Key Points
Inhibition of Bruton’s tyrosine kinase is as effective in vitro against AML as chronic lymphocytic leukemia.
Ibrutinib shows activity in AML because Bruton’s tyrosine kinase is constitutively active.
Abstract
Bruton’s tyrosine kinase (BTK) is a cytoplasmic protein found in all hematopoietic cell lineages except for T cells. BTK mediates signaling downstream of a number of receptors. Pharmacologic targeting of BTK using ibrutinib (previously PCI-32765) has recently shown encouraging clinical activity in a range of lymphoid malignancies. This study reports for the first time that ibrutinib inhibits blast proliferation from human acute myeloid leukemia (AML) and that treatment with ibrutinib significantly augmented cytotoxic activities of standard AML chemotherapy cytarabine or daunorubicin. Here we describe that BTK is constitutively phosphorylated in the majority of AML samples tested, with BTK phosphorylation correlating highly with the cell’s cytotoxic sensitivity toward ibrutinib. BTK-targeted RNAi knockdown reduced colony-forming capacity of primary AML blasts and proliferation of AML cell lines. We showed that ibrutinib binds at nanomolar range to BTK. Furthermore, we showed ibrutinib’s antiproliferative effects in AML are mediated via an inhibitory effect on downstream nuclear factor-κB survival pathways. Moreover, ibrutinib inhibited AML cell adhesion to bone marrow stroma. Furthermore, these effects of ibrutinib in AML were seen at comparable concentrations efficacious in chronic lymphocytic leukemia. These results provide a biological rationale for clinical evaluation of BTK inhibition in AML patients.
Introduction
Acute myeloid leukemia (AML) is primarily a disease of the elderly.1 In younger patients (<65 years, median diagnosis 72 years), there is improved survival over the decades; however, older patients have seen no similar improvement, with intensive cytotoxic treatment being a dilemma.2 AML is composed of a heterogenous group of tumors. Despite this diversity, AML relies on common programs of self-renewal downstream of the driver oncogenes, suggesting that disease is caused by only a few mutations,3 and mechanistically common therapeutic approaches are broadly useful despite oncogenic involvement.4
Tyrosine kinases (TK) are attractive drugable targets in cancer. In AML, TK-activating mutations occur in 50% of patients.5,6 Furthermore TK-dependent cell survival pathways are dysregulated in most cases.7-10 Bruton’s TK (BTK) has been identified as functionally important in malignant hematopoietic cells. BTK was originally identified functionally in B-cell receptor signaling, with mutations blocking B-cell development.11-13 Other receptors (including Toll-like receptors [TLRs]) are BTK dependent. Recent phase 1 and 2 studies of the irreversible BTK inhibitor ibrutinib have demonstrated promising activity and tolerability against B-cell malignancies including chronic lymphocytic leukemia (CLL), mantle cell lymphoma, hairy cell leukemia, multiple myeloma, and diffuse large B-cell lymphoma.14-20 In addition to lymphoid cells, BTK expression has also been found in hematopoietic stem cells (HSCs), multipotent progenitors, and several other hematopoietic cells including erythroid and megakaryocytic cells.21 Furthermore, it is known that BTK deficiency/inhibition affects myeloid cells including macrophage lipopolysaccharide/TLR-induced tumor necrosis factor (TNF) production,22 dendritic cell function via interleukin (IL)-10 and Stat3,23 neutrophil development,24,25 and collagen-induced platelet aggregation.26,27 Moreover, high BTK phosphorylation/expression are observed in AML.28,29 Here we explain BTK function in human AML and describe the pharmacologic effects of BTK inhibition by ibrutinib on AML proliferation and bone marrow (BM) adhesion.
Methods
Materials
AML-derived cell lines were obtained from European Collection of Cell Cultures and authenticated by DNA fingerprinting. They were used at low passage for 6 months maximum post resuscitation, with regular Mycoplasma testing. Anti–nuclear factor-κB (NF-κB)/protein kinase B (AKT) and total-BTK [D3H5] antibodies were obtained from Cell Signaling Technologies. The phospho (p)-Y223-BTK [EP420Y] and p-Y551-BTK [EP267Y] antibodies were obtained from Abcam. Other antibodies were obtained from Santa Cruz Biotechnology. Ibrutinib was obtained from Selleck Chemicals. Stem cell factor (SCF), IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and TNF were obtained from Invitrogen. All other reagents were obtained from Sigma-Aldrich, unless indicated otherwise.
Cell culture
AML cells were obtained from patients’ BM or blood after informed consent was given in accordance with the Declaration of Helsinki and under approval from the United Kingdom National Research Ethics Service (LRECref07/H0310/146). For primary cell isolation, heparinized blood was collected from volunteers, and human peripheral blood mononuclear cells were isolated by histopaque density-gradient centrifugation. AML samples with >80% blasts were purified using CD34+ selection kit (Miltenyi Biotec) (denoted by an asterisk in supplemental Table 1, available on the Blood Web site). We obtained hematopoietic CD34+ cells from 2 sources: Stem Cell Technologies and volunteer donors. For all CD34+ experiments, at least 3 different donors were used to obtain the results presented. Cell type was confirmed by microscopy and flow cytometry.
Human BM stromal cells (BMSCs) were isolated from AML patient BM aspirates. Mononuclear cells were collected by gradient centrifugation and plated in growth media. Nonadherent cells were removed after 2 days. When they were 60% to 80% confluent, adherent cells were trypsinized and expanded for 3 to 5 weeks. BMSCs were checked for expression of CD105, CD73, CD90, and lack of CD45 and CD34 expression.30,31
Real-time polymerase chain reaction
Total RNA was extracted from 5 × 105 cells using the Nucleic Acid PrepStation. Reverse transcription was performed using an RNA polymerase chain reaction (PCR) core-kit (Applied Biosystems). Relative quantitative real-time (qRT)-PCR used SYB-green technology (Roche) on generated complementary DNA. After preamplification (95°C/60 seconds), PCRs were amplified for 45 cycles (95°C/15 seconds, 60°C/10 seconds, 72°C/10 seconds) on a Roche 384-well LightCycler480. Messenger RNA (mRNA) expression was normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Western blot and NF-κB–binding assays
Immunocytochemistry
Primary CD34+ cells, AML lines, and primary samples were fixed, permeabilized, and blocked with goat serum. Samples were stained with primary antibodies against BTK or phosphorylated Y223-BTK (Cell Signaling Technology) and visualized with secondary Alexa Fluor 568- or 488-conjugated immunoglobulin G (heavy and light chain-specific) (Invitrogen), respectively. Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole before samples were mounted with Fluoromount aqueous mounting medium. Cells were imaged by an AxioCam ICm1 monochrome CCD camera attached to the Apotome.2 Imaging System using Axiovision 4.8.2 software (Carl Zeiss). Image staining intensities were analyzed with ImageJ software (n = 20 per sample).
