Key Points
More patients with chronic myeloid leukemia in chronic phase achieve EMR on frontline nilotinib than imatinib.
EMR failure on frontline nilotinib or imatinib predicts poor outcomes in patients with chronic myeloid leukemia in chronic phase.
Abstract
We explored the impact of early molecular response (EMR; BCR-ABL ≤10% on the international scale [BCR-ABLIS] at 3 or 6 months) on outcomes in patients with newly diagnosed chronic myeloid leukemia in chronic phase treated with nilotinib or imatinib based on 4 years of follow up in Evaluating Nilotinib Efficacy and Safety in Clinical Trials—Newly Diagnosed Patients. Patients (n = 846) received nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, or imatinib 400 mg once daily. At 3 months, more patients had EMR failure (ie, BCR-ABLIS >10%) on imatinib (33%) than on nilotinib (9%-11%); similarly at 6 months, 16% of patients in the imatinib arm vs 3% and 7% in the nilotinib arms had EMR failure. In all arms, EMR failure was associated with lower rates of molecular response, an increased risk of progression, and lower overall survival compared with EMR achievement. We also analyzed patient and treatment characteristics associated with EMR and found distinct patterns in the nilotinib arms vs the imatinib arm. High Sokal risk score was associated with a high rate of EMR failure on imatinib, but not on nilotinib. In contrast, reduced dose intensity and dose interruptions were strongly associated with EMR failure in nilotinib-treated, but not imatinib-treated, patients. This study is registered at www.clinicaltrials.gov as #NCT00471497.
Introduction
The advent of imatinib1,2 and the promise of long-term cytogenetic remissions for many patients with Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) has led to efforts exploring whether factors at baseline or within the first few months of imatinib therapy might predict future outcomes. Such factors might enable early identification of patients with a high probability of poor long-term outcomes and the opportunity to evaluate alternative treatment strategies in this setting. BCR-ABL transcript levels of >10% according to the international scale (BCR-ABLIS), as measured by real-time quantitative polymerase chain reaction (PCR), at 3 or 6 months from initiation of imatinib therapy correlate with reduced rates of molecular response, event-free survival, progression-free survival (PFS), and overall survival (OS).3-7 Recent reports suggest that early responses have similar predictive value for patients receiving frontline nilotinib or dasatinib.8,9 However, debate continues regarding the applicability of the BCR-ABLIS ≤10% cutoff to tyrosine kinase inhibitors (TKIs) other than imatinib and the relative value of the 3- vs 6-month time points.6,9-12 Post hoc analyses of data from large, randomized clinical trials, within which parallel groups of patients receiving imatinib or newer TKIs can be assessed, will improve our understanding of these landmarks. Data from these studies will be especially valuable for exploring patient and treatment characteristics associated with achieving BCR-ABLIS ≤10% at 3 or 6 months (ie, an early molecular response [EMR]) on each TKI.
Evaluating Nilotinib Efficacy and Safety in Clinical Trials—Newly Diagnosed Patients (ENESTnd) is a randomized, international, phase 3 trial of nilotinib 300 or 400 mg twice daily vs imatinib 400 mg once daily in patients with newly diagnosed Ph+ CML-CP.13-15 Results of ENESTnd showed that nilotinib significantly reduced the risk of progression to accelerated phase/blast crisis (AP/BC) and afforded superior rates of complete cytogenetic response, major molecular response (MMR), and deep molecular response (ie, molecular responses ≥4-log reduction [MR4] or ≥4.5-log reduction [MR4.5])16 vs imatinib.13-15 Here, we present updated 4-year follow-up data from ENESTnd and analyze the utility of BCR-ABL transcript levels at 3 and 6 months to predict future MMR, MR4.5, PFS, and OS in patients receiving nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, or imatinib 400 mg once daily.
Materials and methods
The design of ENESTnd (ClinicalTrials.gov identifier: NCT00471497) has been previously described.13-15 All patients had Ph+ CML-CP newly diagnosed within the previous 6 months and had received no prior TKI therapy (except imatinib for ≤2 weeks) or other medical treatment of CML (except hydroxyurea or anagrelide).13-15 Patients were stratified by Sokal risk score at baseline. BCR-ABL transcript levels were assessed by real-time quantitative PCR in a centralized laboratory (MolecularMD, Portland, OR).13-15 Patients were monitored for cytogenetic and molecular responses, PFS, OS, progression to AP/BC, and safety as previously described.13-15 MMR was defined as BCR-ABLIS ≤0.1%, MR4 as BCR-ABLIS ≤0.01%, and MR4.5 as BCR-ABLIS ≤0.0032%16 ; negative PCR results were included only if the assay sensitivity was confirmed to be ≥4.5 logs. PFS on study was defined as freedom from progression to AP/BC or death from any cause on study drug or during follow up after study drug discontinuation. OS on study was defined as freedom from death from any cause while on the study drug or during follow up after study drug discontinuation.
Landmark analyses for the 3-month time point included patients with typical b2a2 and/or b3a2 BCR-ABL transcripts and evaluable PCR samples at 3 months. Rates of MMR, MR4.5, PFS, and OS were evaluated among patients grouped by BCR-ABLIS at 3 months (≤1%, >1% to ≤10%, ≤10%, and >10%). Patients with MMR or MR4.5 by 3 months were excluded from the landmark analyses of cumulative incidence of MMR or MR4.5, respectively. Landmark analyses of PFS and OS excluded patients with a PFS or OS event, respectively, or who were censored by 3 months. A separate set of landmark analyses was conducted according to BCR-ABLIS at 6 months (full results are reported in the supplemental Appendix, available on the Blood Web site).
For time-to-event analyses, Kaplan-Meier methods were used, and P values were derived using the log-rank test. According to the study protocol, the primary efficacy end point (MMR at 12 months) and key secondary efficacy end point (durable MMR at 24 months) were formally adjusted for type I error because of multiple comparisons, and thus can be claimed to be statistically significant. The landmark analyses presented here are exploratory and were not preplanned in the protocol. All P values related to these analyses and presented here were thus post hoc and were not adjusted for multiple comparisons.
Baseline characteristics and early treatment exposure according to BCR-ABL levels at 3 months and the impact of baseline Sokal risk score or early dose interruption on progression in patients with EMR failure (ie, BCR-ABLIS >10%) were assessed by treatment arm using descriptive statistics. This analysis had a data cutoff of July 27, 2012, when all patients had a minimum of 4 years of follow up.
