Key Points
Expression of exhaustion markers is decreased on CMV-specific CD8+ T cells from CLL patients as compared with those from age-matched HCs.
Functionality of CMV-specific CD8+ T cells in CLL with respect to cytokine production, cytotoxicity, and immune synapse formation is preserved.
Abstract
In chronic lymphocytic leukemia (CLL), CD8+ T cells exhibit features of exhaustion and impaired functionality. Yet, reactivations of latent viruses such as cytomegalovirus (CMV) are uncommon in untreated CLL, suggesting that antiviral responses are uncompromised. We analyzed phenotypical and functional characteristics of CMV-specific CD8+ T cells in CLL patients in comparison with age-matched healthy controls (HCs). Despite increased expression of the inhibitory receptors PD1, CD160, and CD244 on total CD8+ T cells in CLL, expression levels of these markers were decreased on CMV-tetramer+CD8+ T cells. Second, cytokine production upon stimulation with both phorbol 12-myristate 13-acetate/ionomycin and CMV-peptide–loaded antigen-presenting cells was intact in CMV-tetramer+CD8+ T cells. Third, CMV-tetramer+CD8+ T cells of CLL patients and HCs were equally effective in killing CMV-peptide–loaded target cells. Finally, quantitative imaging flow cytometry revealed that the proportion of CD8+ T cells forming immunologic synapses with CMV-peptide–loaded B cells was intact. In conclusion, despite evidence for global T-cell dysfunction in CLL, we show here that CLL-derived CMV-specific CD8+ T cells display lower expression of exhaustion markers and are functionally intact. These data indicate that the changes in the T-cell compartment in CLL may be more heterogeneous than presently assumed.
Introduction
The clinical course of chronic lymphocytic leukemia (CLL) is characterized by considerable morbidity due to disturbances in the immune system, reflected by an increased susceptibility to (opportunistic) infections and the occurrence of autoimmune phenomena. The nature of the involved pathogens suggests that acquired defects in T-cell function play an important role in the evolving immunodeficiency,1,2 as indeed has been reported in CLL.3-7 Importantly, the profound defects in T-cell immunity in CLL may also affect cancer immunosurveillance.8
Several studies have described an expansion of the CD4+ and CD8+ T-cell compartments in CLL.9,10 We have previously shown that the increase in CD8+ T-cell numbers in CLL is caused by an increase in T cells exhibiting the cytotoxic CD45RA+/−CD27− phenotype.11 These cells were mainly found in cytomegalovirus (CMV)-seropositive patients, suggesting that the expansion of the T-cell compartment may be CMV driven.12 Indeed, using CMV-specific tetramers, we could confirm that in CLL patients, the expanded CD8+ T-cell compartment largely consists of CMV-specific T cells.11 In addition, a higher frequency of CMV-directed CD4+ T cells exhibiting the late CD45R0+CD27−CD28−CCR7− phenotype has been found in CLL.13
It is currently unknown whether the reported quantitative changes in the T-cell compartment reflect increased antigenic pressure of the virus due to failure of other components of the immune system or represent a compensatory mechanism for intrinsic changes in T cells in these patients.4 The latter hypothesis is supported by recent findings that T cells isolated from CLL patients show an impaired ability to form immune synapses (ISs) with antigen-presenting cells (APCs).5,6 Moreover, CD8+ T cells derived from CLL patients were found to exhibit features of T-cell exhaustion with increased expression of inhibitory receptors including PD1, CD160, and CD244. In addition, Riches et al found that CD8+ T cells harbor defects in proliferative and cytotoxic capacity, irrespective of CMV serostatus.7
Despite these global T-cell defects, clinically relevant reactivations of latent viruses such as the highly immunodominant CMV are uncommon in untreated CLL patients. No (sub)clinical CMV reactivations were found in 2 independent cohorts of untreated CLL patients.13,14 In addition, in contrast to the reported increased expression of PD1 on total CD8+ T cells, we recently reported a decreased expression of PD1 on effector CD8+ (CD8+CD45RA+/−CD27−) and CD4+ (CD4+CD45RA+/−CD28−) T cells in CLL patients.14 Because these populations are enriched for CMV-specific T cells in CMV-seropositive patients,12 this implies that the expression of exhaustion markers including PD1 might also be decreased on CMV-tetramer+CD8+ T cells from CLL patients. Taken together, these data indicate that functional defects observed in total T cells of CLL patients may not apply to all (virus-specific) T-cell subsets to the same extent. Thus far, studies examining phenotypical and functional features of T cells specifically directed at CMV in CLL patients are lacking.
We thoroughly characterized CMV-specific CD8+ T cells in CLL patients and found that, in contrast to the increased expression on total CD8+ T cells, the expression of the inhibitory molecules PD1, CD160, and CD244 was decreased on CMV-tetramer+CD8+ T cells when compared with the expression on these cells from age-matched healthy controls (HCs). Moreover, we found that CMV-directed CD8+ T-cell function was intact with respect to cytokine production, cytotoxic capacity, and the ability to form adequate ISs.