Proliferation/death assays
Cells were treated with different doses of ibrutinib and then viable numbers measured with Cell-Titer GLO (Promega). For prosurvival assays, cells were cultured in serum-free media as described,34 with indicated ibrutinib concentrations and cytokines, and then measured for proliferative activity using a Cell-Titer GLO or bromodeoxyuridine proliferation assay (Cell Signaling). Apoptosis measurements were performed by Accuri-C6 flow cytometry (BD Biosciences) with Annexin-V/propidium iodide (PI) (Abcam). For AML-BMSC cocultures, AML cell viability was measured by flow cytometry with BMSC forward-scatter exclusion gating.
Virus construction and infection
MicroRNA (miRNA) sequences miRNA-BTK437 (5′-TTCACTGGACTCTTCACCTCT-3′) and miRNA-BTK1092 (5′-TGACAATGAAACCTCCTTCTT-3′) targeting human BTK were selected with Invitrogen Block-iT RNAi designer software (www.invitrogen.com/rnai) and plasmid pcDNATM6.2-GW/EmGFP-miR-neg (Invitrogen) was used as a negative control. MiRNA-encoding sequences were cloned into a Block-iT Pol II miR-RNAi vector (Invitrogen) then EmGFP–pre-miRNA fragments were subcloned into the BamHI/XhoI site of LNT/SffvMCS (gift from Penny Powell, University of East Anglia, Norwich, United Kingdom). MiRNA-encoding viruses were produced as described,35 using packaging plasmids pCMVΔR8.91 (expressing gag-pol) and pMD.G (expressing VSV-G) (provided by Ariberto Fassati, University College, London, United Kingdom). Lentiviral stocks were concentrated using Lenti-X Concentrator (Clontech) and titers were obtained with a Lenti-X qRT-PCR Titration kit (Clontech). For transduction, cells plated onto 12-well plates (5 × 104 cells/well per 0.5 mL) were infected with lentiviral stocks (multiplicity of infection of 15) with 8 μg/mL Polybrene.
Clonogenic methylcellulose assays
Control CD34+ HSCs, AML cell lines, and primary AML cells (1-50 × 103 cells) were plated in methylcellulose (R&D). Colonies were visualized and counted after 10 to 15 days.
BMSC-AML cell adhesion assay
BMSCs were grown in 96-well plates. AML cell lines and primary cells were incubated for 1 hour with 2.5 µM calcein-AM. Fluorescently-labeled AML cells were added into BMSCs and incubated for the indicated times. Gentle washing removed nonadherent calcein-labeled cells. Adherent cells were quantitated with a fluorescence plate reader.
PCR gene array
CD34+ control cells, AML cell lines, and primary cells were pretreated with ±5 µM ibrutinib (16 hours). Resultant complementary DNA was incubated on an NF-κB Signaling Pathway RT2 qPCR array (SABiosciences, United Kingdom) containing 84 key NF-κB genes normalized to GAPDH expression.
BTK occupancy assay
Primary AML cells and U937 were treated with increasing concentrations of ibrutinib (0.3-1000 nM) for 1 hour. Cells were then washed in phosphate-buffered saline and stored at −80°C until a BTK occupancy assay was performed as described.36
Statistics
Student t test was performed, with P < .05 considered statistically significant (*). Results represent means ± standard deviation (SD) of 3 independent experiments. Western blotting data are representative of 3 independent experiments.
Results
BTK is expressed and constitutively phosphorylated in AML
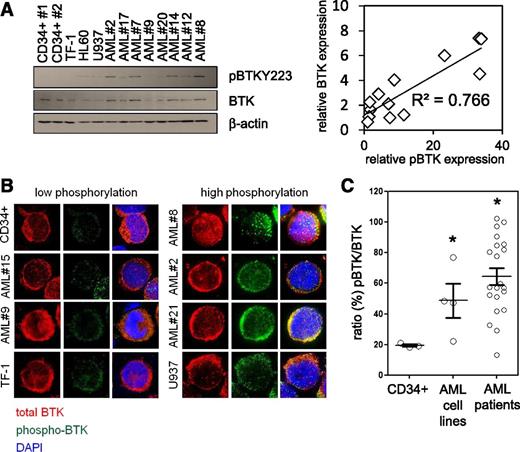
It has been recently reported that the nonreceptor TKs LYN and SYK play an important role in normal B-cell differentiation and hematopoietic signaling and are potential targets for AML therapy.37,38 Because both LYN and SYK are known to activate BTK in hematologic cells39,40 and BTK mRNA had previously been reported to be expressed in AML,28 we examined basal activity levels of BTK in human AML. We determined the level of BTK expression in human primary AML blasts comparing them with normal CD34+ HSC. RT-PCR assessment of BTK mRNA in 25 primary human AML patients and 3 AML cell lines showed BTK expressed at comparable levels with that of control CD34+ HSCs (not shown). Furthermore, using phosphorylation at Y223 as a marker of BTK activation (Figure 1A), we identified a significant correlation between p-BTK and total-BTK expression in AML samples. Immunocytochemical analysis showed a median 3.1-fold greater p-BTK activity in primary AML cells than in comparator nonmalignant CD34+ cells (Figure 1B-C). We also tested phosphorylation at Y551 as a marker of BTK activity. Although we could not detect a signal by western blotting analysis, immunocytochemical analysis of AML cell lines showed similar results to those of the Y223 probe (not shown). These observations show that BTK is ubiquitously expressed in AML, with increased activity (as measured by phosphorylation relative to nonmalignant CD34+ cells) in >90% of the primary AML patient samples tested. Together this implies that BTK is functionally significant in human AML.
