Key Points
MGZL with pathologic features in between NSHL and PMBL is very rare and most frequently occurs in young patients.
A prospective study of DA-EPOCH-R without mediastinal radiation in MGZL demonstrated an inferior outcome compared to patients with PMBL.
Abstract
Mediastinal B-cell lymphomas present in the mediastinum and are most frequent in young patients. Nodular sclerosis Hodgkin lymphoma (NSHL) and primary mediastinal B-cell lymphoma (PMBL) are the common types, whereas mediastinal gray-zone lymphoma (MGZL) is extremely rare and has pathological features intermediate between NSHL and PMBL. The indeterminate pathobiology of MGZL has led to uncertainty regarding therapeutic strategy, and its clinical characteristics and treatment have not been characterized. We conducted a prospective study of infusional dose-adjusted etoposide, doxorubicin, and cyclophosphamide with vincristine, prednisone, and rituximab (DA-EPOCH-R) and filgrastim in untreated MGZL. We analyzed biomarkers of outcome and compared their clinical and biological characteristics to PMBL. Twenty-four MGZL patients had a median age of 33 years (range, 14 to 59 years), and 46% had mediastinal masses ≥10 cm. At 59 months median follow-up, the event-free survival and overall survival were 62% and 74%, respectively. The serum absolute lymphocyte count, the presence of tumor-infiltrating dendritic cells, CD15 expression on the malignant cells, and tumor morphology were biomarkers of outcome in MGZL. Compared with PMBL, MGZL patients were more likely to be male, express CD15, have lower expression of CD20, and have a worse outcome. DA-EPOCH-R alone is effective in MGZL. The trial was registered at ClinicalTrials.gov (NCT00001337).
Introduction
Mediastinal B-cell lymphomas present in the mediastinum of young patients and are mostly represented by nodular sclerosis Hodgkin lymphoma (NSHL) and primary mediastinal B-cell lymphoma (PMBL).1-3 The most recent World Health Organization classification of lymphoid tissues recognized mediastinal gray-zone lymphoma (MGZL), a rare lymphoma with features intermediate between PMBL and NSHL, as a new pathological entity.3-5 Historically, these patients were often included in series of Hodgkin-like anaplastic large cell lymphoma, which was a heterogeneous group.6-8
The clinical characteristics and treatment of MGZL have yet to be defined because of its recent identification and rarity. NSHL and PMBL have both overlapping and distinct clinical features, raising the question of where MGZL lies within the pathological and clinical spectrum of mediastinal B-cell lymphomas. Therapeutically, the Hodgkin-like pathological features of MGZL suggest they should be treated like Hodgkin lymphoma (HL), whereas the strong expression of the CD20 B-cell protein by most MGZLs, a feature of PMBL but not NSHL, suggests they should be treated with rituximab-based regimens as with PMBL.
As a group, mediastinal B-cell lymphomas are hypothesized to derive from a thymic B-cell.2,4,7,9 A significant proportion of PMBL and NSHL patients have amplification of the JAK2/PDL2 locus, which has also been reported in MGZL.9 PMBL and NSHL also have overlapping gene expression profiles, indicating that they lie along a pathobiological continuum.2 MGZL has been described as the missing link between NSHL and PMBL.4 We undertook a study of untreated MGZL to describe its clinical outcome with the immunochemotherapy regimen of infusional dose-adjusted etoposide, doxorubicin, and cyclophosphamide with vincristine, prednisone, and rituximab (DA-EPOCH-R), an effective treatment of PMBL, and to describe its biological characteristics.10
Patients and methods
Study design and treatment
Twenty-four patients with untreated MGZL were prospectively enrolled between November 1999 and February 2013 on a study of DA-EPOCH-R at the National Cancer Institute. Because of the recent recognition of MGZL as a distinct entity by our center in 2004, we amended our prospective study of DA-EPOCH-R in PMBL to add a separate cohort for MGZL. All but three patients were enrolled after this date. Objectives included event-free survival (EFS), overall survival (OS), and immunohistochemical analysis. Pathology was confirmed by S.P. or E.S.J. according to World Health Organization criteria.3 Eligibility included all disease stages and performance statuses, negative tests for HIV and pregnancy, and adequate organ function unless it was the result of involvement by lymphoma. Evaluation included standard blood tests, whole-body computed tomography (CT) scans, and bone marrow biopsy. DA-EPOCH-R was administered as previously described.11,12 Disease sites were evaluated by CT scan after cycles 4 and 6 by using standard response criteria.13,14 The institutional review board approved the study, and all patients provided consent in accordance with the Declaration of Helsinki.