Methods
Patient materials and cell lines
Peripheral blood samples from previously untreated CLL patients were collected at routine follow-up visits at the Department of Hematology at the Academic Medical Center in Amsterdam. The control group consisted of age-matched HCs; all control subjects had normal lymphocyte counts and monoclonal B-cell lymphocytosis was excluded by CD19, CD5, κ, and λ immunophenotyping. All samples used for functional experiments were derived from CLL patients and HCs with positive CMV serology and HLA-A2 or HLA-B7 expression. Ethical approval was confirmed by the medical ethical committee at the Academic Medical Center Amsterdam and written informed consent was obtained in accordance with the Declaration of Helsinki. Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell lines (EBV-LCLs) derived from normal B lymphocytes (HLA-A2 or HLA-B7) were used for antigen presentation.15
Immunofluorescence staining and flow cytometry of exhaustion markers
Peripheral blood mononuclear cells (PBMCs) from CLL patients and HCs were isolated and frozen as described earlier.11 For the determination of CMV specificity, PBMCs were washed twice with ice-cold phosphate-buffered saline containing 0.5% bovine serum albumin and stained for 45 minutes on ice with the appropriate CMV-specific HLA-peptide tetrameric complexes (HLA-A2/NLVPMVATV or HLA-B7/TPRVTGGGAM; Sanquin Reagents), followed by 30 minutes of incubation using saturating amounts of CD8 PE-Cy7, CD45RA fluorescein isothiocyanate, PD1 phycoerythrin (PE), CD160 PE, CD244 PE (eBioscience), CD3 V500 (Becton Dickinson), and CD27 APC-Alexa fluor 750 (Invitrogen). Cells were washed and acquired on a FACSCanto using FACSDiva Software (BD Biosciences). Results were analyzed using FlowJo MacV9.4.11 (Tree Star). Percentages of cells expressing PD1 were determined using CD3− cells from HCs as negative controls as described previously.16
Functional assays
Enrichment of CD8+ T cells
After thawing, CMV-specific CD8+ T cells were enriched via negative depletion using CD19 immunomagnetic microbeads (Miltenyi Biotec), α-CD4 (10-A-12, nr 69), α-CD19 (11G1, nr 156), α-CD14 (CLB-mon/1, nr 143, 8G3), α-CD16 (CLB-FCRgran1, nr142, 5D2) (CLB, Amsterdam), and sheep anti–mouse immunoglobulin-coated magnetic beads (Invitrogen Dynal A.S.) in HCs and CLL as described earlier.17 Purity of CMV-specific CD8+ T cells was around 1% to 2% within total PBMCs following negative selection with comparable percentages between CLL patients and HCs. Cells were left to recover overnight at 37°C.
Cytokine assay
EBV-LCLs, CLL cells, or B cells derived from HCs were loaded with the appropriate CMV-pp65 peptides (HLA-A2: NLVPMVATV or HLA-B7: TPRVTGGGAM; IHB-LUMC peptide synthesis library facility, Leiden) in increasing concentrations (0.001-10 ng/mL), incubated overnight at 37°C, and washed. Enriched CD8+ T cells (1 × 106 T lymphocytes) from CLL patients or HCs were stimulated with phorbol-12-myristate-13-acetate (PMA; 10 ng/mL)/ionomycin (1 µg/mL; Sigma-Aldrich) or CMV-peptide–loaded EBV-LCLs, CMV-peptide–loaded CLL cells, or CMV-peptide–loaded HC B cells in culture medium in a fixed ratio of T:B cells of 5:1 in the presence of αCD28 (15E8; 2 µg/mL), αCD29 (TS 2/16; 1 µg/mL), brefeldin A (Invitrogen; 10 µg/mL), and GolgiStop (BD Biosciences) for 6 hours at 37°C as described previously.18,19 Subsequently, cells were washed twice and incubated with the appropriate CMV-specific HLA-peptide tetrameric complexes for 45 minutes followed by incubation with CD3 V500, CD8 pacific blue, CD16 fluorescein isothiocyanate (BD Biosciences), and Live/Dead fixable red cell stain (Invitrogen) for 30 minutes at 4°C. The cells were then washed twice, fixated, and permeabilized (Cytofix/Cytoperm reagent; BD Biosciences) and subsequently incubated with the following intracellular monoclonal antibodies: anti–interferon (IFN)-γ APC-Alexa fluor 750 (Invitrogen), anti–tumor necrosis factor (TNF)-α Alexa fluor 700, and anti–interleukin-2 (IL-2) PE (BD Biosciences) for 30 minutes at 4°C. Cells were washed twice and measured on an LSRFortessa flow cytometer. Cytokine production was related to cytokine production in CMV-specific CD8+ T cells stimulated with nonloaded EBV-LCLs.