BTK is highly expressed and constitutively phosphorylated in AML. (A) Control CD34+ cells, AML patient cells, and AML cell lines measured for constitutive levels of BTK phosphorylation by western blotting analysis, blots re-probed for wild-type BTK and β-actin to show BTK expression, and sample loading, respectively. Correlation analysis of p-BTK/BTK expression using densitometry is shown. (B) Primary CD34+ cells, AML cells, and AML cell lines were analyzed for phosphorylated BTK (Y223) (green) and total BTK (red) by immunocytochemistry. 4′,6-diamidino-2-phenylindole nuclear stain is shown in blue. (C) Using the immunocytochemical images captured, p-BTK was calculated as a percentage of total BTK. Values indicate the mean ± standard error of the mean from at least 5 individual experiments, sampling at least 10 representative cells from each view. *Statistical significance of P < .05 between the different treatment groups using Student t test.
BTK is highly expressed and constitutively phosphorylated in AML. (A) Control CD34+ cells, AML patient cells, and AML cell lines measured for constitutive levels of BTK phosphorylation by western blotting analysis, blots re-probed for wild-type BTK and β-actin to show BTK expression, and sample loading, respectively. Correlation analysis of p-BTK/BTK expression using densitometry is shown. (B) Primary CD34+ cells, AML cells, and AML cell lines were analyzed for phosphorylated BTK (Y223) (green) and total BTK (red) by immunocytochemistry. 4′,6-diamidino-2-phenylindole nuclear stain is shown in blue. (C) Using the immunocytochemical images captured, p-BTK was calculated as a percentage of total BTK. Values indicate the mean ± standard error of the mean from at least 5 individual experiments, sampling at least 10 representative cells from each view. *Statistical significance of P < .05 between the different treatment groups using Student t test.
Pharmacologic inhibition of BTK in primary AML blasts
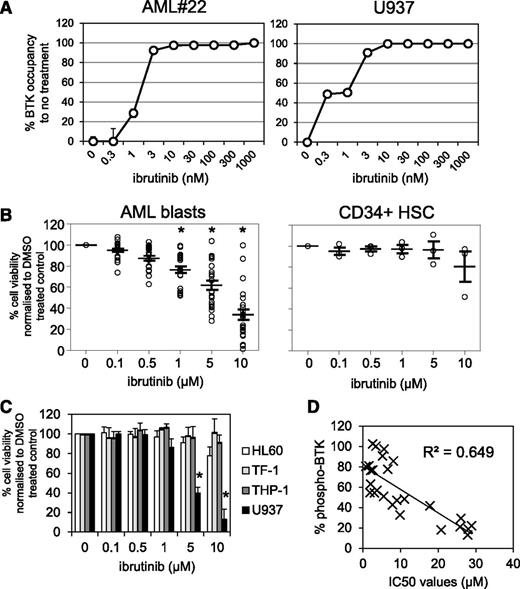
Because the irreversible BTK inhibitor ibrutinib has been shown to inhibit proliferation in vitro in CLL, MCL, and multiple myeloma,20,41,42 we tested whether BTK inhibition would reduce cell viability in primary AML blasts. First we established the level of occupancy of the BTK active site by ibrutinib in AML cells. To do this, we treated primary AML sample #22 and AML cell line U937 with increasing concentrations of ibrutinib (3-1000 nM) for 1 hour. Using the fluorescently-tagged ibrutinib derivative PCI-33380,36 we found that 10 nM ibrutinib was sufficient to fully occupy BTK in both AML #22 and U937 cells (Figure 2A). Half maximal effective concentrations between 0.3 and 3 nM were observed in AML cells, which is comparable with the BTK occupancy data in CLL.36 Next we tested the in vitro activity of ibrutinib in primary AML cells from a broad age range of adult patients (26-92 years) and across a spectrum of World Health Organization AML subclasses (supplemental Table 1). AML cells from 25 patients were treated with increasing concentrations of ibrutinib for 72 hours and compared with nonmalignant CD34+ cells. We found that ibrutinib exhibited concentration-dependent cytotoxicity in AML patient cells (Figure 2B). IC50s were calculated for all AML patient samples, with AML cells exhibiting greater sensitivity to ibrutinib than comparator nonmalignant CD34+ cells (supplemental Tables 1 and 2). Separate Annexin-V/PI staining found 1 to 5 µM ibrutinib-induced AML apoptosis (supplemental Figure 1). Examination of the viability of 4 AML cell lines showed that only U937 had significant cytotoxic response to ibrutinib (IC50 ∼2.6 µM) (Figure 2C and supplemental Table 2). Correlation analysis of BTK phosphorylation (Figure 1C) and ibrutinib IC50s (supplemental Tables 1 and 2) showed a high correlation between high p-BTK and sensitivity toward ibrutinib (Figure 2D). As a control/comparator, we tested ibrutinib cytotoxicity in 6 primary human CLL samples that showed comparable sensitivity (IC50s ∼5 µM) with that achieved in AML (supplemental Figure 2) and that were compatible with in vitro CLL data previously published (cytotoxic IC50s 5-50 µM).43
Pharmacologic inhibition of BTK in primary AML blasts. (A) AML blasts and the AML cell line U937 were treated with increasing doses of ibrutinib for 1 hour and then assayed for occupancy of the BTK active site. (B) AML blasts and CD34+ control cells were treated with increasing doses of ibrutinib (0.1-10 µM) for 72 hours and then assessed by Cell TitreGlo. Data were normalized to dimethyl sulfoxide (DMSO)-treated cells and represent the mean ± SD of 3 experiments. (C) AML cell lines were treated with increasing doses of ibrutinib (0.1-10 µM) for 72 hours and then assessed by Cell TitreGlo. Data were normalized to DMSO-treated cells and represent the mean ± SD of 3 experiments. (D) Correlation analysis of 50% inhibitory concentration (IC50) values of AML blasts treated with ibrutinib and percent of BTK phosphorylation.
Pharmacologic inhibition of BTK in primary AML blasts. (A) AML blasts and the AML cell line U937 were treated with increasing doses of ibrutinib for 1 hour and then assayed for occupancy of the BTK active site. (B) AML blasts and CD34+ control cells were treated with increasing doses of ibrutinib (0.1-10 µM) for 72 hours and then assessed by Cell TitreGlo. Data were normalized to dimethyl sulfoxide (DMSO)-treated cells and represent the mean ± SD of 3 experiments. (C) AML cell lines were treated with increasing doses of ibrutinib (0.1-10 µM) for 72 hours and then assessed by Cell TitreGlo. Data were normalized to DMSO-treated cells and represent the mean ± SD of 3 experiments. (D) Correlation analysis of 50% inhibitory concentration (IC50) values of AML blasts treated with ibrutinib and percent of BTK phosphorylation.