Patients began on dose level 1 of DA-EPOCH-R (rituximab 375 mg/m2 day 1, doxorubicin 10 mg/m2 per day, etoposide 50 mg/m2 per day, and vincristine 0.4 mg/m2 per day [no cap]; continuous infusion days 1, 2, 3, and 4 (96-hour total); cyclophosphamide 750 mg/m2 30-minute infusion day 5; and prednisone 120 mg/m2 divided and administered in two separate doses on days 1, 2, 3, 4, and 5, as previously described.10 Patients received filgrastim 300 µg on day 6 and continued until the absolute neutrophil count (ANC) reached ≥5000 cells per µL past the nadir. Dose adjustments were based on the neutrophil nadir, which was monitored with complete blood counts performed 2 times per week, and were made in 20% increments. Dose adjustments above the starting dose level applied to etoposide, doxorubicin, and cyclophosphamide, and adjustments below the starting dose level applied only to cyclophosphamide. Doses were increased above the previous cycle if the nadir ANC was ≥500 per µL, and only reduced below the previous cycle if the ANC was <500 per µL on ≥3 measurements or the nadir platelet count was <25 000 per µL. Patients received 2 cycles beyond complete response or stable changes for 6 to 8 cycles. Patients with >1 extranodal site and elevated lactate dehydrogenase (LDH) received intrathecal methotrexate 12 mg on day 1 and 5 of cycles 3 to 6. Sulfamethoxazole and trimethoprim was administered 3 days per week.
FDG-PET-CT scan evaluation
To assess whether there was disease at the end of treatment, patients with a residual mediastinal mass underwent [18F]-fluorodeoxyglucose positron emission tomography-CT ([18F]FDG-PET-CT) scans. One patient who was treated early in the series received a gallium scan. Patients with standard uptake values above the mediastinal blood pool received repeat scans at approximately 6-week intervals until they normalized or stabilized. Patients with significantly worsening FDG-PET abnormalities underwent a biopsy in most cases to assess the presence of disease. FDG-PET scans were not repeated in patients with negative posttreatment scans. The FDG-PET scans were scored according to the 5-point Deauville scoring system with scores of 1 to 3 considered negative and scores of 4 to 5 considered positive for disease.15,16 Patients diagnosed with disease after treatment with DA-EPOCH-R received radiotherapy and were classified as events.
Immunohistochemical analysis
Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded tissue sections and included CD20, CD3, CD79a, PAX-5, OCT-2, Bob-1, BCL-6, CD68, and dendritic cell–specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN). For DC-SIGN, tissue sections underwent deparaffinization and rehydration followed by antigen retrieval using low-pH Dako Target Retrieval Solution (Dako, Carpinteria, CA) in a microwave oven. The slides were incubated with primary antibody (1:100 dilution) for 2 hours. I-View DAB Detection Kit (Ventana Medical Systems, Tucson, AZ) was used as a detection system. The other IHC stains were performed as previously described.9 Positive controls were run with each set of slides and showed appropriate staining patterns.
CD20 and CD15 immunoreactivity in the malignant cells was recorded as 0 (no positive cells observed), 1+ (1% to 25%), 2+ (26% to 50%), 3+ (51% to 75%), and 4+ (76% to 100%)(Figure 1A). DC-SIGN and CD68 stains on infiltrating bystander cells were scored as 0 (no positive cells observed), 1+ (1% to 25%), 2+ (26% to 50%) and 3+ (>50%) (Figure 1B). We also determined that at least 5% of infiltrating bystander cells expressed CD68 in all MGZL, a cutoff shown to be prognostic in HL.17 CD30 and BCL-6 immunoreactivity was not quantitated and scored as positive for CD30 if there was any staining on the malignant cells or was scored as positive for BCL-6 if there was staining on >10% of the malignant cells. All slides were independently reviewed, and the scores were agreed upon by joint rereview (S.P. and A.N.).
MGZL IHC photomicrographs. (A) CD15+ biopsies showing low (1+) and high (4+) staining of malignant cells. (B) DC-SIGN–positive biopsies showing low (1+) and high (3+) staining of infiltrating dendritic/macrophage cells.