All authors are in compliance and have received appropriate institutional review board approval from their respective institutions. This study was conducted in accordance with the Declaration of Helsinki.
Results
Summary of overall ENESTnd 4-year results
After a minimum follow up of 4 years, rates of MMR, MR4, and MR4.5 remained significantly higher in the nilotinib 300 mg twice-daily and nilotinib 400 mg twice-daily arms compared with the imatinib arm (Table 1). Estimated 4-year rates of freedom from progression to AP/BC on study were significantly higher on nilotinib (96.7% [P = .0497] and 97.8% [P = .0074]; nilotinib 300 mg and 400 mg twice daily, respectively) compared with imatinib (93.1%). Estimated rates of 4-year OS on study were 94.3%, 96.7%, and 93.3% for nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, and imatinib, respectively. The safety profiles of nilotinib and imatinib remained similar to those previously reported,13-15 with no new safety signals and few newly occurring grade 3/4 adverse events reported.
ENESTnd 4-year overall results: patient disposition and long-term end points
| . | Nilotinib 300 mg twice daily (n = 282) . | Nilotinib 400 mg twice daily (n = 281) . | Imatinib 400 mg once daily (n = 283) . |
|---|---|---|---|
| Patient disposition | |||
| Remaining on active follow up or died, % | 94 | 95 | 93 |
| Remaining on core treatment, % | 66 | 69 | 57 |
| Cumulative incidence of molecular response | |||
| MMR by 4 years, % (P value vs imatinib) | 76 (<.0001) | 73 (<.0001) | 56 |
| MR4 by 4 years, % (P value vs imatinib) | 56 (<.0001) | 50 (<.0001) | 32 |
| MR4.5 by 4 years, % (P value vs imatinib) | 40 (<.0001) | 37 (.0002) | 23 |
| Progression to AP/BC on study* | |||
| Number of events, n | 9 | 6 | 19 |
| Estimated 4-year freedom from progression to AP/BC on study, % (P value vs imatinib)† | 96.7 (.0497) | 97.8 (.0074) | 93.1 |
| HR (95% CI) | 0.46 (0.21-1.02) | 0.31 (0.12-0.77) | |
| PFS on study‡ | |||
| Number of events, n | 19 | 10 | 22 |
| Estimated 4-year PFS, % (P value vs imatinib)† | 92.7 (.5643) | 96.3 (.0264) | 92.0 |
| HR (95% CI) | 0.84 (0.45-1.54) | 0.44 (0.21-0.93) | |
| OS on study§ | |||
| Number of events, n | 15 | 9 | 19 |
| Estimated 4-year OS, % (P value vs imatinib)† | 94.3 (.4636) | 96.7 (.0498) | 93.3 |
| HR (95% CI) | 0.78 (0.39-1.53) | 0.46 (0.21-1.02) | |
| . | Nilotinib 300 mg twice daily (n = 282) . | Nilotinib 400 mg twice daily (n = 281) . | Imatinib 400 mg once daily (n = 283) . |
|---|---|---|---|
| Patient disposition | |||
| Remaining on active follow up or died, % | 94 | 95 | 93 |
| Remaining on core treatment, % | 66 | 69 | 57 |
| Cumulative incidence of molecular response | |||
| MMR by 4 years, % (P value vs imatinib) | 76 (<.0001) | 73 (<.0001) | 56 |
| MR4 by 4 years, % (P value vs imatinib) | 56 (<.0001) | 50 (<.0001) | 32 |
| MR4.5 by 4 years, % (P value vs imatinib) | 40 (<.0001) | 37 (.0002) | 23 |
| Progression to AP/BC on study* | |||
| Number of events, n | 9 | 6 | 19 |
| Estimated 4-year freedom from progression to AP/BC on study, % (P value vs imatinib)† | 96.7 (.0497) | 97.8 (.0074) | 93.1 |
| HR (95% CI) | 0.46 (0.21-1.02) | 0.31 (0.12-0.77) | |
| PFS on study‡ | |||
| Number of events, n | 19 | 10 | 22 |
| Estimated 4-year PFS, % (P value vs imatinib)† | 92.7 (.5643) | 96.3 (.0264) | 92.0 |
| HR (95% CI) | 0.84 (0.45-1.54) | 0.44 (0.21-0.93) | |
| OS on study§ | |||
| Number of events, n | 15 | 9 | 19 |
| Estimated 4-year OS, % (P value vs imatinib)† | 94.3 (.4636) | 96.7 (.0498) | 93.3 |
| HR (95% CI) | 0.78 (0.39-1.53) | 0.46 (0.21-1.02) | |
CI, confidence interval; HR, hazard ratio.
Progression to AP/BC events include progression to AP/BC or CML-related death on core or extension treatment or any progression to AP/BC reported during the follow up after discontinuation of treatment.
Kaplan-Meier estimated rates.
PFS events include progression to AP/BC or death from any cause on core or extension treatment or during follow up after discontinuation of treatment.
OS events include death from any cause on core or extension treatment or during follow up after discontinuation of treatment.
Landmark analyses: patients
The 3-month landmark analyses included 258, 260, and 264 patients in the nilotinib 300 mg twice-daily, nilotinib 400 mg twice-daily, and imatinib arms, respectively, with typical BCR-ABL transcripts and evaluable 3-month PCR samples. The analysis of MMR excluded an additional 25 patients on nilotinib 300 mg twice daily, 14 patients on nilotinib 400 mg twice daily, and 2 patients on imatinib with MMR by 3 months. The analysis of MR4.5 excluded an additional patient in each nilotinib arm with MR4.5 by 3 months. The analysis of PFS on study excluded 2 additional patients in the nilotinib 400 mg twice-daily arm who were censored by 3 months.