Cytotoxicity assay
EBV-LCLs were labeled with dimethyldodecylamine oxide (DDAO; Invitrogen) and subsequently loaded with the appropriate CMV-pp65 peptides (10 µg/mL), incubated overnight at 37°C, and washed as previously described.17,20,21 Enriched CD8+ T cells containing 0.3 × 103 to 2 × 104 CMV-specific CD8+ T cells (effectors) from CLL patients or HCs were incubated with 1 × 104 DDAO-labeled CMV-peptide-pp65–loaded EBV-LCLs (targets) at various effector:target ratios at 37°C for 6 hours. Subsequently, cell death in target cells was measured by flow cytometry using 3,3′-Dihexyloxacarbocyanine iodide (Invitrogen) and propidium iodide (Sigma-Aldrich). As a negative control, enriched CD8+ T cells stimulated with nonloaded EBV-LCLs at the highest effector:target ratio were taken along. Cell death in the CMV-peptide–loaded EBV-LCLs (stimulated cells) was corrected for background cell death in the nonloaded EBV-LCLs (unstimulated cells) and calculated as (% apoptosisstimulated cells − % apoptosisunstimulated cells)/% viableunstimulated cells. Mean percentage of background cell death in CMV-peptide–nonloaded EBV-LCLs in all experiments was 36% (n = 17) and samples exceeding ×1.5 of the standard deviation (SD) from that percentage were excluded from the analysis. As a consequence, 1 HC was excluded from the analysis.
Immunological synapse assay
EBV-LCLs were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) and subsequently loaded with the appropriate CMV-pp65 peptides (10 µg/mL), incubated overnight at 37°C, and washed. Enriched CD8+ T cells containing 1 × 104 CMV-specific CD8+ T cells and 1 × 105 CFSE-labeled CMV-peptide-pp65–loaded EBV-LCLs were incubated at 37°C for 30 minutes. Cells were fixated with 2% paraformaldehyde for 15 minutes, washed, and stained with CD8 APC (Becton Dickinson) for 30 minutes at 4°C. Cells were then washed twice, fixated and permeabilized (Cytofix/Cytoperm reagent; BD Biosciences), and subsequently incubated with Hoechst (Invitrogen) to stain the nucleus and phalloidin-Texas red (PhTR; Invitrogen) to stain the actin cytoskeleton for 30 minutes at 4°C. Cells were washed twice and acquired using ImageStream X (0.5-1.0 × 105 events; Amnis). Data were analyzed using IDEAS software (Amnis). To identify properly formed ISs, a synapse score was computed as a ratio of the maximum pixel intensity of the PhTR signal at the T-cell/B-cell interface versus the mean pixel intensity of the PhTR signal on the CD8+ T cell. As a negative control, CMV-specific CD8+ T cells stimulated with nonloaded EBV-LCLs were taken along. The percentage of specific adequately formed synapses within doublets (T+B cell) was calculated as (% ISstimulated cells − % ISunstimulated cells)/% non-ISunstimulated cells.
Statistical analysis
The d’Agostino and Pearson omnibus normality test was performed to assess normal distribution of data sets. Because data sets were not normally distributed, a 2-tailed Mann-Whitney U test was used to analyze differences between groups. A P value < .05 was considered statistically significant.
Results
Expression of exhaustion markers on CMV-specific CD8+ T cells
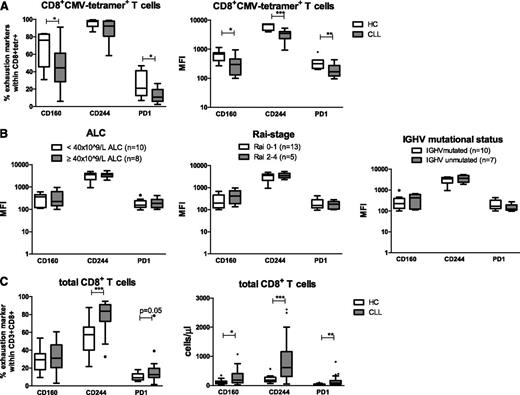
Expression levels of the inhibitory molecules CD160, CD244 and PD1 were measured on CMV-tetramer+CD8+ T cells from CLL patients (n = 18) and age-matched HCs (n = 7). Characteristics of the CLL patients and HCs are presented in supplemental Table 1, available on the Blood Web site. The proportion of CMV-tetramer+CD8+ T cells expressing CD160 and PD1 in CLL patients was decreased. Also, the geometric mean fluorescence intensity (MFI) of CD160, CD244, and PD1 on CMV-tetramer+CD8+ T cells from CLL patients was significantly decreased in comparison with the MFI of CMV-tetramer+CD8+ T cells from age-matched HCs (Figure 1A).