MGZL IHC photomicrographs. (A) CD15+ biopsies showing low (1+) and high (4+) staining of malignant cells. (B) DC-SIGN–positive biopsies showing low (1+) and high (3+) staining of infiltrating dendritic/macrophage cells.
Comparison with PMBL
We compared the MGZL patients to a cohort of untreated patients with PMBL who were prospectively treated with DA-EPOCH-R. Patient eligibility was identical for both groups except that patients with PMBL were required to have a mediastinal mass of at least 5 cm. Treatment and follow-up were identical in both groups. The clinical outcome of patients with PMBL was previously published whereas the immunohistochemical end points for PMBL represent new information.10
Statistical analysis
OS was calculated from on-study date until death or last follow-up, and EFS was calculated from on-study date until progression, radiotherapy, death, or last follow-up. The probabilities of OS and EFS were determined by the Kaplan-Meier method.18 The significance of the difference between pairs of Kaplan-Meier curves was determined by an asymptotic or exact log-rank test as appropriate. In general, P values were unadjusted for multiple comparisons because of their being prespecified or exploratory. However, when patients were analyzed initially by grouped marker values and the results suggested that a preferred, dichotomous division in the groupings would indicate a better prognostic association with the outcome, the resulting P values were adjusted by multiplying by the implicit number of such comparisons performed to identify the final grouping. The individual prognostic factors and the scores from the International Prognostic Index (IPI) and International Prognostic Score (IPS) were analyzed for prognostic significance. The prognostic significance of maximum mediastinal tumor size was dichotomized into <10-cm and ≥10-cm groups. All P values are 2-tailed. The median potential follow-up was calculated between on-study date and date of analysis.
Results
Patient characteristics and tumor pathology
Twenty-four patients were enrolled (Table 1). The median patient age was 33 years (range, 14 to 59 years), and 63% were male. Forty-six percent of patients had a mediastinal mass larger than 10 cm, and 50% had elevated LDH. A minority of patients had extranodal involvement or pleural or pericardial effusions. Most patients had low peripheral blood absolute lymphocyte counts (ALCs) with a median of 0.88 cells per µL (range, 0.3 to 2.88 cells per µL; normal, >1.18 cells per µL).
Clinical and pathological characteristics
| Characteristics . | MGZL . | PMBL10 . | 2-tailed P . | ||
|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||
| Total patients | 24 | 51 | |||
| Male gender | 15 | 63 | 21 | 41 | .14 |
| Age, years | .61 | ||||
| Median | 33 | 30 | |||
| Range | 14-59 | 19-52 | |||
| Bulky tumor ≥10 cm | 11 | 46 | 33 | 65 | .14 |
| Range | 1.3-20 | 5-18 | |||
| Stage IV disease | 3 | 13 | 15 | 29 | .15 |
| LDH >normal | 12 | 50 | 40 | 78 | .017 |
| Extranodal site | 6 | 25 | 27 | 53 | .027 |
| Pleural or pericardial effusion | 5 | 21 | 28 | 55 | .006 |
| ALC cells per µl | 1.01 (0.24-2.15) | .78 | |||
| Median | 0.88 | 1.01 | |||
| Range | 0.3-2.88 | 0.24-2.15 | |||
| Predominant tumor morphology | |||||
| PMBL-like | 8 | 33 | NA | ||
| NSHL-like | 15 | 63 | NA | ||
| Composite (BMBL and NSHL) | 1 | 4 | NA | ||
| IHC | |||||
| CD20+ (malignant cells) | |||||