BCR-ABL levels at 3 months: impact of baseline characteristics and treatment
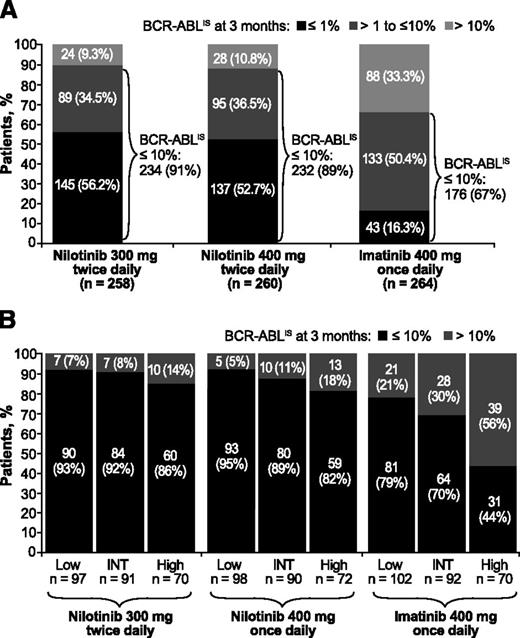
More patients achieved BCR-ABLIS ≤10% and BCR-ABLIS ≤1% at 3 months on nilotinib than on imatinib (BCR-ABLIS ≤10%: 91%, nilotinib 300 mg twice daily; 89%, nilotinib 400 mg twice daily; 67%, imatinib; BCR-ABLIS ≤1%: 56%, 53%, and 16%, respectively; Figure 1A). Patients on imatinib had higher rates of EMR failure than those on nilotinib across all 3 Sokal risk score groups, but this difference was greatest in patients with high Sokal risk scores (EMR failure in 14%, 18%, and 56% of patients with high Sokal risk scores on nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, and imatinib, respectively). In patients receiving nilotinib, Sokal risk score had little impact on achievement of EMR at 3 months (Figure 1B).
BCR-ABL levels at 3 months from start of treatment in evaluable patients. Panels show BCR-ABL levels at 3 months from start of treatment overall (A) and by Sokal risk score at baseline (B) in patients treated with nilotinib 300 mg twice daily (n = 258), nilotinib 400 mg twice daily (n = 260), or imatinib 400 mg once daily (n = 264). Patients with unevaluable BCR-ABL transcript levels (n = 24 in the nilotinib 300 mg twice-daily arm, 21 in the nilotinib 400 mg twice-daily arm, and 19 in the imatinib arm) were excluded from the landmark analyses for the following reasons: atypical transcripts at baseline: nilotinib 300 mg twice daily (n = 5), nilotinib 400 mg twice daily (n = 1), and imatinib (n = 2); missing samples at 3 months: nilotinib 300 mg twice daily (n = 4), nilotinib 400 mg twice daily (n = 3), and imatinib (n = 5); or discontinued treatment by 3 months (3-month PCR analysis not performed): nilotinib 300 mg twice daily (n = 15, including 1 progression event), nilotinib 400 mg twice daily (n = 17), and imatinib (n = 12, including 1 progression event). INT, intermediate.
BCR-ABL levels at 3 months from start of treatment in evaluable patients. Panels show BCR-ABL levels at 3 months from start of treatment overall (A) and by Sokal risk score at baseline (B) in patients treated with nilotinib 300 mg twice daily (n = 258), nilotinib 400 mg twice daily (n = 260), or imatinib 400 mg once daily (n = 264). Patients with unevaluable BCR-ABL transcript levels (n = 24 in the nilotinib 300 mg twice-daily arm, 21 in the nilotinib 400 mg twice-daily arm, and 19 in the imatinib arm) were excluded from the landmark analyses for the following reasons: atypical transcripts at baseline: nilotinib 300 mg twice daily (n = 5), nilotinib 400 mg twice daily (n = 1), and imatinib (n = 2); missing samples at 3 months: nilotinib 300 mg twice daily (n = 4), nilotinib 400 mg twice daily (n = 3), and imatinib (n = 5); or discontinued treatment by 3 months (3-month PCR analysis not performed): nilotinib 300 mg twice daily (n = 15, including 1 progression event), nilotinib 400 mg twice daily (n = 17), and imatinib (n = 12, including 1 progression event). INT, intermediate.
In all 3 treatment arms, patients with EMR failure at 3 months had larger median spleen sizes than those who achieved EMR; in the nilotinib 300 mg twice-daily arm and the imatinib arm, patients with EMR failure had higher median white cell counts at study start than those who achieved EMR (Table 2). Patients with a dose interruption lasting ≥5 consecutive days were more likely to have EMR failure than those with no dose interruption or a dose interruption lasting <5 consecutive days (nilotinib 300 mg twice daily: 16% vs 6%; nilotinib 400 mg twice daily: 23% vs 4%; imatinib: 39% vs 32%). However, patients with a dose interruption lasting ≥5 days constituted more than half of patients with EMR failure on nilotinib (nilotinib 300 mg twice daily: 14/24 patients [58%]; nilotinib 400 mg twice daily: 22/28 patients [79%]) compared with 22% of those on imatinib (19/88). In both nilotinib arms, the median dose intensity during the first 3 months was lower in patients with EMR failure than in patients with EMR at 3 months (nilotinib 300 mg twice daily: 79% vs 100% of planned dose; nilotinib 400 mg twice daily: 62% vs 100% of planned dose); there was no apparent difference in dose intensity between the 2 groups in the imatinib arm.