Ibrutinib inhibits AML proliferation
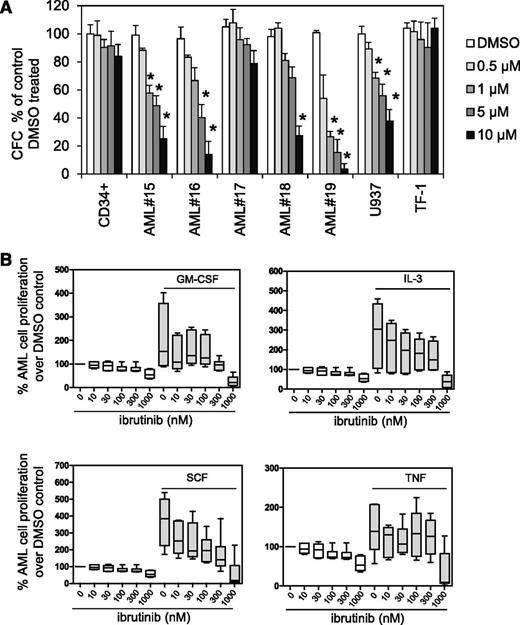
We explored ibrutinib’s effect on AML blast colony formation in methylcellulose compared with normal CD34+ myeloid progenitor cells. We assayed colony formation across a range of AML samples that are both sensitive and resistant to ibrutinib in assays (supplemental Table 1). We found in AML samples and U937 cells that ibrutinib inhibited colony-forming cellular proliferation (Figure 3A). Because ibrutinib was shown to inhibit prosurvival signals derived from the CLL microenvironment, we examined ibrutinib’s effects on AML prosurvival signals.34,43,44 AML cells were pretreated with ibrutinib (10-1000 nM) for 1 hour and then cultured with IL-3, GM-CSF, SCF, or TNF for 72 hours. Ibrutinib inhibited proliferative responses to exogenous IL-3, GM-CSF, and SCF, but not TNF (Figure 3B). Similarly, a bromodeoxyuridine assay measured AML cell proliferation with 0.5 μM ibrutinib pretreatment before the addition of IL-3, GM-CSF, SCF, or TNF (supplemental Figure 3), also showing that ibrutinib pretreatment inhibited proliferative responses to IL-3, GM-CSF, and SCF, but not TNF.
Ibrutinib inhibits AML proliferation. (A) AML blasts, AML cell lines, and CD34+ control cells were treated with 0.5, 1, 5, and 10 µM ibrutinib, and colony-forming assays were performed to show the number of colonies or colony-forming cells (CFC). Data were normalized to DMSO-treated cells. (B) Primary AML blasts (n = 6) were pretreated with increasing doses of ibrutinib (0.01-1 µM) for 1 hour and then treated with GM-CSF (10 ng/mL), IL-3 (10 ng/mL), SCF (50 ng/mL), or TNF (10 ng/mL) for 72 hours and then assessed by Cell TitreGlo. Data were normalized to DMSO-treated cells.
Ibrutinib inhibits AML proliferation. (A) AML blasts, AML cell lines, and CD34+ control cells were treated with 0.5, 1, 5, and 10 µM ibrutinib, and colony-forming assays were performed to show the number of colonies or colony-forming cells (CFC). Data were normalized to DMSO-treated cells. (B) Primary AML blasts (n = 6) were pretreated with increasing doses of ibrutinib (0.01-1 µM) for 1 hour and then treated with GM-CSF (10 ng/mL), IL-3 (10 ng/mL), SCF (50 ng/mL), or TNF (10 ng/mL) for 72 hours and then assessed by Cell TitreGlo. Data were normalized to DMSO-treated cells.
Genetic inhibition of BTK reduces AML cell colony formation
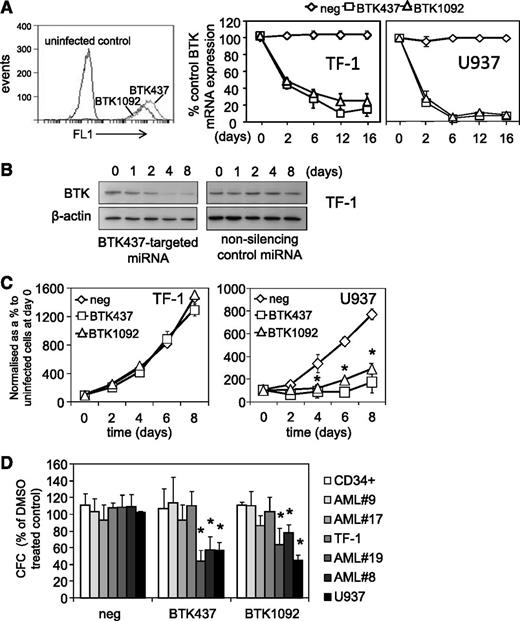
Genetic inhibition of BTK in AML cell lines and AML blasts was achieved by lentiviral-mediated long-term BTK knockdown using targeted artificial miRNA (BTK-targeted miRNA) and visualization of infected cells via a concurrently expressed GFP signal. These constructs induced GFP expression and BTK knockdown for up to 16 days (Figure 4A-B). The role of BTK in cell viability clonogenicity was assessed. Introduction of BTK-targeted miRNA dramatically inhibited U937 proliferation but had no effect on TF-1 cells (Figure 4C), which show control levels of p-BTK activity. With BTK-miRNA–targeted knockdown, we observed reduced methylcellulose colony formation in high p-BTK–expressing primary AML blasts and U937 (3/3 samples tested), but not in low p-BTK–expressing AML blasts and TF-1 (3/3 samples tested) or nonmalignant CD34+ HSCs, compared with control miRNA–targeted cells (Figure 4D). This suggests that p-BTK plays a central role in AML proliferation and maintenance.