| First to fourth quartiles | 24/24 | 100 | 51/51 | 100 | 1.0 |
| ≥Fourth quartile | 17/24 | 71 | 51/51 | 100 | <.0001 |
| CD30+ (malignant cells) | 24/24 | 100 | 30/44 | 68 | .001 |
| CD15+ (malignant cells) | |||||
| ≥First quartile | 13/24 | 54 | 2/34 | 6 | <.0001 |
| BCL-6-positive (malignant cells) | 13/15 | 86 | 33/37 | 89 | 1.0 |
| DC-SIGN-positive (bystander cells) | |||||
| ≥1+ staining | 10/19 | 53 | 12/35 | 34 | .25 |
| CD68+ (bystander cells) | |||||
| ≥ 5% staining | 16/16 | 100 | 26/28 | 93 | 1.0 |
| Characteristics . | MGZL . | PMBL10 . | 2-tailed P . | ||
|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||
| Total patients | 24 | 51 | |||
| Male gender | 15 | 63 | 21 | 41 | .14 |
| Age, years | .61 | ||||
| Median | 33 | 30 | |||
| Range | 14-59 | 19-52 | |||
| Bulky tumor ≥10 cm | 11 | 46 | 33 | 65 | .14 |
| Range | 1.3-20 | 5-18 | |||
| Stage IV disease | 3 | 13 | 15 | 29 | .15 |
| LDH >normal | 12 | 50 | 40 | 78 | .017 |
| Extranodal site | 6 | 25 | 27 | 53 | .027 |
| Pleural or pericardial effusion | 5 | 21 | 28 | 55 | .006 |
| ALC cells per µl | 1.01 (0.24-2.15) | .78 | |||
| Median | 0.88 | 1.01 | |||
| Range | 0.3-2.88 | 0.24-2.15 | |||
| Predominant tumor morphology | |||||
| PMBL-like | 8 | 33 | NA | ||
| NSHL-like | 15 | 63 | NA | ||
| Composite (BMBL and NSHL) | 1 | 4 | NA | ||
| IHC | |||||
| CD20+ (malignant cells) | |||||
| First to fourth quartiles | 24/24 | 100 | 51/51 | 100 | 1.0 |
| ≥Fourth quartile | 17/24 | 71 | 51/51 | 100 | <.0001 |
| CD30+ (malignant cells) | 24/24 | 100 | 30/44 | 68 | .001 |
| CD15+ (malignant cells) | |||||
| ≥First quartile | 13/24 | 54 | 2/34 | 6 | <.0001 |
| BCL-6-positive (malignant cells) | 13/15 | 86 | 33/37 | 89 | 1.0 |
| DC-SIGN-positive (bystander cells) | |||||
| ≥1+ staining | 10/19 | 53 | 12/35 | 34 | .25 |
| CD68+ (bystander cells) | |||||
| ≥ 5% staining | 16/16 | 100 | 26/28 | 93 | 1.0 |
All patients had histologic and/or phenotypic features intermediate between PMBL and NSHL. Usually, the tumors had a predominant morphology, which was either PMBL-like in 33% (8 of 24), or more frequently Hodgkin-like in 63% (15 of 24) of the patients, and one patient’s disease was classified as composite NSHL and PMBL (Table 1). Although the neoplastic cells from all patients showed some degree of CD20 expression, 71% had strong diffuse staining in all the malignant cells (Table 1). As shown in Table 1, the neoplastic cells in 86% of tested patients also expressed the germinal center transcription factor BCL-6. CD30 and CD15, which are typically expressed by the neoplastic cells in NSHL, were expressed by 100% and 54% of the MGZL patients, respectively (Figure 1B). For most patient samples, the malignant cells showed robust CD30 staining, but CD15 intensity was variable.
Patient outcome
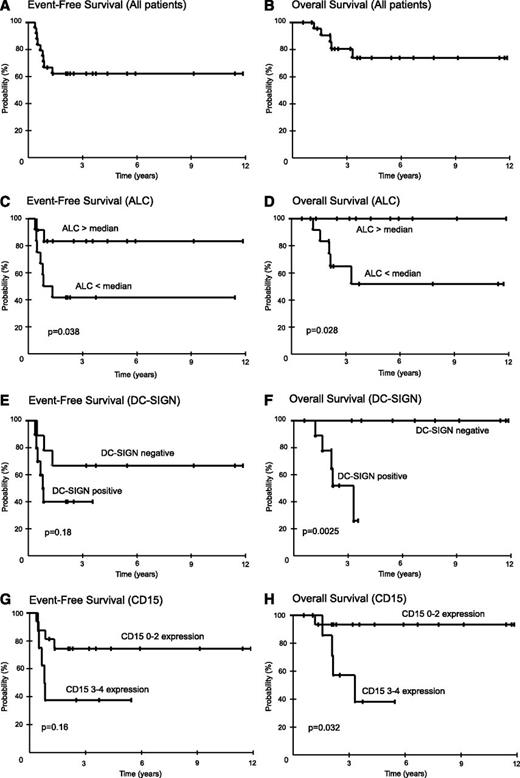
All patients responded: 19 complete remissions and 5 partial remissions. Nine patients were diagnosed with active disease at a median of 3 months (range, 1 to 6 months) after completing therapy. Presence of disease was diagnosed by biopsy in 7 patients and by progressive disease on scans in 2 patients. On the basis of PET-CT evaluation showing disease limited to the mediastinal area, all 9 patients received involved-field salvage radiotherapy and 4 were in continuous remission at 3, 73, 91, and 128 months. At the median follow-up of 59 months (range, 7 to 142 months), the EFS and OS were 62% and 74%, respectively (Figure 2A-B).