Characteristics at study start and early treatment exposure according to BCR-ABL transcript level at 3 months
| . | Nilotinib 300 mg twice daily . | Nilotinib 400 mg twice daily . | Imatinib 400 mg once daily . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics at study start . | . | BCR-ABLIS at 3 months . | . | BCR-ABLIS at 3 months . | . | BCR-ABLIS at 3 months . | |||
| n . | ≤10% . | >10% . | n . | ≤10% . | >10% . | n . | ≤10% . | >10% . | |
| Patients, n (%)* | 258 | 234 (91) | 24 (9) | 260 | 232 (89) | 28 (11) | 264 | 176 (67) | 88 (33) |
| Median spleen size below costal margin (range), cm | 5.0 (1-27) | 8.0 (2-19) | 6.0 (1-25) | 8.0 (1-20) | 3.5 (1-15) | 10.5 (1-25) | |||
| Median platelet count (range), ×109/L | 431 (90-3880) | 355 (101-1385) | 368 (103-1817) | 490 (126-1819) | 370 (66-1400) | 362 (84-2232) | |||
| Median white cell count (range), ×109/L | 24 (2-247) | 40 (3-167) | 23 (2-435) | 28 (4-254) | 23 (3-181) | 34 (3-482) | |||
| Median blasts in peripheral blood (range), % | 0 (0-10) | 0 (0-15) | 0 (0-12) | 0 (0-6) | 0 (0-14) | 0 (0-12) | |||
| Splenomegaly, n (%) | 109 | 91 (83) | 18 (17) | 103 | 89 (86) | 14 (14) | 99 | 47 (47) | 52 (53) |
| Chromosomal abnormalities in addition to the Philadelphia chromosome, n (%) | 26 | 22 (85) | 4 (15) | 39 | 33 (85) | 6 (15) | 30 | 17 (57) | 13 (43) |
| Clonal evolution (major route aberrations), n (%)† | 5 | 3 (60) | 2 (40) | 3 | 3 (100) | 0 | 4 | 1 (25) | 3 (75) |
| Treatment exposure in the first 3 months | |||||||||
| Patients, n (%)* | 258 | 234 (91) | 24 (9) | 260 | 232 (89) | 28 (11) | 264 | 176 (67) | 88 (33) |
| Median dose intensity (range; % of planned dose), mg/day‡ | 600 (210-604; 100) | 474 (270-600; 79) | 800 (222-800; 100) | 492 (270-800; 62) | 400 (255-405; 100) | 400 (215-400; 100) | |||
| Dose interruption for ≥5 consecutive days, n (%) | 85 | 71 (84) | 14 (16) | 94 | 72 (77) | 22 (23) | 49 | 30 (61) | 19 (39) |
| No dose interruption or dose interruption for <5 consecutive days, n (%) | 173 | 163 (94) | 10 (6) | 166 | 160 (96) | 6 (4) | 215 | 146 (68) | 69 (32) |
| . | Nilotinib 300 mg twice daily . | Nilotinib 400 mg twice daily . | Imatinib 400 mg once daily . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics at study start . | . | BCR-ABLIS at 3 months . | . | BCR-ABLIS at 3 months . | . | BCR-ABLIS at 3 months . | |||
| n . | ≤10% . | >10% . | n . | ≤10% . | >10% . | n . | ≤10% . | >10% . | |
| Patients, n (%)* | 258 | 234 (91) | 24 (9) | 260 | 232 (89) | 28 (11) | 264 | 176 (67) | 88 (33) |
| Median spleen size below costal margin (range), cm | 5.0 (1-27) | 8.0 (2-19) | 6.0 (1-25) | 8.0 (1-20) | 3.5 (1-15) | 10.5 (1-25) | |||
| Median platelet count (range), ×109/L | 431 (90-3880) | 355 (101-1385) | 368 (103-1817) | 490 (126-1819) | 370 (66-1400) | 362 (84-2232) | |||
| Median white cell count (range), ×109/L | 24 (2-247) | 40 (3-167) | 23 (2-435) | 28 (4-254) | 23 (3-181) | 34 (3-482) | |||
| Median blasts in peripheral blood (range), % | 0 (0-10) | 0 (0-15) | 0 (0-12) | 0 (0-6) | 0 (0-14) | 0 (0-12) | |||
| Splenomegaly, n (%) | 109 | 91 (83) | 18 (17) | 103 | 89 (86) | 14 (14) | 99 | 47 (47) | 52 (53) |
| Chromosomal abnormalities in addition to the Philadelphia chromosome, n (%) | 26 | 22 (85) | 4 (15) | 39 | 33 (85) | 6 (15) | 30 | 17 (57) | 13 (43) |
| Clonal evolution (major route aberrations), n (%)† | 5 | 3 (60) | 2 (40) | 3 | 3 (100) | 0 | 4 | 1 (25) | 3 (75) |
| Treatment exposure in the first 3 months | |||||||||
| Patients, n (%)* | 258 | 234 (91) | 24 (9) | 260 | 232 (89) | 28 (11) | 264 | 176 (67) | 88 (33) |
| Median dose intensity (range; % of planned dose), mg/day‡ | 600 (210-604; 100) | 474 (270-600; 79) | 800 (222-800; 100) | 492 (270-800; 62) | 400 (255-405; 100) | 400 (215-400; 100) | |||
| Dose interruption for ≥5 consecutive days, n (%) | 85 | 71 (84) | 14 (16) | 94 | 72 (77) | 22 (23) | 49 | 30 (61) | 19 (39) |
| No dose interruption or dose interruption for <5 consecutive days, n (%) | 173 | 163 (94) | 10 (6) | 166 | 160 (96) | 6 (4) | 215 | 146 (68) | 69 (32) |
Evaluable patients at 3 months (ie, patients with typical transcripts at baseline and evaluable PCR samples at 3 months).
Includes trisomy 8 or 19, second Philadelphia chromosome, or isochromosome 17.
None of the patients in the imatinib arm had their imatinib dose escalated to 800 mg by 3 months.
BCR-ABL levels ≤10% at 3 months predicted future MMR and MR4.5
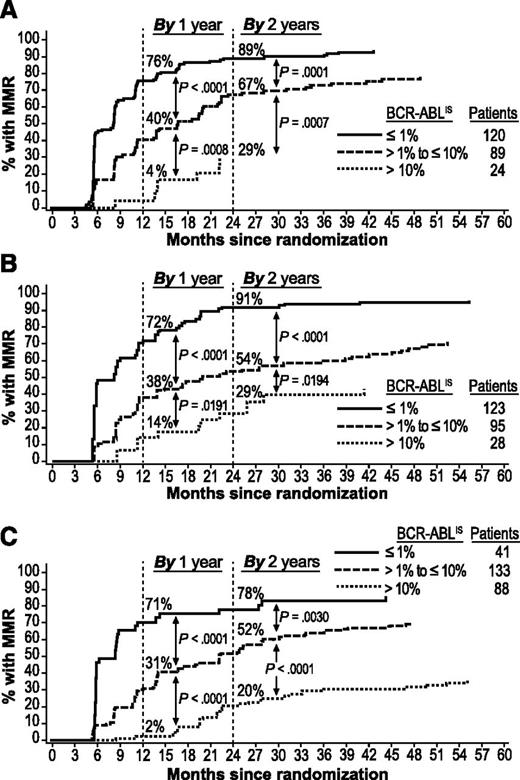
Patients who achieved EMR at 3 months were more likely to achieve MMR by 2 years than were patients with EMR failure. Among patients with EMR (but without MMR) vs patients with EMR failure, respectively, 80% (167/209) vs 29% (7/24; P < .0001) on nilotinib 300 mg twice daily, 75% (163/218) vs 29% (8/28; P < .0001) on nilotinib 400 mg twice daily, and 58% (101/174) vs 20% (18/88; P < .0001) on imatinib achieved MMR by 2 years.