Genetic inhibition of BTK inhibits cell proliferation in AML cells. AML cell lines (TF-1 and U937) were transduced with BTK-targeted miRNA GFP-tagged lentiviral constructs. (A) Transfected cells were measured for GFP expression using flow cytometry for 2 BTK-targeted miRNA (BTK437 and BTK1092) in TF-1 cells. RNA was extracted from TF-1 and U937 cells transduced with BTK-targeted and nonsilencing miRNA control constructs and examined for BTK expression by RT-PCR at the indicated times. mRNA expression was normalized to GAPDH mRNA levels. (B) Protein extracts were also obtained and western blot analysis was conducted for p-BTK and BTK protein levels. (C) TF-1 and U937 were transduced with either BTK-targeted miRNA or nonsilencing control miRNA construct for 72 hours. Cell number was assessed by Cell TitreGlo assay. (D) AML blasts, AML cell lines, and CD34+ HSCs were transduced with BTK-targeted miRNA and control miRNA constructs, and colony-forming assays were performed to show the number of colonies detected. In all panels, the values indicate the mean ± SD from 3 independent experiments. *Statistical significance of P < .05 between the different treatment groups.
Genetic inhibition of BTK inhibits cell proliferation in AML cells. AML cell lines (TF-1 and U937) were transduced with BTK-targeted miRNA GFP-tagged lentiviral constructs. (A) Transfected cells were measured for GFP expression using flow cytometry for 2 BTK-targeted miRNA (BTK437 and BTK1092) in TF-1 cells. RNA was extracted from TF-1 and U937 cells transduced with BTK-targeted and nonsilencing miRNA control constructs and examined for BTK expression by RT-PCR at the indicated times. mRNA expression was normalized to GAPDH mRNA levels. (B) Protein extracts were also obtained and western blot analysis was conducted for p-BTK and BTK protein levels. (C) TF-1 and U937 were transduced with either BTK-targeted miRNA or nonsilencing control miRNA construct for 72 hours. Cell number was assessed by Cell TitreGlo assay. (D) AML blasts, AML cell lines, and CD34+ HSCs were transduced with BTK-targeted miRNA and control miRNA constructs, and colony-forming assays were performed to show the number of colonies detected. In all panels, the values indicate the mean ± SD from 3 independent experiments. *Statistical significance of P < .05 between the different treatment groups.
Ibrutinib inhibits AML NF-κB survival genes
We and others previously reported that BTK is involved in p65-mediated transactivation during NF-κB activation in macrophages and malignant plasma cells.14,45 Because p65 phosphorylation is necessary for the induction of NF-κB/p65-dependent gene expression in other hematologic cells,9 we determined whether a similar role for BTK exists in AML. We used a PCR-based NF-κB gene expression array to examine the expression of 84 NF-κB genes from control CD34+ HSCs, primary AML cells, and AML cell lines, treated with 5 µM ibrutinib for 16 hours. We found that ibrutinib dramatically reduced the expression of NF-κB target genes from AML patient cells with high BTK phosphorylation but not CD34+ HSCs and low BTK phosphorylation–level AML cells (supplemental Figure 4A). A similar pattern of genes regulation was observed in U937 and TF-1, in which BTK was knocked down by BTK-targeted miRNA (supplemental Figure 5). To validate PCR NF-κB array results, we examined the expression of TNF, FLICE (caspase-8)-inhibitory protein, AKT, and NF-κB1 (which the array highlighted as BTK dependent) by qRT-PCR. This analysis confirmed that initial observations that decreased NF-κB survival genes and NF-κB transcription factors are seen in response to ibrutinib (supplemental Figure 4B). Thus BTK expression or activity regulates selective known NF-κB target survival genes.
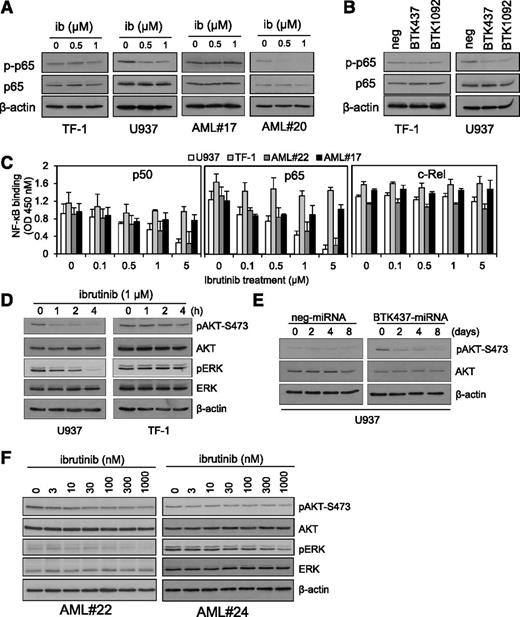
To further verify the involvement of NF-κB, and specifically p65, we treated AML cells with ibrutinib and examined p65 phosphorylation. In primary AML blasts and cell lines, ibrutinib was found to inhibit p-p65 expression in high p-BTK cells (2/2 samples tested) but not in low p-BTK expression AML cells (2/2 samples tested) (Figure 5A). This observation was confirmed on AML cell lines U937 and TF-1 with BTK knockdown (Figure 5B). To determine whether BTK inhibition had an effect on NF-κB nuclear activity, we examined p50, p65, and c-Rel binding activities in AML blasts and cell lines. Ibrutinib significantly inhibited p50 and p65 κB binding activity but not c-Rel (Figure 5C). Taken together, these results confirm that BTK is involved in p65-mediated transactivation during NF-κB activation in human AML.
AKT, ERK, and NF-κB activity in AML cells is augmented by BTK inhibition. (A) AML cell lines and AML blasts were treated with 0.5 and 1 µM of ibrutinib for 8 hours and then whole-cell extracts were prepared and western blot analysis was conducted for p-p65, p65, and β-actin protein levels. (B) AML cell lines (TF-1 and U937) were transduced with BTK-targeted miRNA GFP-tagged lentiviral constructs (BTK437 and BTK1092) as well as negative control. Protein extracts were also obtained and western blot analysis was conducted for p-p65 and β-actin protein levels. (C) AML cell lines and AML blasts were treated with 0.1 to 5 µM of ibrutinib for 8 hours, and nuclear extracts were prepared and an NF-κB binding assay was performed for p50, p65, and c-Rel. (D) AML cell lines were treated with 1 µM ibrutinib for various times and then whole cell extracts were obtained. Western blot analysis was conducted for p-AKT-S473, total AKT, p-ERK and total ERK, and β-actin protein levels. (E) U937 lines were transduced with miRNA GFP-tagged lentiviral constructs (Neg-miRNA and BTK437-miRNA) for up to 8 days. Whole-cell extracts were prepared and western blot analysis was conducted for p-AKT-S473, total AKT, and β-actin protein levels. (F) Primary AML cell lines (AML #22 and AML #24) were treated with 3 to 1000 nM of ibrutinib for 8 hours. Whole-cell extracts were prepared and western blot analysis was conducted for p-AKT-S473, total AKT, p-ERK and total ERK, and β-actin protein levels.