Kaplan-Meier plots of EFS and OS in MGZL. Twenty-four patients were prospectively treated with DA-EPOCH-R. Patients were observed for a median of 59 months (range, 7 to 142 months) and results are presented at 3 years. (A) Overall, EFS was 62% (95% CI, 42% to 79%), and (B) OS was 74% (95% CI, 51% to 89%). (C) EFS for patients with ALCs >880 cells per μL (the median) was 83% (95% CI, 55% to 95%), and for <880 cells per μL, it was 42% (95% CI, 19% to 68%), respectively (P = .038). (D) OS for patients with ALCs ≥880 cells per μL was 100% (95% CI, 74% to 100%), and for <880 cells per μL, it was 52% (95% CI, 24% to 78%), respectively (P = .028). (E) EFS for patients without or with tumor-infiltrating DC-SIGN–positive cells was 67% (95% CI, 35% to 88%) and 40% (95% CI, 17% to 69%), respectively (P = .18). (F) OS for patients without or with tumor-infiltrating DC-SIGN–positive cells was 100% (95% CI, 66% to 100%) and 52% (95% CI, 5% to 68%), respectively (P = .0025). (G) EFS for patients with CD15 scores of 0 to 2 vs 3 to 4 was 74% (95% CI, 50% to 90%) and 38% (95% CI, 14% to 69%), respectively (P = .16). (H) OS for patients with CD15 scores of 0 to 2 vs 3 to 4 was 93% (95% CI, 70% to 99%) and 38% (95% CI, 12% to 74%), respectively (P = .032).
Kaplan-Meier plots of EFS and OS in MGZL. Twenty-four patients were prospectively treated with DA-EPOCH-R. Patients were observed for a median of 59 months (range, 7 to 142 months) and results are presented at 3 years. (A) Overall, EFS was 62% (95% CI, 42% to 79%), and (B) OS was 74% (95% CI, 51% to 89%). (C) EFS for patients with ALCs >880 cells per μL (the median) was 83% (95% CI, 55% to 95%), and for <880 cells per μL, it was 42% (95% CI, 19% to 68%), respectively (P = .038). (D) OS for patients with ALCs ≥880 cells per μL was 100% (95% CI, 74% to 100%), and for <880 cells per μL, it was 52% (95% CI, 24% to 78%), respectively (P = .028). (E) EFS for patients without or with tumor-infiltrating DC-SIGN–positive cells was 67% (95% CI, 35% to 88%) and 40% (95% CI, 17% to 69%), respectively (P = .18). (F) OS for patients without or with tumor-infiltrating DC-SIGN–positive cells was 100% (95% CI, 66% to 100%) and 52% (95% CI, 5% to 68%), respectively (P = .0025). (G) EFS for patients with CD15 scores of 0 to 2 vs 3 to 4 was 74% (95% CI, 50% to 90%) and 38% (95% CI, 14% to 69%), respectively (P = .16). (H) OS for patients with CD15 scores of 0 to 2 vs 3 to 4 was 93% (95% CI, 70% to 99%) and 38% (95% CI, 12% to 74%), respectively (P = .032).
Twenty-one patients with residual mediastinal masses underwent posttreatment PET-CT scans (Table 2). Maximum standard uptake values below or above the mediastinal blood pool were present in the residual mediastinal masses of 11 and 10 patients, corresponding to Deauville scores of 1 to 2 vs 3 to 5, respectively. When considering a Deauville score of 1 to 3 as negative and 4 to 5 as positive, FDG-PET had a sensitivity and specificity of 63% and 100%, respectively, and a positive and negative predictive value of 100% and 81%.