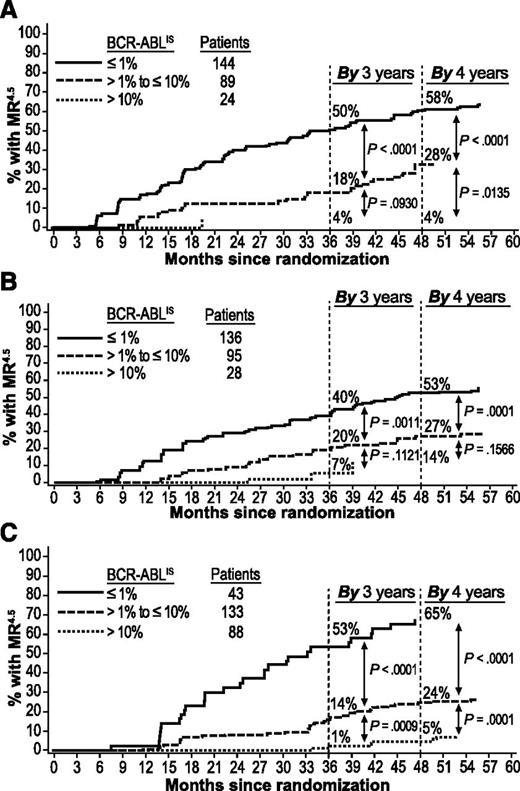
Achievement of MR4.5 by 4 years was also more likely in patients who achieved EMR at 3 months than in patients with EMR failure. Among patients with EMR (but without MR4.5) vs patients with EMR failure, respectively, 47% (109/233) vs 4% (1/24; P < .0001) on nilotinib 300 mg twice daily, 42% (98/231) vs 14% (4/28; P = .0040) on nilotinib 400 mg twice daily, and 34% (60/176) vs 5% (4/88; P < .0001) on imatinib achieved MR4.5 by 4 years.
Patients with BCR-ABLIS ≤1% at 3 months had higher rates of MMR by 1 and 2 years (Figure 2) and MR4.5 by 3 and 4 years (Figure 3) than patients with BCR-ABLIS >1% to ≤10% or BCR-ABLIS >10%. Among patients with BCR-ABLIS ≤1% (but without MMR) at 3 months, 89% (107/120), 91% (112/123), and 78% (32/41) of those on nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, and imatinib, respectively, achieved MMR by 2 years; among patients with BCR-ABLIS ≤1% (but without MR4.5) at 3 months, 58% (84/144), 53% (72/136), and 65% (28/43), respectively, achieved MR4.5 by 4 years.
Cumulative incidence of MMR by BCR-ABL levels at 3 months in evaluable patients. Panels show MMR achieved in the nilotinib 300 mg twice-daily (A), nilotinib 400 mg twice-daily (B), and imatinib 400 mg once-daily (C) arms. Patients with atypical transcripts at baseline or unevaluable or missing PCR assessments at 3 months, or who achieved MMR by 3 months, were excluded.
Cumulative incidence of MMR by BCR-ABL levels at 3 months in evaluable patients. Panels show MMR achieved in the nilotinib 300 mg twice-daily (A), nilotinib 400 mg twice-daily (B), and imatinib 400 mg once-daily (C) arms. Patients with atypical transcripts at baseline or unevaluable or missing PCR assessments at 3 months, or who achieved MMR by 3 months, were excluded.
Cumulative incidence of MR4.5by BCR-ABL levels at 3 months in evaluable patients. Panels show MR4.5 achieved in the nilotinib 300 mg twice-daily (A), nilotinib 400 mg twice-daily (B), and imatinib once-daily (C) arms. Patients with atypical transcripts at baseline or unevaluable or missing PCR assessments at 3 months, or who achieved MR4.5 by 3 months, were excluded.
Cumulative incidence of MR4.5by BCR-ABL levels at 3 months in evaluable patients. Panels show MR4.5 achieved in the nilotinib 300 mg twice-daily (A), nilotinib 400 mg twice-daily (B), and imatinib once-daily (C) arms. Patients with atypical transcripts at baseline or unevaluable or missing PCR assessments at 3 months, or who achieved MR4.5 by 3 months, were excluded.
BCR-ABL levels ≤10% at 3 months predicted improved PFS and OS
Patients who achieved EMR at 3 months were significantly more likely to be alive and free from progression at 4 years compared with patients with EMR failure (Table 3). Estimated PFS rates at 4 years in patients with EMR and EMR failure, respectively, were 95.2% and 82.9% (nilotinib 300 mg twice daily; P = .0061), 96.9% and 89.0% (nilotinib 400 mg twice daily; P = .0399), and 97.7% and 82.6% (imatinib; P < .0001).
PFS and OS at 4 years by BCR-ABL level at 3 months in evaluable* patients
| . | Nilotinib 300 mg twice daily (n = 282) . | Nilotinib 400 mg twice daily (n = 281) . | Imatinib 400 mg once daily (n = 283) . |
|---|---|---|---|
| PFS on study† | |||
| BCR-ABLIS at 3 months | |||
| ≤1% | |||
| Evaluable patients/events, n | 145/6 | 136/3 | 43/2 |
| Estimated 4-year PFS, % | 95.8 | 97.8 | 95.3 |
| P value vs >1% to ≤10% | .8269 | .3660 | .2338 |
| >1% to ≤10% | |||
| Evaluable patients/events, n | 89/4 | 94/4 | 133/2 |
| Estimated 4-year PFS, % | 94.2 | 95.7 | 98.5 |
| P value vs >10% | .0351 | .1907 | <.0001 |
| ≤10% | |||
| Evaluable patients/events, n | 234/10 | 230/7 | 176/4 |
| Estimated 4-year PFS, % | 95.2 | 96.9 | 97.7 |
| P value vs >10% | .0061 | .0399 | <.0001 |
| >10% | |||
| Evaluable patients/events, n | 24/4 | 28/3 | 88/15 |
| Estimated 4-y PFS, % | 82.9 | 89.0 | 82.6 |
| OS on study‡ | |||
| BCR-ABLIS at 3 months | |||
| ≤1% | |||
| Evaluable patients/events, n | 145/5 | 137/3 | 43/2 |
| Estimated 4-year OS, % | 96.5 | 97.8 | 95.3 |
| P value vs >1% to ≤10% | .6348 | .3653 | .0982 |
| >1% to ≤10% | |||
| Evaluable patients/events, n | 89/2 | 95/4 | 133/1 |
| Estimated 4-year OS, % | 97.2 | 95.7 | 100 |
| P value vs >10% | .0219 | .5219 | <.0001 |
| ≤10% | |||
| Evaluable patients/events, n | 234/7 | 232/7 | 176/3 |
| Estimated 4-year OS, % | 96.7 | 96.9 | 98.9 |
| P value vs >10% | .0116 | .2483 | <.0001 |
| >10% | |||
| Evaluable patients/events, n | 24/3 | 28/2 | 88/14 |
| Estimated 4-year OS, % | 86.7 | 92.7 | 83.6 |
| . | Nilotinib 300 mg twice daily (n = 282) . | Nilotinib 400 mg twice daily (n = 281) . | Imatinib 400 mg once daily (n = 283) . |
|---|---|---|---|
| PFS on study† | |||
| BCR-ABLIS at 3 months | |||
| ≤1% | |||
| Evaluable patients/events, n | 145/6 | 136/3 | 43/2 |