AKT, ERK, and NF-κB activity in AML cells is augmented by BTK inhibition. (A) AML cell lines and AML blasts were treated with 0.5 and 1 µM of ibrutinib for 8 hours and then whole-cell extracts were prepared and western blot analysis was conducted for p-p65, p65, and β-actin protein levels. (B) AML cell lines (TF-1 and U937) were transduced with BTK-targeted miRNA GFP-tagged lentiviral constructs (BTK437 and BTK1092) as well as negative control. Protein extracts were also obtained and western blot analysis was conducted for p-p65 and β-actin protein levels. (C) AML cell lines and AML blasts were treated with 0.1 to 5 µM of ibrutinib for 8 hours, and nuclear extracts were prepared and an NF-κB binding assay was performed for p50, p65, and c-Rel. (D) AML cell lines were treated with 1 µM ibrutinib for various times and then whole cell extracts were obtained. Western blot analysis was conducted for p-AKT-S473, total AKT, p-ERK and total ERK, and β-actin protein levels. (E) U937 lines were transduced with miRNA GFP-tagged lentiviral constructs (Neg-miRNA and BTK437-miRNA) for up to 8 days. Whole-cell extracts were prepared and western blot analysis was conducted for p-AKT-S473, total AKT, and β-actin protein levels. (F) Primary AML cell lines (AML #22 and AML #24) were treated with 3 to 1000 nM of ibrutinib for 8 hours. Whole-cell extracts were prepared and western blot analysis was conducted for p-AKT-S473, total AKT, p-ERK and total ERK, and β-actin protein levels.
Silencing BTK inhibits AKT pathway in AML cells
The phosphoinositide 3-kinase (PI3K)/AKT pathway is frequently activated in AML.46,47 AKT phosphorylation on Ser473 can be detected in 50% to 80% of AML patients.48,49 Mechanisms leading to PI3K/AKT activation in AML are unclear. The p110δ isoform of class IA PI3K is always expressed in AML cells, whereas p110α and p110β isoforms are heterogeneously expressed, with the frequency of p110γ isoforms unknown.50,51 Moreover, inhibition of BTK by ibrutinib has been shown to inhibit AKT phosphorylation in CLL and MCL.43,52
We hypothesized that BTK was upstream of PI3K/AKT. To verify the role of BTK in constitutively-active PI3K/AKT, we treated U937 and TF-1 cells with 1 µM ibrutinib for various times and then analyzed extracts for ser473 p-AKT. Because ERK signaling was also inhibited by ibrutinib, we also examined p-ERK. Figure 5D shows that expression of p-AKT and p-ERK in U937, but not TF-1, is reduced by ibrutinib when compared with total AKT, total ERK, and β-actin. To validate the results obtained with ibrutinib, we also analyzed p-AKT in U937 with BTK437 knockdown. This showed that U937 treated with BTK437 knockdown had significantly reduced p-AKT (Figure 5E). To further validate these observations, we examined p-AKT and p-ERK levels in primary AML in response to increasing concentrations of ibrutinib (3-1000 nM). Figure 5F shows that low concentrations of ibrutinib inhibit p-AKT and p-ERK in primary AML (supplemental Figure 6). Moreover, to determine whether ibrutinib had off-target effects at concentrations of between 30 nM and 10 000 nM, we examined p38MAPK activity in AML cells, because ibrutinib does not directly inhibit p38 activity.53 Ibrutinib had no effect on p-p38 in AML cells (supplemental Figure 7A-B). These data place BTK upstream of constitutively active PI3K/AKT and p-ERK signaling in human AML.
BTK inhibition enhances conventional chemotherapy’s effects in AML
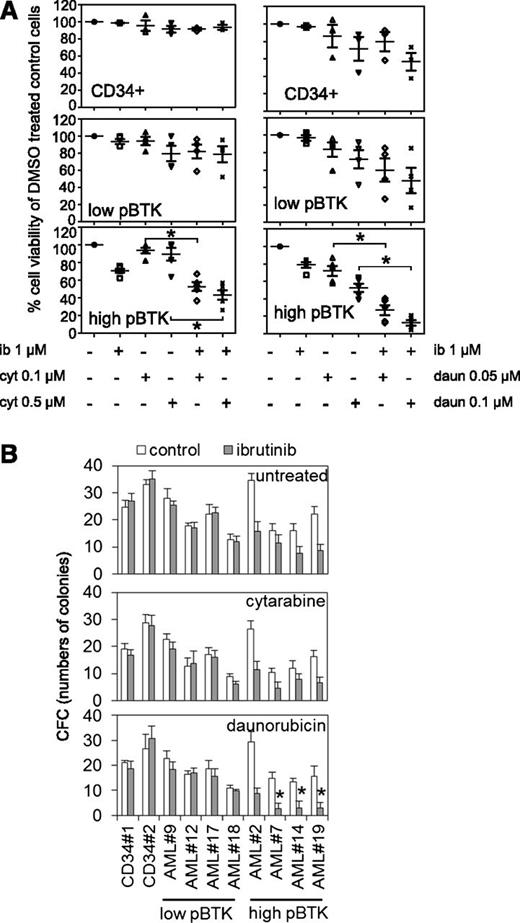
Chemotherapeutics frequently act synergistically and are often used clinically in combination. Here we determined whether ibrutinib could act synergistically with either cytarabine or daunorubicin, 2 widely used AML front-line treatments. In AML patients’ cells and cell lines, ibrutinib significantly increased cytotoxic responses, in combination with cytarabine (0.1-0.5 μM) or daunorubicin (0.05-0.1 μM) in high p-BTK AML cells (Figure 6A). Furthermore, in high p-BTK human AML blasts, ibrutinib, when added to cytarabine, reduced its IC50 by a median of 1.5-fold (range, 0.18-3.55, n = 5), or when added to daunorubicin, it reduced IC50s by a median of 3.1-fold (range, 0.72-15.14, n = 5). Importantly, ibrutinib had no effect on cytarabine and daunorubicin IC50s in nonmalignant CD34+ cells (supplemental Table 3). Similarly, ibrutinib augmented the cytotoxic effects of both cytarabine and daunorubicin to high p-BTK AML cells in colony-forming assays but had no additional effect on nonmalignant CD34+ cells (Figure 6B).