End of treatment FDG-PET-CT
| Variable . | SUVmax ≤mediastinal blood pool (n = 11) . | SUVmax >mediastinal blood pool (n = 10) . | ||
|---|---|---|---|---|
| No uptake . | SUVmax ≤mediastinal blood pool . | SUVmax <liver . | SUVmax ≥liver . | |
| Deauville score | 1 | 2 | 3 | 4-5 |
| Disease absent | 7 | 2 | 4 | 0 |
| Disease documented | 1 | 1 | 1 | 5 |
| Variable . | SUVmax ≤mediastinal blood pool (n = 11) . | SUVmax >mediastinal blood pool (n = 10) . | ||
|---|---|---|---|---|
| No uptake . | SUVmax ≤mediastinal blood pool . | SUVmax <liver . | SUVmax ≥liver . | |
| Deauville score | 1 | 2 | 3 | 4-5 |
| Disease absent | 7 | 2 | 4 | 0 |
| Disease documented | 1 | 1 | 1 | 5 |
| Outcome measures . | |||
|---|---|---|---|
| Sensitivity . | Specificity . | Positive predictive value . | Negative predictive value . |
| 63% | 100% | 100% | 81% |
| Outcome measures . | |||
|---|---|---|---|
| Sensitivity . | Specificity . | Positive predictive value . | Negative predictive value . |
| 63% | 100% | 100% | 81% |
Deauville score 1 to 3 interpreted as negative and 4 to 5 as positive.
SUVmax, maximal standardized uptake value.
Toxicity was assessed on all 145 cycles of DA-EPOCH-R. The ANC pharmacodynamic target of <500 cells per mm3 was achieved on 53% of cycles, with infrequent ANC <100 cells per mm3 (9%) and thrombocytopenia <25 000 cells per mm3 (2%). Fever and neutropenia occurred on 12% of cycles. Grade 3 or higher nonhematopoietic toxicities including ileus and neurosensory toxicity occurred in <4% of patients and were similar to those in prior reports.10 There were no treatment-related deaths.
Clinical and molecular prognostic markers
The overlapping features of MGZL with PMBL and NSHL suggest that they may share prognostic features. Because of the small sample size and the absence of a validation cohort, our analyses are exploratory and hypothesis generating. We first analyzed the clinical IPI developed for diffuse large B-cell lymphoma and the IPS developed for HL, as well as tumor mass size, and we found no associations with EFS or OS in MGZL.19,20 On the basis of our finding of reduced peripheral blood lymphocytes in MGZL, which is prognostic in HL, we analyzed the effect on EFS and OS. We assessed the outcome of patients with ALC above and below the median of 880 cells per µL and observed a plateau in EFS of 83% and 42% (P = .038), respectively, and OS of 100% and 52% (P = .028) (Figure 2C-D).
The frequent expression of CD15 and CD30 by MGZL, commonly expressed on Reed-Sternberg cells, raised the hypothesis that immunohistochemical biomarkers for HL may be prognostic in MGZL.3 On the basis of recent data that CD68+ tumor-associated macrophages are biomarkers of poor survival in HL, we analyzed CD68 expression in MGZL.17 Bu using the 5% immunohistochemical expression cutoff that was prognostic in HL, all MGZL patient samples contained CD68+ tumor-associated macrophages (Table 1). To further assess whether CD68+ tumor-associated macrophages are predictive in MGZL, on the basis of the limited data available, we divided the patients into those with 1+, 2+, and 3+ scores and explored the optimum cutoff point. This analysis showed that with a division of 1+ to 2+ (n = 11) vs 3+ (n = 5), the EFS was 73% and 20% (P = .063), respectively, and the OS was 82% and 50% (P = .29) at 3 years, suggesting a trend that as with HL, CD68+ tumor-associated macrophages are adverse in MGZL. Because of the limited number of patients, these results need to be assessed in a larger series.
On the basis of a gene expression analysis of MGZL, PMBL, and NSHL, we identified a dendritic cell gene expression signature that distinguished MGZL and NSHL from PMBL.21 This signature includes CD209, which encodes DC-SIGN, a marker of dendritic cells and activated macrophages.22 Interestingly, DC-SIGN gene expression was significantly associated with poor survival in NSHL, suggesting that it may be a biomarker of macrophage activity in MGZL.21 To investigate this hypothesis, we performed IHC and observed DC-SIGN–positive tumor-associated dendritic/activated macrophage cells ≥1+ in 53% of our MGZL patients (Table 1; Figure 1). Patients with and without DC-SIGN–positive tumor-infiltrating dendritic/activated macrophage cells showed an EFS of 67% and 40% (P = .18), respectively, and an OS of 100% and 52% (P = .0025) at 3 years, indicating that these infiltrating cells are associated with a worse outcome in MGZL (Figure 2E-F).
