively. Four of the 8 patients with HD achieved an unconfirmed PR (at doses of 100 to 150 mg); these responders continued receiving afuresertib for 231, 483, 651, and 105 days. The duration of study treatment of various malignancies is presented in Table 4. Although reduction in tumor burden was observed in patients with CLL, no patient with CLL met the 2008 International Workshop on CLL criteria for a PR or complete response. One patient with LCH at 150 mg achieved a confirmed PR; this responder continued receiving afuresertib for 830 days while on this trial and was subsequently offered continuation of treatment on another trial. The single patient with ALL came off study before response could be assessed. The 9 patients with AML either experienced treatment failure (n = 5) or did not have response assessed (n = 4).
No. of days on study by type of malignancy
| Type of malignancy . | No. of patients . | Days on study . | ||
|---|---|---|---|---|
| Median . | Minimum . | Maximum . | ||
| ALL | 1 | 27 | 27 | 27 |
| AML | 9 | 22 | 19 | 119 |
| CLL | 7 | 66 | 24 | 801 |
| HD | 8 | 265.5 | 41 | 751 |
| LCH | 1 | 833 | 833 | 833 |
| NHL | 13 | 42 | 8 | 746 |
| MM | 34 | 97.5 | 8 | 541 |
| Type of malignancy . | No. of patients . | Days on study . | ||
|---|---|---|---|---|
| Median . | Minimum . | Maximum . | ||
| ALL | 1 | 27 | 27 | 27 |
| AML | 9 | 22 | 19 | 119 |
| CLL | 7 | 66 | 24 | 801 |
| HD | 8 | 265.5 | 41 | 751 |
| LCH | 1 | 833 | 833 | 833 |
| NHL | 13 | 42 | 8 | 746 |
| MM | 34 | 97.5 | 8 | 541 |
Discussion
From this first-time-in-human trial, we conclude that afuresertib is safe and well tolerated and has a favorable PK profile. On the basis of 2 DLTs in the 150-mg cohort, the recommended monotherapy phase 2 dose from this study was established at an MTD of 125 mg per day. The safety profile of afuresertib is consistent with other PI3K/AKT/mTOR pathway inhibitors in the clinic, with a majority of side effects related to the GI tract (diarrhea, nausea, dyspepsia) or fatigue.22 Moreover, the safety profile for the MM population (34 patients) does not differ significantly from the overall population with various hematologic malignancies (Table 3).
PK parameters were generally dose proportional, with a prolonged effective half-life of approximately 1.7 days, and did not show time dependency. The variability of the PK for afuresertib in this study was low to moderate, with coefficient of variation for doses >75 mg ranging from 23% to 65% and 24% to 48% for maximum plasma concentration and area under the plasma concentration-time curve from time 0 to 24 hours following single and repeat doses, respectively. There were only 2 patients at the 25-mg and 75-mg doses, and coefficient of variation percent could not be adequately determined. The favorable PK profile enables further clinical development of afuresertib.
Typically, with potent AKT inhibition (especially inhibition of the AKT2 isoform), one would expect perturbations in glucose metabolism resulting in hyperglycemia accompanied by increased insulin secretion.23 This mechanism-based toxicity has been postulated as a valid surrogate pharmacodynamic marker for this class of drugs, with the advantage of being minimally invasive and easily accessible.24 The differences in the magnitude of hyperglycemia seen with different drugs can be associated with the differences in PK profile, duration, and magnitude of AKT pathway inhibition, and the relative kinase selectivity of these compounds. The improved kinase selectivity and potency of afuresertib for AKT versus other protein kinase C isoforms may explain the relatively low incidence and magnitude of hyperglycemia with afuresertib; specifically, only 2 cases of hyperglycemia (one each at grade 1 and grade 2) were reported in our study. This is in contrast to previous reports with other pan-AKT inhibitors in which hyperglycemia was seen in approximately 15% to 20% of patients.25,26 Of note, serial serum glucose sampling and plasma insulin monitoring were planned in this study only in those patients achieving serum drug concentrations corresponding to those leading to changes in insulin concentrations in preclinical animal models. Since only a few patients in our study fulfilled these criteria, correlation between plasma afuresertib concentrations and glucose or insulin levels could not be demonstrated.