Reduced viability and colony formation of AML cells after inhibition of BTK in combination with conventional chemotherapy. (A) AML blasts and CD34+ control cells were either untreated or treated with ibrutinib (1 µM) for 8 hours and then treated with either cytarabine (0.1 µM or 0.5 µM) or daunorubicin (0.05 µM or 0.1 µM) for 48 hours and then assessed by Cell TitreGlo. Values indicate mean ± SD of 3 experiments. (B) AML cells and control cells were either untreated or treated with ibrutinib (1 µM) for 8 hours and then treated with either cytarabine (0.1 µM) or daunorubicin (0.05 µM), and then colony-forming assays were performed to show the number of colonies. In all panels, the values indicate the mean ± SD from 3 independent experiments. *Statistical significance of P < .05 between the different treatment groups using Student t test.
Reduced viability and colony formation of AML cells after inhibition of BTK in combination with conventional chemotherapy. (A) AML blasts and CD34+ control cells were either untreated or treated with ibrutinib (1 µM) for 8 hours and then treated with either cytarabine (0.1 µM or 0.5 µM) or daunorubicin (0.05 µM or 0.1 µM) for 48 hours and then assessed by Cell TitreGlo. Values indicate mean ± SD of 3 experiments. (B) AML cells and control cells were either untreated or treated with ibrutinib (1 µM) for 8 hours and then treated with either cytarabine (0.1 µM) or daunorubicin (0.05 µM), and then colony-forming assays were performed to show the number of colonies. In all panels, the values indicate the mean ± SD from 3 independent experiments. *Statistical significance of P < .05 between the different treatment groups using Student t test.
Ibrutinib inhibits AML cell adhesion to BMSCs
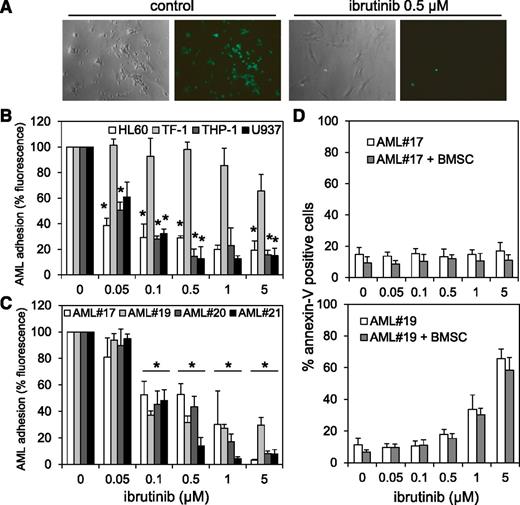
Interaction between AML blasts and its BM microenvironment is critical in regulating tumor survival and chemotherapy resistance. Inhibiting AML blast adhesion to BMSCs is associated with improved tumor cytotoxicity.54,55 Because we found that BTK functions directly upstream of AKT in AML blasts, and because BTK inhibition with ibrutinib is known to perturb interactions between CLL and myeloma tumor cell and microenvironment,17,43 we established whether BTK also functions between AML tumor cells and their BM-stromal niche that protects them. A calcein-AM fluorescence-based adhesion assay determined whether BTK inhibition by ibrutinib affects AML cell lines and blasts binding to BMSCs. Figure 7A shows a representative example of calcein-AM–treated THP-1 cells on BMSCs after 8 hours coculture ± ibrutinib. Overall, treatment with ibrutinib concentrations ≥0.1 µM significantly reduced adhesion of AML cell lines and primary blasts to BMSCs (Figure 7B-C), regardless of BTK phosphorylation status. Moreover, concentrations needed to inhibit AML-BMSC adhesion of 0.1 µM have little or no effect on the cytotoxicity of primary AML cell and AML cell lines (Figure 2A,C), demonstrating that ibrutinib effectively disrupts interactions between AML cells and BMSCs, independent of AML p-BTK expression, and at concentrations much lower than those required to cause cytotoxicity.
AML-BMSC adhesion and protection is disrupted by ibrutinib. (A) Light and fluorescence microscopic images show cocultured calcein-AM–treated THP-1 cells and BMSCs with and without 0.5 µM ibrutinib treatment for 8 hours. (B) Percentage of AML cell lines and (C) primary AML blasts attached to the primary AML BMSCs in the coculture setting in the presence and absence of various concentrations of ibrutinib for 8 hours. (D) AML blasts from AML #17 (low p-BTK) and AML #19 (high p-BTK) were left alone or cocultured with BMSCs in the presence or absence of various concentrations of ibrutinib for 48 hours and then stained for Annexin-V and analyzed by flow cytometry. In all panels, the values indicate the mean ± SD from 3 independent experiments. *Statistical significance of P < .05 between the different treatment groups.
AML-BMSC adhesion and protection is disrupted by ibrutinib. (A) Light and fluorescence microscopic images show cocultured calcein-AM–treated THP-1 cells and BMSCs with and without 0.5 µM ibrutinib treatment for 8 hours. (B) Percentage of AML cell lines and (C) primary AML blasts attached to the primary AML BMSCs in the coculture setting in the presence and absence of various concentrations of ibrutinib for 8 hours. (D) AML blasts from AML #17 (low p-BTK) and AML #19 (high p-BTK) were left alone or cocultured with BMSCs in the presence or absence of various concentrations of ibrutinib for 48 hours and then stained for Annexin-V and analyzed by flow cytometry. In all panels, the values indicate the mean ± SD from 3 independent experiments. *Statistical significance of P < .05 between the different treatment groups.
BMSCs provide no protection for AML blasts from ibrutinib-induced apoptosis
Because others showed that BMSCs can protect AML cells from chemotherapy-induced apoptosis,54-56 we assessed the antileukemic efficacy of ibrutinib in AML blasts under BMSC coculture conditions. We cultured AML blasts from AML #17 (low p-BTK) and AML #19 (high p-BTK) alone or cocultured with the AML patient’s very own BMSCs, with or without ibrutinib. Ibrutinib induced apoptosis in a concentration-dependent manner in AML #19 cells cultured alone or cocultured with their isotypic BMSCs (Figure 7D). No apoptosis was observed in AML #17 cells treated with ibrutinib, either cultured alone or cocultured with BMSCs. The effect of ibrutinib on AML #17 was anticipated because ibrutinib had not previously been shown to be cytotoxic to this sample. These findings show that ibrutinib inhibition of BTK activity induces apoptosis in AML cells alone, even when cocultured with BMSCs. However, because the stromal cells are known to protect AML from conventional cytotoxic drugs such as cytarabine, ibrutinib delivers a dual antileukemic effect by (1) releasing leukemic cells from its protective microenvironment and (2) antiproliferative/cytotoxic responses, independent of any stromal detachment.