The association of a Hodgkin-like microenvironment and poor outcome in MGZL raised the hypothesis that a predominant HL-like morphology and/or phenotype would also be associated with a worse outcome. To address this question, we first looked at the association between the predominant morphology and EFS and OS. Contrary to our hypothesis, there was a significant trend between the presence of a predominant PMBL-like morphology and lower EFS (P = .025) but a weaker trend with respect to OS (P = .21). Additionally, the intensity of CD20 staining on the malignant MGZL cells was not associated with outcome. In contrast, CD15 staining was associated with outcome. To provide a quantitative estimate of CD15 staining, patients were scored in quartiles. We analyzed two cutoff points for CD15 staining and outcome and identified a preferred cutoff between 0 to 2 and 3 to 4, which showed an EFS of 74% and 38% (P = .078 unadjusted; P = .16 adjusted), respectively, and an OS of 93% and 38% (P = .016 unadjusted; P = .032 adjusted) at 3 years, indicating that robust expression of CD15 identifies a poor prognosis group in MGZL (Figure 2G-H). Notably, there was no difference in the intensity or frequency of CD15 staining between PMBL-like and Hodgkin-like MGZL, indicating that the prognostic effect of CD15 was unrelated to the predominant morphology.
Comparison of MGZL and PMBL
Because of the high malignant cell expression of CD20 MGZL, suggesting it is more similar to an aggressive B-cell lymphoma, we were interested in comparing MGZL to PMBL patients enrolled on the same DA-EPOCH-R protocol, albeit in a separate cohort (Table 1).10 Interestingly, the MGZL patients were significantly less likely to have an elevated LDH, extranodal disease, or pleural or pericardial effusions and were more likely to be male, clinical characteristics more frequent in massive mediastinal NSHL.23 Additionally, MGZL patients were significantly more likely to express CD30 and CD15, phenotypic hallmarks of NSHL, but commonly had strong diffuse CD20 expression, which is not found in NSHL. Clinically, patients with MGZL had a significantly worse EFS and OS compared with our recently published series of 51 patients with PMBL who received identical treatment.10 With a median follow-up of 5 years, patients with MGZL compared with those who had PMBL achieved a significantly lower EFS (62% vs 93%; P = .0005) and OS (74% vs 97%; P = .0012), respectively (Figure 3A-B). Expectedly, because of the low rate of treatment failure in PMBL, none of the biomarkers of outcome in MGZL, including ALC, were prognostic in PMBL.
Kaplan-Meier plots of EFS and OS of MGZL and PMBL. (A) EFS was 62% (95% CI, 42% to 79%) for MGZL (blue curve) compared with 93% (95% CI, 81% to 98%) for PMBL (red curve) at 5 years (P = .0005). (B) OS was 74% (95% CI, 51% to 89%) for MGZL (blue curve) compared with 97% (95% CI, 83% to 99%) for PMBL (red curve) at 5 years (P = .0012).
Kaplan-Meier plots of EFS and OS of MGZL and PMBL. (A) EFS was 62% (95% CI, 42% to 79%) for MGZL (blue curve) compared with 93% (95% CI, 81% to 98%) for PMBL (red curve) at 5 years (P = .0005). (B) OS was 74% (95% CI, 51% to 89%) for MGZL (blue curve) compared with 97% (95% CI, 83% to 99%) for PMBL (red curve) at 5 years (P = .0012).