CMV-specific CD8+T cells from CLL patients exhibit decreased expression of the exhaustion markers PD1, CD160, and CD244 as compared with those from HCs. (A) Percentages and geometric MFI of CD160, CD244, and PD1 within CMV-specific CD8+ T cells using CMV tetramers in untreated CLL patients (n = 18) and age-matched HCs (n = 7) were measured by flow cytometry. (B) Geometric MFI of CD160, CD244, and PD1 within CMV-specific CD8+ T cells, stratified according to different clinical parameters. (C) Percentages of CD160, CD244, and PD1 within total CD8+ T cells and absolute numbers of CD160, CD244, and PD1 in total CD8+ T cells from CLL patients (n = 38) and age-matched HCs (n = 13). For panels A to C, horizontal bars, boxes, whiskers, and dots represent median, 25%/75% quartiles, range, and outliers, respectively. Significant differences in the expression of different markers between CLL and HCs are presented as *.01 ≤ P < .05; **.001 ≤ P < .01; ***P < .001 (Mann-Whitney U test).
CMV-specific CD8+T cells from CLL patients exhibit decreased expression of the exhaustion markers PD1, CD160, and CD244 as compared with those from HCs. (A) Percentages and geometric MFI of CD160, CD244, and PD1 within CMV-specific CD8+ T cells using CMV tetramers in untreated CLL patients (n = 18) and age-matched HCs (n = 7) were measured by flow cytometry. (B) Geometric MFI of CD160, CD244, and PD1 within CMV-specific CD8+ T cells, stratified according to different clinical parameters. (C) Percentages of CD160, CD244, and PD1 within total CD8+ T cells and absolute numbers of CD160, CD244, and PD1 in total CD8+ T cells from CLL patients (n = 38) and age-matched HCs (n = 13). For panels A to C, horizontal bars, boxes, whiskers, and dots represent median, 25%/75% quartiles, range, and outliers, respectively. Significant differences in the expression of different markers between CLL and HCs are presented as *.01 ≤ P < .05; **.001 ≤ P < .01; ***P < .001 (Mann-Whitney U test).
Expression of these inhibitory molecules was not affected by the disease burden, because no differences were observed in the expression, both MFI and percentages, of CD160, CD244 or PD1 in relation to absolute lymphocyte count (ALC) or Rai stage. Also, no differences were observed between patients with mutated and unmutated IGHV mutational status (Figure 1B and supplemental Figure 1).
In contrast, analysis of expression levels of exhaustion markers on total CD8+ T cells from CLL patients (n = 38) in comparison with total CD8+ T cells from HCs (n = 13) revealed a significant increase in absolute numbers of CD8+ T cells expressing CD160 and significant increases in both the proportion and absolute numbers of CD8+ T cells expressing CD244 and PD1 (Figure 1C), in agreement with Riches et al.7
Thus, in contrast to total CD8+ T cells, CMV-tetramer+CD8+ T cells from CLL patients show a decreased expression of exhaustion markers compared with CMV-tetramer+CD8+ T cells derived from age-matched HCs.
Cytokine responses of CMV-specific CD8+ T cells
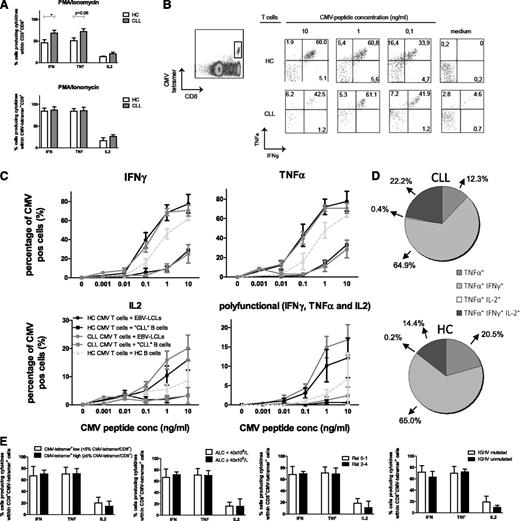
Stimulus-induced cytokine production was investigated in CMV-tetramer+CD8+ T cells. First, cytokine production in total CD8+ and CMV-tetramer+CD8+ T cells after 6 hours of PMA/ionomycin stimulation was measured. This stimulus bypasses the T-cell receptor, and hence the ensuing response is independent of (potentially impaired) IS formation. In accordance with recent data from Riches et al,7 a significantly increased production of IFN-γ and a tendency to increased production of TNF-α was observed in total CD8+ T cells from CLL patients in comparison with HCs (Figure 2A). On average, more than 80% of the CMV-tetramer+ cells produced IFN-γ and TNF-α, whereas lower percentages (varying between 50% and 60%) of total CD8+ T cells did (Figure 2A). No differences were found in cytokine production between CMV-specific CD8+ T cells from CLL patients and HCs.