| Estimated 4-year PFS, % | 95.8 | 97.8 | 95.3 |
| P value vs >1% to ≤10% | .8269 | .3660 | .2338 |
| >1% to ≤10% | |||
| Evaluable patients/events, n | 89/4 | 94/4 | 133/2 |
| Estimated 4-year PFS, % | 94.2 | 95.7 | 98.5 |
| P value vs >10% | .0351 | .1907 | <.0001 |
| ≤10% | |||
| Evaluable patients/events, n | 234/10 | 230/7 | 176/4 |
| Estimated 4-year PFS, % | 95.2 | 96.9 | 97.7 |
| P value vs >10% | .0061 | .0399 | <.0001 |
| >10% | |||
| Evaluable patients/events, n | 24/4 | 28/3 | 88/15 |
| Estimated 4-y PFS, % | 82.9 | 89.0 | 82.6 |
| OS on study‡ | |||
| BCR-ABLIS at 3 months | |||
| ≤1% | |||
| Evaluable patients/events, n | 145/5 | 137/3 | 43/2 |
| Estimated 4-year OS, % | 96.5 | 97.8 | 95.3 |
| P value vs >1% to ≤10% | .6348 | .3653 | .0982 |
| >1% to ≤10% | |||
| Evaluable patients/events, n | 89/2 | 95/4 | 133/1 |
| Estimated 4-year OS, % | 97.2 | 95.7 | 100 |
| P value vs >10% | .0219 | .5219 | <.0001 |
| ≤10% | |||
| Evaluable patients/events, n | 234/7 | 232/7 | 176/3 |
| Estimated 4-year OS, % | 96.7 | 96.9 | 98.9 |
| P value vs >10% | .0116 | .2483 | <.0001 |
| >10% | |||
| Evaluable patients/events, n | 24/3 | 28/2 | 88/14 |
| Estimated 4-year OS, % | 86.7 | 92.7 | 83.6 |
Patients with atypical transcripts at baseline or unevaluable PCR samples at 3 months were excluded. Patients who had a PFS event or who were censored by 3 months were excluded from the analysis of PFS. Patients with an OS event or who were censored by 3 months were excluded from the analysis of OS.
PFS events include progression to AP/BC or death from any cause on core or extension treatment or during follow up after discontinuation of treatment.
OS events include death from any cause on core or extension treatment or during follow up after discontinuation of treatment.
Patients who achieved EMR at 3 months also had higher rates of OS at 4 years compared with patients with EMR failure (Table 3). Estimated OS rates at 4 years in patients with EMR and EMR failure, respectively, were 96.7% and 86.7% (nilotinib 300 mg twice-daily arm; P = .0116), 96.9% and 92.7% (nilotinib 400 mg twice daily; P = .2483), and 98.9% and 83.6% (imatinib; P < .0001).
Outcomes in patients with BCR-ABL levels >10% at 3 months
Of the 24 patients with EMR failure at 3 months on nilotinib 300 mg twice daily, 19 (79%) achieved EMR at 6 months and 5 (21%) had EMR failure at 6 months. Of the 28 patients with EMR failure at 3 months on nilotinib 400 mg twice daily, 15 (54%) achieved EMR at 6 months, 12 (43%) had EMR failure at 6 months, and 1 (4%) had a missing assessment at 6 months. Of the 88 imatinib-treated patients with EMR failure at 3 months, 48 (55%) achieved EMR at 6 months, 32 (36%) had EMR failure at 6 months, and 8 (9%) had missing assessments at 6 months.
With a minimum 4 years of follow up, progression events (ie, progression to AP/BC or death from any cause) had occurred on core treatment or during follow up in 4 of 24 patients (17%), 3 of 28 patients (11%), and 15 of 88 patients (17%) with EMR failure at 3 months on nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, and imatinib, respectively. Of these 22 progressions, 9 (41%; 2 on nilotinib 300 mg twice daily, 0 on nilotinib 400 mg twice daily, and 7 on imatinib) occurred between 3 and 6 months of treatment. Patients with EMR failure at 3 months accounted for 21% (4/19) of total progressions on study in the nilotinib 300 mg twice-daily arm, 30% (3/10) of total progressions on study in the nilotinib 400 mg twice-daily arm, and 68% (15/22) of total progressions on study in the imatinib arm.
Patients with EMR failure at 3 months were further stratified by Sokal risk score and treatment exposure during the first 3 months (with or without a dose interruption of ≥5 consecutive days) to explore potential effects of these factors on progression (Table 4). The small number of patients with EMR failure who progressed on nilotinib 300 mg twice daily (4/24) or nilotinib 400 mg twice daily (3/28) prevented meaningful assessment of differences in progression rates according to these factors. Of the 4 patients who progressed on nilotinib 300 mg twice daily, 1 and 3 had high and intermediate/low Sokal risk scores, respectively, and 2 had significant dose interruptions in the first 3 months. Of the 3 patients who progressed on nilotinib 400 mg twice daily, 2 and 1 had high and intermediate/low Sokal risk scores, respectively, and all 3 had significant dose interruptions in the first 3 months. In the imatinib arm, among the 88 patients with EMR failure, there were more progressions in patients with high vs intermediate/low Sokal risk scores (8/39 [21%] vs 7/49 [14%]) and fewer progressions in patients with vs without a significant dose interruption in the first 3 months (2/19 [11%] vs 13/69 [19%]).