Discussion
We present the first prospective study of MGZL and describe its clinical and immunophenotypic characteristics and treatment outcome. MGZL shares clinical characteristics with PMBL and NSHL, including young age and bulky mediastinal masses, but more like mediastinal NSHL, it has a predominance of males, and a lower frequency of elevated LDH, extranodal disease, and effusions.10,23 Clinically, 62% of patients achieved continuous complete remissions with DA-EPOCH-R, indicating that it is an effective treatment. Furthermore, among the 9 patients who did not achieve a durable remission, 4 (44%) received salvage therapy with involved-field radiation alone and are in continuous remission. The remaining 5 patients were aggressively treated with salvage chemotherapy and/or allogeneic transplantation and all died of disease, highlighting the chemotherapy resistance of this subgroup. This contrasts with our findings in PMBL in which the EFS and OS were 93% and 97%, respectively, at 60 months with DA-EPOCH-R.10
The occurrence of treatment failure in one third of MGZL patients and the curative potential of salvage radiotherapy makes early identification of treatment failure important. FDG-PET provided excellent specificity but sensitivity of only 63%. Clinical prognostic factors such as IPI and IPS and mass size were not predictive. However, the ALC and the presence of DC-SIGN–positive dendritic/activated macrophage cells were relatively robust biomarkers of clinical outcome in which pretreatment ALC above the median and the absence of DC-SIGN–positive cells in the tumor biopsies were associated with a 100% survival. Notably, pretreatment ALC and tumor-infiltrating macrophages are prognostic biomarkers in classical HL, consistent with a biological connection between MGZL and NSHL and a shared pathobiology.17,19
The MGZL malignant cells expressed CD15 in more than half the patients, and all expressed CD30, which is characteristically expressed on Reed-Sternberg cells.3 Interestingly, the robust expression of CD15 (scores 3 to 4) on the malignant cells was associated with a worse outcome, suggesting that the resistant tumors may be more Hodgkin-like. However, this was not the case. Tumors with a predominant PMBL-like morphology had a worse outcome but a level of CD15 expression similar to that of tumors with a predominant HL-like morphology, consistent with the intermediate histopathology of MGZL. Most MGZL patients also expressed the B-lineage–specific proteins CD20 and BCL-6, which is unlike HL, in which loss of the B-cell program is a fundamental biological feature.24 Although our results show that MGZL lies along a morphologic and immunophenotypic continuum between NSHL and PMBL, the clinical outcomes indicate that biological characteristics associated with HL, including low ALC, tumor-associated dendritic/macrophage cells, and CD15 expression, were associated with a worse outcome.
Although our results are promising compared with standard HL-based chemotherapy plus radiation in bulky NSHL in which more than 30% of patients progress, we cannot rigorously determine whether DA-EPOCH-R is optimal treatment for MGZL, given the limited number of patients and without a randomized study design.23,25 To help address the question of therapeutic strategy, it is useful to review the treatment outcome of Hodgkin-like anaplastic large cell lymphoma, which historically included some patients that might be considered MGZL today.3 Zinzani et al reported the largest prospective series of Hodgkin-like anaplastic large cell lymphoma, but that series specifically excluded tumors with B-cell markers.8 In that study, patients were randomly assigned to receive either methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin or doxorubicin, bleomycin, vinblastine, and dacarbazine to assess whether these borderline tumors should be treated as aggressive high-grade non-HLs or HLs; 85% of patients had a mediastinal mass, and patients with bulky tumors received consolidation radiation therapy. The relapse-free survival was 94% and 92% at 32 months for the two arms, respectively, indicating that both treatment approaches were equally effective when combined with radiation therapy for most patients. Although this series likely did not include patients we would classify as having MGZL, because CD20 was an exclusion criterion, this study provides cogent evidence that histologically borderline tumors are not advantaged by the use of HL-based treatments.
A recent abstract from Evens et al suggests that gray-zone lymphomas have a relatively poor outcome compared with other aggressive B-cell lymphomas.26,27 In this study of 96 patients, 44% had mediastinal masses, suggesting a diagnosis of MGZL, and 56% had non-MGZL, which is not the same disease. At a median follow-up of approximately 2 years, the progression-free survival and OS were 41% and 84%, respectively, for all patients, and the outcome was similar in patients with MGZL and non-MGZL, as well as in patients who received non-HL–based or HL-based regimens. These studies are consistent with our results, which show that MGZL has a poorer outcome, likely the result of higher drug resistance and not the result of using a non-HL–based treatment. Nonetheless, DA-EPOCH-R alone produced durable remissions in most patients, indicating that it is an effective treatment of these relatively resistant lymphomas.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.H.W. conceived and conducted the study, analyzed the data, and wrote the manuscript; S.P. and A.N. conceived the study, analyzed the data, and helped write the manuscript; K.C. treated, analyzed the data, and helped write the manuscript; M.S., S.M.S., and M.R. analyzed the data and helped write the manuscript; L.M.S. and E.S.J. conceived the study, analyzed the data, and helped write the manuscript; K.D. treated, analyzed the data, and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wyndham H. Wilson, Lymphoid Malignancy Branch, Center for Cancer Research, National Cancer Institute, Building 10, Room 4N/115, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: wilsonw@mail.nih.gov.