CMV-specific CD8+T cells from CLL patients are equally effective in cytokine production as those from HCs. (A) Enriched CD8+ T cells from CLL patients and HCs were stimulated with PMA/ionomycin, and cytokine production was measured within total CD3+CD8+ and CD3+CD8+CMV-tetramer+ cells after 6 hours by flow cytometry. Bars represent mean ± standard error of the mean (SEM) of n = 9 CLL and n = 5 HCs. Significant differences are presented as *.01 ≤ P < .05; **.001 ≤ P < .01; ***P < .001 (Mann-Whitney U test). (B-E) Enriched CD8+ T cells from CLL patients or HCs were incubated with HLA-I–matched EBV-LCLs, CLL cells, or B cells derived from HCs and loaded with increasing concentrations of the corresponding CMV pp65 peptide (HLA-A2 or HLA-B7) in the presence of αCD28, αCD29, brefeldin A, and GolgiStop. After 6 hours, cytokine production was analyzed by flow cytometry in CD3+CD8+tetramer+ cells. (B) Representative dot plots from 1 CLL patient and 1 HC. (C) Percentage of IFN-γ+, TNF-α+, and IL-2+ cells within CD3+CD8+tetramer+ cells and polyfunctional cells producing IFN-γ+, TNF-α+, and IL-2+ within the CD3+CD8+tetramer+ cells are presented. Results are shown as mean ± SEM of n = 9 CLL and n = 5 HCs. (D) Percentage of TNF-α+, TNF-α+IFN-γ+, TNF-α+IL-2+, and TNF-α+IFN-γ+IL-2+ (polyfunctional) cells within the CD3+CD8+tetramer+TNFα+ cells stimulated with CMV-peptide–loaded (10 ng/mL) EBV-LCLs. Results are shown as mean percentages of n = 9 CLL and n = 5 HCs. (E) Percentage of IFN-γ+, TNF-α+, and IL-2+ cells within the CD3+CD8+CMV-tetramer+ cells stimulated with 1.0 ng/mL CMV-peptide–loaded EBV-LCLs, stratified according to CMV-tetramer+CD8+ low and high cells defined as < or ≥ 5% CMV-tetramer+CD8+ cells within CD8+ cells, ALC, Rai stage, or IGHV mutation status. Bars represent mean ± SEM. conc, concentration; pos, positive.
CMV-specific CD8+T cells from CLL patients are equally effective in cytokine production as those from HCs. (A) Enriched CD8+ T cells from CLL patients and HCs were stimulated with PMA/ionomycin, and cytokine production was measured within total CD3+CD8+ and CD3+CD8+CMV-tetramer+ cells after 6 hours by flow cytometry. Bars represent mean ± standard error of the mean (SEM) of n = 9 CLL and n = 5 HCs. Significant differences are presented as *.01 ≤ P < .05; **.001 ≤ P < .01; ***P < .001 (Mann-Whitney U test). (B-E) Enriched CD8+ T cells from CLL patients or HCs were incubated with HLA-I–matched EBV-LCLs, CLL cells, or B cells derived from HCs and loaded with increasing concentrations of the corresponding CMV pp65 peptide (HLA-A2 or HLA-B7) in the presence of αCD28, αCD29, brefeldin A, and GolgiStop. After 6 hours, cytokine production was analyzed by flow cytometry in CD3+CD8+tetramer+ cells. (B) Representative dot plots from 1 CLL patient and 1 HC. (C) Percentage of IFN-γ+, TNF-α+, and IL-2+ cells within CD3+CD8+tetramer+ cells and polyfunctional cells producing IFN-γ+, TNF-α+, and IL-2+ within the CD3+CD8+tetramer+ cells are presented. Results are shown as mean ± SEM of n = 9 CLL and n = 5 HCs. (D) Percentage of TNF-α+, TNF-α+IFN-γ+, TNF-α+IL-2+, and TNF-α+IFN-γ+IL-2+ (polyfunctional) cells within the CD3+CD8+tetramer+TNFα+ cells stimulated with CMV-peptide–loaded (10 ng/mL) EBV-LCLs. Results are shown as mean percentages of n = 9 CLL and n = 5 HCs. (E) Percentage of IFN-γ+, TNF-α+, and IL-2+ cells within the CD3+CD8+CMV-tetramer+ cells stimulated with 1.0 ng/mL CMV-peptide–loaded EBV-LCLs, stratified according to CMV-tetramer+CD8+ low and high cells defined as < or ≥ 5% CMV-tetramer+CD8+ cells within CD8+ cells, ALC, Rai stage, or IGHV mutation status. Bars represent mean ± SEM. conc, concentration; pos, positive.
Secondly, cytokine production by CMV-tetramer+CD8+ T cells was assessed after stimulation with EBV-LCLs loaded with CMV-pp65 peptide. The proportion of CMV-tetramer+CD8+ T cells producing IFN-γ, TNF-α, and IL-2 did not differ between CMV-tetramer+CD8+ T cells from CLL patients and those from HCs (Figure 2B-C). The vast majority of cells produced IFN-γ and TNF-α simultaneously, while considerable percentages of triple-positive and TNF-α single-positive cells were also found (Figure 2D). The percentages of single-, double-, or triple-positive (polyfunctional) tetramer+CD8+ T cells did not differ between CLL patients and HCs (Figure 2C-D), suggesting that CMV-specific T-cell function is not impaired in terms of cytokine production. When CLL cells were used for antigen presentation, the proportion of cells producing IFN-γ, TNF-α, and IL-2 was significantly lower in both CLL- and HC-derived CMV-tetramer+CD8+ cells in comparison with cytokine production upon stimulation with EBV-LCLs and also with HC-derived B cells (Figure 2C), emphasizing the poor antigen-presenting capacity of CLL cells.8
Cytokine production was not affected by the amount of CMV-tetramer+ cells within CD8+ cells or by disease burden (ALC, Rai stage) or IGHV mutational status (Figure 2E).