Progressions* on study in patients with EMR failure (BCR-ABLIS >10% at 3 months) on nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, or imatinib 400 mg once daily by Sokal risk score (high, intermediate/low) and early dose interruption ≥5 consecutive days (yes, no)
| . | BCR-ABLIS >10% at 3 months . | ||
|---|---|---|---|
| . | Nilotinib 300 mg twice daily (n = 24) . | Nilotinib 400 mg twice daily (n = 28) . | Imatinib 400 mg once daily (n = 88) . |
| Sokal risk score at study start | |||
| High | n = 10 | n = 13 | n = 39 |
| Progressions, n (%)* | 1 (10) | 2 (15) | 8 (21) |
| Intermediate/low | n = 14 | n = 15 | n = 49 |
| Progressions, n (%)* | 3 (21) | 1 (7) | 7 (14) |
| Dose interruption ≥5 consecutive days in the first 3 months | |||
| Yes | n = 14 | n = 22 | n = 19 |
| Progressions, n (%)* | 2 (14) | 3 (14) | 2 (11) |
| No | n = 10 | n = 6 | n = 69 |
| Progressions, n (%)* | 2 (20) | 0 | 13 (19) |
| . | BCR-ABLIS >10% at 3 months . | ||
|---|---|---|---|
| . | Nilotinib 300 mg twice daily (n = 24) . | Nilotinib 400 mg twice daily (n = 28) . | Imatinib 400 mg once daily (n = 88) . |
| Sokal risk score at study start | |||
| High | n = 10 | n = 13 | n = 39 |
| Progressions, n (%)* | 1 (10) | 2 (15) | 8 (21) |
| Intermediate/low | n = 14 | n = 15 | n = 49 |
| Progressions, n (%)* | 3 (21) | 1 (7) | 7 (14) |
| Dose interruption ≥5 consecutive days in the first 3 months | |||
| Yes | n = 14 | n = 22 | n = 19 |
| Progressions, n (%)* | 2 (14) | 3 (14) | 2 (11) |
| No | n = 10 | n = 6 | n = 69 |
| Progressions, n (%)* | 2 (20) | 0 | 13 (19) |
Progressions shown are PFS events on study (ie, progression AP/BC or death from any cause on core or extension treatment or during follow up after discontinuation of treatment).
At the time of data cutoff, 16/24 patients (67%) with EMR failure at 3 months on nilotinib 300 mg twice daily had discontinued for the following reasons: disease progression (n = 1), suboptimal response or treatment failure (n = 11), adverse event or laboratory abnormality (n = 2), withdrawal of consent (n = 1), or death (n = 1). Thirteen of 28 patients (46%) with EMR failure on nilotinib 400 mg twice daily had discontinued for the following reasons: adverse event or laboratory abnormality (n = 9), suboptimal response or treatment failure (n = 2), death (n = 1), or disease progression (n = 1). Fifty-four of the 88 patients (61%) with EMR failure on imatinib had discontinued for the following reasons: disease progression (n = 8), suboptimal response or treatment failure (n = 25), adverse event or laboratory abnormality (n = 15), withdrawal of consent (n = 5), or administrative problems (n = 1). Three patients (13%) with EMR failure on nilotinib 300 mg twice daily, 2 patients (7%) with EMR failure on nilotinib 400 mg twice daily, and 14 patients (16%) with EMR failure on imatinib had died.
Prediction of long-term outcomes using 3- vs 6-month landmarks
Landmark analyses of MMR by 1 and 2 years, MR4.5 by 3 and 4 years, PFS, and OS using BCR-ABL levels at 6 months showed generally similar results to those at 3 months. Patients with EMR failure at 6 months had lower rates of molecular response, PFS, and OS compared with patients with EMR at 6 months across all 3 treatment arms (see the supplemental Appendix for full results). However, fewer patients overall had EMR failure at 6 months vs 3 months (nilotinib 300 mg twice daily: 7 [3%] vs 24 [9%]; nilotinib 400 mg twice daily: 17 [7%] vs 28 [11%]; imatinib: 40 [16%] vs 88 [33%]).
To evaluate the relative ability of the 3- and 6-month landmarks to accurately identify patients with poor long-term outcomes, qualitative comparison of estimated 4-year PFS on study and achievement of MR4.5 by 4 years in patients with or without EMR at the 3- and 6-month landmarks was performed (supplemental Appendix, Table 1). The ability to positively predict a PFS event by 4 years in patients with EMR failure was generally similar using the 3- vs 6-month landmarks (estimated 4-year PFS in patients with EMR failure at 3 vs 6 months, respectively—nilotinib 300 mg twice daily: 82.9% vs 75.0%; nilotinib 400 mg twice daily: 89.0% vs 81.9%; imatinib: 82.6% vs 78.6%). Very few patients with EMR at either 3 or 6 months progressed or died (estimated 4-year PFS in patients with EMR at 3 vs 6 months, respectively—nilotinib 300 mg twice daily: 95.2% vs 95.1%; nilotinib 400 mg twice daily: 96.9% vs 97.0%; imatinib: 97.7% vs 97.2%). EMR failure at 3 or 6 months was highly predictive of failure to achieve MR4.5 (percentage of patients with EMR failure at 3 vs 6 months, respectively, without MR4.5 by 4 years—nilotinib 300 mg twice daily: 96% vs 100%; nilotinib 400 mg twice daily: 86% vs 100%, imatinib: 95% vs 98%).
Discussion
The rate of initial decline in BCR-ABL transcript levels has proven to be a reliable predictor of subsequent outcomes in patients with CML-CP on frontline imatinib4-7 and may also be a reliable predictor in patients receiving frontline nilotinib or dasatinib.8,9 Furthermore, achievement of BCR-ABLIS ≤10% at 3 months on second-line nilotinib was recently reported to be predictive of complete cytogenetic response, MMR, and improved event-free survival in imatinib-resistant or -intolerant patients with CML-CP.17
Using data from a large, randomized clinical trial comparing frontline nilotinib vs imatinib, we confirmed the strong predictive value of 3-month BCR-ABL levels in patients with newly diagnosed CML-CP treated with nilotinib. Importantly, in landmark analyses of this type, patients are excluded if they achieve the target responses (eg, MMR or MR4.5) before the specified landmarks. Thus, the superior rates of molecular response on nilotinib compared with imatinib by each landmark time point were not captured by these results.
The percentage of patients with EMR failure was far lower in the nilotinib arms than in the imatinib arm. However, for this smaller subset of patients, the outlook was equally poor. Rates of molecular response, PFS, and OS were all lower in patients with EMR failure than in patients who achieved EMR. Patients with EMR failure had virtually no prospect of achieving MR4.5 (which is associated with improved long-term outcomes18-21 and is a key eligibility criterion for studies of treatment-free remission22-25 ) by 4 years and had a substantial risk of progression. These results support the notion that BCR-ABLIS >10% at 3 months is an important predictor of treatment failure on nilotinib as well as on imatinib.