Discussion
Outcomes for the 75% of patients diagnosed with AML over 60 years of age remain generally poor, largely because of the intensity and side effects of existing curative therapeutic strategies (which are commonly used to treat younger, fitter patients) coupled with patient comorbidities, which frequently limit their use in this older, less fit population. Consequently, there is an urgent need to identify pharmacologic strategies in AML, which are not only effective but can be tolerated by the older, less well patient. It is envisaged that treatments which target tumor-specific biology will help realize this goal.
In this work, we build on the observations that SYK and LYN have been identified as possible AML targets.37,38 Downstream of SYK and LYN is BTK, widely expressed in hematopoietic cells and long known in B-cell differentiation and survival. A Btk/Tec member,57 it contains a pleckstrin-homology domain and SH2&3 Src-homology domains.21 BTK activation has been implicated in a variety of hematopoietic cellular responses, and there is a growth in literature supporting the role of BTK in a spectrum of B-cell–derived hematologic malignancies.20 Our studies demonstrate that BTK is expressed and constitutively active (p-BTK) in ∼90% of AML samples tested (relative to normal CD34+ cells) and, furthermore, that AML proliferation and survival appear to be partly dependent on BTK.
Ibrutinib (formally PCI-32765) is a selective covalent inhibitor of BTK. It is rapidly absorbed, potently irreversibly binds to BTK and then is eliminated primarily through metabolism, and shows selectivity for BTK against a panel of kinase enzymes.36 We have determined BTK target occupancy of ibrutinib in primary AML and an AML cell line (Figure 2A), showing that 99% of BTK is occupied by ibrutinib at 10 nM in AML. We have correlated this with partial inhibition of SCF-, GM-CSF–, and IL-3–induced AML proliferation at 10 nM. Moreover, downstream signaling pathways including AKT and ERK are also inhibited at 10 nM ibrutinib (Figure 5F). Together, these results show a correlation between BTK-ibrutinib binding with a cellular response in AML.
Ibrutinib reduced the IC50s of daunorubicin and cytarabine by 3.5- and 1.5-fold, respectively, in primary AML samples but did not change sensitivity of control nonmalignant CD34+ cells to these drugs. This leads us to hypothesize about whether future regimens may include BTK inhibition to permit dose reductions of cytotoxic drugs but maintain cytotoxic efficacy. If such approaches were associated with more favorable side effect profiles, there would be a scope for their use across a greater spectrum (including age and comorbidities) of AML patients than the present standard dose of cytotoxics alone.
In this study we describe a significant correlation between BTK Y223 phosphorylation and viability of AML cells to ibrutinib (Figures 2B-D and 3A-B). In contrast, ibrutinib had minimal effects on apoptosis in control CD34+ HSCs, AML samples, and cell lines with low BTK Y223 phosphorylation. However, some AML samples that exhibited Y223 phosphorylation, such as AML #17, were relatively insensitive to ibrutinib, whereas AML#7 with lower levels of Y223 phosphorylation were very sensitive, suggesting that at least in vitro there are factors other than the degree of BTK phosphorylation that modify responsiveness to ibrutinib.
Interestingly, we showed that AML blasts express BTK mRNA or protein at levels comparable with those observed in human CD34+ HSCs. This demonstrates that the high BTK activity seen in this study is not primarily a result of differential BTK expression, but probably more the result of upstream BTK regulators. Likely candidates include SYK and LYN, both of which have been shown to have constitutive activity in AML. Determining the mechanism of activation of BTK in AML is under investigation; however, we have demonstrated evidence of both AKT signaling alteration and NF-κB gene expression and activity after ibrutinib treatment or BTK knockdown. This suggests that BTK is involved in prosurvival signals within AML.
Leukemia stem cells can infiltrate the BM niche to hijack normal homeostatic processes, leading to enhanced self-renewal, proliferation, and chemotherapeutic resistance.56 Moreover, drug strategies disrupting interactions between AML tumor cell and its microenvironment appear to increase the cytotoxicity of conventional chemotherapies.54,55 In the lymphoid malignancies, ibrutinib appears to function in part by disrupting interaction between the tumor cell and BM/lymph node niche. In AML, we report that ibrutinib, in addition to inhibiting cytokine/chemokine-induced proliferation in AML cells in vitro, also inhibits cell adhesion to BMSCs, and that the same BMSCs do not confer any protection from ibrutinib-induced apoptosis. These data lead us to hypothesize that ibrutinib will improve the efficacy of standard chemotherapeutic drugs in AML patients, not only by directly inhibiting proliferation but also by perturbing tumor cell adhesion to microenvironment stromal cells that protect and maintain them.
In conclusion, we show that BTK is activated and functional in primary AML blasts. We demonstrate that the majority of primary AML blasts display therapeutic responses to BTK inhibition and are efficacious on cell growth, adhesion, and colony formation. With well-tolerated BTK inhibitors currently in clinical trials for other hematologic malignancies, these results should have immediate relevance for clinical testing in AML patients on ibrutinib and/or other BTK inhibitors either alone or combined.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Association for International Cancer Research, National Institutes for Health Research, and The Big C for funding; Richard Ball, Norfolk and Norwich University Hospital tissue bank, for primary tissue collection assistance; Drs Betty Chang and Stella Chang (Pharmacyclics Inc., Sunnyvale, CA) for target occupancy work and manuscript comments; and the Liverpool Lymphocyte Malignancy Group (particularly Joseph Slupsky and Kathy Till) for discussions regarding cell adhesion.
Authorship
Contribution: S.A.R., K.M.B., and D.J.M. designed the research; S.A.R., L.Z., and M.Y.M. performed the research; and S.A.R., K.M.B., and D.J.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David J. MacEwan, Department of Molecular and Clinical Pharmacology, Institute of Translational Medicine, University of Liverpool, Liverpool, L69 3GE, United Kingdom; e-mail: macewan@liverpool.ac.uk.
References
Author notes
K.M.B. and D.J.M. contributed equally to this study.