In order to test the sensitivity of this assay in the detection of impaired cytokine production, we mimicked intrinsic T-cell defects in vitro by administering cyclosporine to CD8+ T cells. Cyclosporine is an immunosuppressant agent that inhibits dephosphorylation of the transcription factor of activated T cells by binding to cyclophilin22 and has been shown to inhibit T-cell proliferation as well as cytotoxicity in vitro.23,24 At cyclosporine concentrations of ≥0.2 µg/mL, similar to those achieved in vivo, a clear decrease in the proportion of CMV-tetramer+CD8+ T cells producing IFN-γ and TNF-α following stimulation with CMV-pp65-peptide–loaded EBV-LCLs was observed. In addition, impaired cytokine production in both CMV-tetramer+ and total CD8+ T cells after PMA/ionomycin stimulation and the addition of cyclosporine ≥0.2 µg/mL was examined (supplemental Figure 2A).
In summary, cytokine responses of CMV-specific CD8+ T cells derived from CLL patients were found to be intact.
Cytotoxic responses of CMV-specific CD8+ T cells
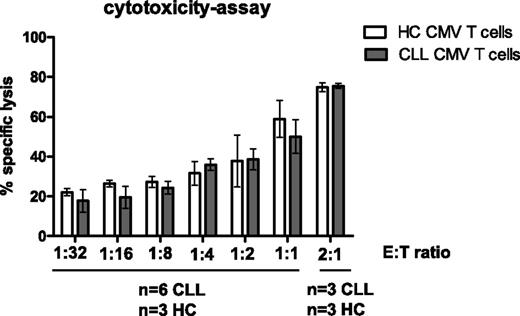
The cytotoxic potential of CMV-specific CD8+ T cells from CLL patients was examined using EBV-LCLs loaded with CMV peptide at different effector:target ratios. CMV-tetramer+CD8+ T cells from CLL patients were found to be as effective as those from HCs in killing CMV-peptide–loaded EBV-LCLs (Figure 3), indicating that the cytotoxic potential of these T cells is intact. We tested whether defects in cytotoxic activity could be observed by this assay using cyclosporine in vitro as described above. Indeed, specific lysis could be blocked up to almost 50% in the presence of cyclosporine (supplemental Figure 2B).
CMV-specific CD8+T cells from CLL patients are as cytotoxic as those from HCs. Enriched CD8+ T cells from CLL patients and HCs (effectors) were incubated with DDAO-labeled CMV-peptide–loaded HLA-I–matched EBV-LCLs (targets) at various effector-to-target ratios. After 6 hours of incubation, cell death in the target cells was measured using 3,3′-Dihexyloxacarbocyanine iodide and propidium iodide. As a negative control, enriched CD8+ T cells and nonloaded EBV-LCLs with the highest effector-to-target ratio were taken along. Results are presented as the percentage of specific lysis, calculated by subtraction of spontaneous cell death within CMV-peptide–nonloaded EBV-LCLs with mean ± SEM of n = 6 CLL and n = 3 HCs. No significant differences were observed between HCs and CLL at any effector-to-target ratio (Mann-Whitney U test).
CMV-specific CD8+T cells from CLL patients are as cytotoxic as those from HCs. Enriched CD8+ T cells from CLL patients and HCs (effectors) were incubated with DDAO-labeled CMV-peptide–loaded HLA-I–matched EBV-LCLs (targets) at various effector-to-target ratios. After 6 hours of incubation, cell death in the target cells was measured using 3,3′-Dihexyloxacarbocyanine iodide and propidium iodide. As a negative control, enriched CD8+ T cells and nonloaded EBV-LCLs with the highest effector-to-target ratio were taken along. Results are presented as the percentage of specific lysis, calculated by subtraction of spontaneous cell death within CMV-peptide–nonloaded EBV-LCLs with mean ± SEM of n = 6 CLL and n = 3 HCs. No significant differences were observed between HCs and CLL at any effector-to-target ratio (Mann-Whitney U test).
Taken together, these data show that the function of CMV-specific CD8+ T cells is intact, also with respect to cytotoxic capability.