The MTD was defined on the basis of dose-limiting hepatotoxicity in 2 of the 6 patients at the 150-mg dose level. Study treatment was withdrawn from both patients, and the liver enzyme abnormalities resolved within 33 days in the first of the 2 patients. The protocol did not allow rechallenge; therefore, the patient was removed from study. Subsequent review of the second patient revealed extensive liver involvement by lymphoma at the time of study enrollment, which confounded interpretation of the DLT. As such, although we cannot exclude the possibility that afuresertib contributed to this DLT, the exact causation remains unclear. Given the complexity of attributing drug relatedness to the second DLT in conjunction with the observation of minimal hyperglycemia, we feel that further exploration of dose in conjunction with tissue-based pharmacodynamic biomarkers be recommended for subsequent studies.
Signs of clinical activity with afuresertib were demonstrated in a number of hematologic malignancies in this trial. This is not unexpected if one considers the central role of AKT signaling in the pathogenesis of those diseases. The most encouraging clinical activity was seen early in MM patients, and this population was preferentially enrolled into the expansion cohort. Perifosine, a putative AKT inhibitor, recently failed to demonstrate superiority when combined with bortezomib in a phase 3 trial. This drug has many potential mechanisms of action, making it difficult to assess the importance of pure AKT inhibition in MM. We believe our study is important because afuresertib remains the only highly specific AKT inhibitor tested in MM in the clinical setting. Among 34 MM patients enrolled in the trial, 3 patients achieved PR (9%), 3 achieved MR (9%), and a number of patients had disease stabilization over prolonged periods of time. The median duration on study for all 32 MM patients at the 125-mg dose was 103.5 days (range, 8 to 541 days). It is worth noting that the responses in MM patients occurred early in the treatment course (usually within 1 to 2 cycles) and were durable among heavily pretreated patients who, on average, had 5 prior lines of treatment. For 6 patients who achieved PR or MR, the median time on study treatment (ie, without disease progression) was 319 days (range, 210 to 569 days) and 357 days (range, 158 to 485 days), respectively. Four MM patients were receiving afuresertib at the time of study closure and were transitioned to a follow-up study.
To the best of our knowledge, afuresertib is the first small-molecule AKT inhibitor to demonstrate single-agent clinical activity against MM. There is also emerging data that afuresertib in combination with bortezomib and dexamethasone has synergistic antimyeloma effects.27 The evidence comes from a phase 1/2 study recently reported at the meeting of the American Society of Hematology demonstrating an overall response rate of 61% and clinical benefit rate of 78% in a population of 67 relapsed MM patients.27 This highlights the clinical potential of afuresertib as a partner for combination therapies for MM patients. In conclusion, this phase 1 study demonstrated favorable clinical activity in the context of an attractive PK profile and tolerable safety profile, which suggests that AKT inhibition with afuresertib may provide a novel therapeutic approach for MM.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Funding for this study was provided by GlaxoSmithKline. All listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors and retained full control of the manuscript content. Writing and editorial support, in the form of assembling tables and figures, collating author comments, copyediting, fact checking, and referencing, was done by Cactus Communications and was funded by GlaxoSmithKline.
Authorship
Contribution: A.S., S.R.M., R.A.B., C.C., and J.B.O. were involved in the conception and design of the manuscript; A.S., S.J.H., S.R.M., D.A.S., J.G., R.K., J.B.O., and C.C. analyzed and interpreted the data; S.-S.Y., S.J.H., S.R.M., R.A.B., R.K., J.B.O., and C.C. collected and assembled the data; S.-S.Y. provided the study material; all authors were involved in manuscript writing and final approval of the manuscript.
Conflict-of-interest disclosure: S.R.M., D.A.S., R.A.B., J.G., R.K., and J.B.O. are employees of GlaxoSmithKline (GSK) and hold GSK stock. R.K. holds a US patent, application number 20120258933. C.C. has received honoraria from Celgene, Lundbeck, and Roche in addition to research funding from Celgene and GSK. The remaining authors declare no competing financial interests.
Correspondence: Christine Chen, Suite 5-220, 610 University Ave, Toronto, ON, Canada M5G2M9; e-mail: christine.chen@uhn.ca.