Although achievement of EMR is determined after randomization and cannot be isolated from other confounding factors, the comparative nature of the randomized trial allows us to make inferences about the relationship between EMR and improved long-term outcomes that were not possible in prior studies that focused only on imatinib-treated patients. A substantially higher percentage of patients achieved EMR on nilotinib compared with imatinib, yet patients achieving EMR on either treatment had equally favorable prognoses; these results suggest that achieving EMR actually drives down the risk of progression, rather than simply being a marker of favorable disease biology.
Recent results from the Therapeutic Intensification in De Novo Leukemia (TIDEL) II trial showed that patients with BCR-ABLIS >10% at 3 months on imatinib had increased risk of progression to BC and low rates of MMR and MR4.5 by 1 and 2 years, despite either having their imatinib dose escalated or switching to nilotinib after 3 months.26 In ENESTnd, nearly half of all progressions on study in patients with EMR failure occurred between 3 and 6 months of treatment. Taken together, these observations suggest that for any modification in therapy to have a meaningful impact on the rate of progression in patients with EMR failure, it needs to be initiated without delay. However, simply switching to a more potent TKI may not be an adequate intervention. More innovative strategies, such as combining TKI therapy with inhibition of another key signaling pathway, need to be investigated in this high-risk group.
In ENESTnd, rates of EMR failure at 3 months were highest among patients with high Sokal risk scores at baseline; however, EMR failures were observed in all risk groups. Patients with high Sokal risk scores were much more likely to achieve EMR on nilotinib than imatinib, with EMR failure observed in more than half (56%) of patients with high Sokal risk scores in the imatinib arm vs 14% and 18% in the nilotinib arms. Rates of EMR failure were also lower on nilotinib than imatinib among patients with low and intermediate Sokal risk scores. In all treatment arms, progressions after EMR failure were observed in patients with high and intermediate/low Sokal risk scores; our data did not provide sufficient evidence for further refinement of EMR risk categories based on Sokal risk score.
In all 3 treatment arms, EMR failure at 3 months was associated with larger spleen size but appeared more strongly related to dose reductions or interruptions in the nilotinib arms than in the imatinib arm. Patients on nilotinib with EMR failure had median dose intensities well below the planned dose during their first 3 months of treatment, and more than half of these patients had a dose interruption lasting ≥5 days. This suggests that suboptimal dosing and dose interruptions played a key causative role in EMR failure in patients treated with nilotinib. In the imatinib arm, median dose intensity did not differ among patients who did or did not achieve EMR, suggesting that treatment exposure was not the dominant factor in patients receiving imatinib. In the imatinib arm, we also observed a trend toward fewer progressions in patients who had a significant dose interruption; however, these results were limited by small sample size. The impact of early dose interruptions on progression risk following EMR failure warrants further exploration with a larger data set.
Our results suggest that EMR failure at 3 and 6 months are similarly predictive of poor long-term outcomes in patients treated with frontline nilotinib or imatinib. However, rates of EMR failure were lower at 6 months than at 3 months, and the number of patients with EMR failure at 6 months, particularly in the nilotinib arms, was small. Importantly, many of the progression events on study occurred between 3 and 6 months of treatment, suggesting a clear advantage for use of the earlier time point as a clinically relevant molecular landmark for prediction of progression. Because patients in ENESTnd with EMR failure were kept on the same treatment, our results cannot ascertain the relative value of switching patients with EMR failure at either time point to alternate therapies.
In summary, this landmark analysis of ENESTnd confirmed that achievement of EMR is predictive of future clinical outcomes in patients with newly diagnosed CML-CP treated with frontline nilotinib or imatinib. Patients with EMR failure had lower rates of molecular response, PFS, and OS compared with patients who achieved EMR. More patients achieved EMR on nilotinib than on imatinib, and with 4 years of follow up, nilotinib continues to show higher rates of molecular response and lower rates of progression to AP/BC compared with imatinib. These results suggest that treatment with frontline nilotinib may allow more patients with Ph+ CML-CP to achieve early, deep molecular responses and attain improved long-term outcomes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Albert Hoenekopp (formerly of Novartis Pharma AG) for ongoing medical and clinical support. We also thank Karen Miller-Moslin, Pamela Tuttle, and Karen Kaluza (Articulate Science, LLC) for medical editorial assistance with this manuscript.
The ENESTnd study and work presented here were sponsored and funded by Novartis Pharmaceuticals Corporation. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: T.P.H., H.M.K., and A.H. designed and performed research; H.M.K., D.N., C.N., C.A.D.S., S.M., R.A.L., and A.H. collected and assembled data; T.P.H., G.S., H.M.K., F.G., G.R., C.A.D.S., M.E.K., X.F., H.D.M., R.A.L., and A.H. analyzed and interpreted data; T.P.H., H.M.K., C.A.D.S., M.E.K., and R.A.L. provided study materials; and all authors drafted/approved the manuscript.
Conflict-of-interest disclosure: T.P.H. acted as a consultant and received honoraria from Novartis, Bristol-Myers Squibb, and Ariad and received research funding from Novartis, Bristol-Myers Squibb, and CLS Pharmaceuticals. G.S. received honoraria from Novartis, Bristol-Myers Squibb, Pfizer, and Ariad. H.M.K. acted as a consultant for Novartis and received research funding from Novartis, Bristol-Myers Squibb, Sanofi-Aventis, Amgen, Ariad, and Pfizer. F.G. received honoraria from Novartis and Bristol-Myers Squibb and research funding from Novartis. D.N. received honoraria from Novartis. G.R. acted as a consultant for Novartis and Bristol-Myers Squibb; received honoraria from Novartis, Bristol-Myers Squibb, and Pfizer; and received research funding from Novartis. C.N. received research funding from Novartis. C.A.D.S. received research funding from the University of Campinas-Brazil. M.E.K. received research funding from Novartis. S.M., X.F., and H.D.M. are employees of Novartis. R.A.L. acted as a consultant and received research funding from Novartis. A.H. acted as a consultant for and received honoraria and research funding from Novartis, Bristol-Myers Squibb, Pfizer, and Ariad.
Correspondence: Timothy P. Hughes, Department of Haematology, SA Pathology, PO Box 14 Rundle Mall, Adelaide, SA 5000, Australia; e-mail: timothy.hughes@health.sa.gov.au.