IS formation of CMV-specific CD8+ T cells
Other studies suggested that CLL-derived CD4+ and CD8+ T cells are unable to form adequate ISs with APCs upon stimulation by a cocktail of staphylococcal superantigens (SEA and SEB).5,6 The ability of CMV-specific CD8+ T cells to form ISs with CMV-peptide–loaded EBV-LCLs was analyzed. The formation of synapses was visualized and quantified by imaging flow cytometry (Figure 4). This technique has advantages over the current gold standard in the study of ISs, confocal laser-scanning microscopy, because it combines quantitative and qualitative data in an automated fashion. In order to identify properly formed ISs, a synapse score was computed based on the fluorescence signal intensity of PhTR (an F-actin marker) at the interface of B-T–cell doublets as described in “Methods” and Figure 4A-D. Doublets displaying a synapse score >1.5 (Figure 4D) were considered to have a reorganized cytoskeleton at the intercellular contact surface and, therefore, counted as adequate IS events. In this way, we were able to determine the percentage of doublets displaying adequately formed synapses among doublets of 1 B cell and 1 T cell. The percentage of adequate synapses formed was corrected for synapses formed in the absence of CMV peptide. An average of 0.6 × 105 cells were acquired per condition containing approximately 550 doublets in the CMV-peptide–stimulated samples, of which >40% displayed adequate specific-formed ISs. Mean percentages of background-formed synapses (ie, synapses between T cells and nonloaded EBV-LCLs) within doublets for HCs and CLL patients were 22.1 ± 12.0 (mean ± SD) and 21.8 ± 11.7 (mean ± SD), respectively (supplemental Figure 3). The proportion of adequately formed specific synapses within doublets in the presence of CMV peptide was similar in CLL patients compared with HCs (Figure 4E).
CMV-specific CD8+T cells from CLL patients and HCs are equally effective in forming adequate synapses with APCs. (A-E) Enriched CD8+ T cells (1 × 104) from CLL patients and HCs were incubated for 30 minutes with CFSE-labeled CMV-peptide–loaded HLA-I–matched EBV-LCLs (1 × 105) and fixated with 2% paraformaldehyde. Cells were stained for CD8 (CD8-APC), F-actin (PhTR), and a nuclear dye (Hoechst) and acquired using ImageStream X100 (0.5-1.0 × 105 events). As a negative control, enriched CD8+ T cells and nonloaded EBV-LCLs were analyzed. (A) T-cell/B-cell aggregates were identified using CFSE vs CD8-APC bivariate plots. (B) Doublets were gated on their content in the DNA-binding dye Hoechst and cell aggregates were discarded. (C) To identify adequate ISs, a synapse score was computed as a ratio of the maximum pixel intensity of the PhTR (Max Px) signal at the T-cell/B-cell interface vs the mean pixel intensity of the PhTR (Mean Px) signal on the CD8+ T cell. (D) This score identifies doublets in which the PhTR signal has polarized to the IS. (E) Percentage of doublets displaying an adequately formed ISs (relative to all doublets) corrected for background synapse formation. Dots represent individuals; n = 5 CLL, n = 3 HCs with mean ± SEM.
CMV-specific CD8+T cells from CLL patients and HCs are equally effective in forming adequate synapses with APCs. (A-E) Enriched CD8+ T cells (1 × 104) from CLL patients and HCs were incubated for 30 minutes with CFSE-labeled CMV-peptide–loaded HLA-I–matched EBV-LCLs (1 × 105) and fixated with 2% paraformaldehyde. Cells were stained for CD8 (CD8-APC), F-actin (PhTR), and a nuclear dye (Hoechst) and acquired using ImageStream X100 (0.5-1.0 × 105 events). As a negative control, enriched CD8+ T cells and nonloaded EBV-LCLs were analyzed. (A) T-cell/B-cell aggregates were identified using CFSE vs CD8-APC bivariate plots. (B) Doublets were gated on their content in the DNA-binding dye Hoechst and cell aggregates were discarded. (C) To identify adequate ISs, a synapse score was computed as a ratio of the maximum pixel intensity of the PhTR (Max Px) signal at the T-cell/B-cell interface vs the mean pixel intensity of the PhTR (Mean Px) signal on the CD8+ T cell. (D) This score identifies doublets in which the PhTR signal has polarized to the IS. (E) Percentage of doublets displaying an adequately formed ISs (relative to all doublets) corrected for background synapse formation. Dots represent individuals; n = 5 CLL, n = 3 HCs with mean ± SEM.
Discussion
T-cell exhaustion is a state of acquired T-cell dysfunction that arises during chronic viral infections, but it also occurs in the presence of cancer. Exhaustion is characterized by the expression of inhibitory molecules and poor effector function.25 Interestingly, total CD8+ T cells from CLL patients were recently found to show features of exhaustion, namely increased expression of the inhibitory molecules PD1, CD160, and CD244 and impaired proliferative and cytotoxic capacity.7 However, in contrast to what is observed in “classical exhaustion,” cytokine responses in these cells remained intact. Therefore, the state of exhaustion of CD8+ T cells in CLL was termed “pseudoexhaustion,” and it was reasoned that this type of exhaustion might be distinct due to differences in antigen affinity: chronic stimulation by low-affinity self antigens in the case of CLL in contrast to chronic stimulation by high-affinity viral antigens in the case of chronic viral infections. In addition, several other studies have previously shown evidence for impaired T-cell function in CLL with changes in gene expression profiles and impaired IS formation in T cells from CLL patients.3-5 Interestingly, these changes could also be induced in HC T cells by coculturing these cells with CLL cells, indicating a pivotal role for CLL in the occurrence of these T-cell disturbances. We here show that in contrast to the expression on total CD8+ T cells in CLL, CMV-specific CD8+ T cells exhibit lower expression of the exhaustion markers when compared with CMV-specific CD8+ T cells from HCs. In addition, we demonstrate that these CMV-specific CD8+ T cells are functionally intact, not only in terms of cytokine production, but also with respect to cytotoxic capacity and the ability to form adequate ISs.
Approximately 60% to 80% of adults are infected with CMV. In healthy individuals, the infection evolves into asymptomatic latency after the acute phase. In contrast, during immunodeficiency, CMV infection may cause serious complications.26 However, although defects in immune function probably originate early in the course of CLL,2,27,28 clinically relevant CMV reactivations are usually not seen in untreated CLL patients.2 We and others have consistently found negative CMV polymerase chain reactions in CMV-seropositive patients.13,14 This indicates that clinically CMV-specific CD8+ T-cell function is preserved in CLL, which corroborates our findings in vitro.
The discordant expression of exhaustion markers and preserved functionality of CMV-specific CD8+ T cells as compared with total CD8+ T cells in CLL may be related to the unique phenotype and functional properties of CMV-specific T cells. CMV is unique in inducing a vast pool of resting virus-specific T cells with constitutive cytolytic effector function.29-31 There is evidence that replicative senescence, defined as failure to clonally expand in response to reexposure to the antigen, does not occur in these cells. This is supported by the observation that telomere length, which is considered to be indicative of replicative senescence, is preserved in CMV-specific T cells.29,32 In addition, CMV-specific CD8+ T cells can expand during and after reactivation, showing that this population can respond with renewed clonal expansion and that these T cells are effective in mediating a strong antiviral response in vivo.20 Finally, CD45RA+CD27− CMV-directed CD8+ T cells express both perforin and granzyme B and are able to execute direct ex vivo cytolysis without costimulation and CD4 T-cell help in vitro.20,33-35
Phenotypic characteristics of virus-specific T cells vary in different latent virus infections30,31,33 and as a consequence correspond to differences in functionality.8,34,36,37 Whether the observed preserved functionality is unique to CMV-specific CD8+ T cells or also applies to T-cell subsets with other (virus-directed) specificities has not been studied thus far. The fact that, in contrast to CMV, reactivations of the herpesviruses herpes simplex virus and varicella zoster virus are more prevalent in CLL2,38 suggests that this might not be the case. Taken together, in contrast to the current view of a global T-cell dysfunction in CLL,3-5,7,39 our data indicate that functional T-cell subtypes are present in these patients. This observation might imply that the antigenic identity and/or the differentiation status of T cells play a role in the susceptibility to CLL-induced phenotypical and functional changes.
Increased expression of PD1 correlates with exhaustion and impaired T-cell function. However, whether PD1 is an inducer of T-cell exhaustion and impaired functionality or simply a consequence of T-cell exhaustion is less clear. However, several observations support the notion that the expression of PD1 indeed induces impaired T-cell functionality,39-44 which infers that the observed dampening of the upregulation of PD1 on CMV-specific CD8+ T cells might be mechanistically related to the preserved T-cell function in CLL.
CMV-specific T-cell numbers increase over time in a process called “memory inflation.”45,46 Indeed, frequencies of CMV-directed CD8+ T cells are increased in elderly healthy individuals, and this is exaggerated in immunocompromised patients.11,47 The cause of the expansion in CLL patients may be related to failure of other parts of the virus-specific immune response, such as antibodies or natural killer (NK) cell activity. We have previously shown that CMV-specific immunoglobulin G titers do not correlate with effector-type T-cell numbers in both CLL patients and HCs.14 So far, functionality of NK cells in CLL has not been extensively studied. The available literature did show altered NK cell numbers, implicating that NK cells might be affected in CLL patients.48,49 Alternatively, the increase in CMV-specific T cells may be explained by T-cell intrinsic changes such as an extended lifespan or accelerated proliferation of these cells. The latter explanation is supported by our findings of decreased expression of inhibitory molecules on CMV-specific T cells, which influence their ability to proliferate.25
In conclusion, despite defects in functionality in total CD8+ T cells in CLL, we demonstrated intact functionality of CMV-specific CD8+ T cells in vitro in terms of cytokine production, cytotoxic capacity, and the ability to form adequate ISs. In addition, the inhibitory molecules PD1, CD244, and CD160 were decreased on CMV-specific CD8+ T cells, which might be directly related to their preserved function. These data shed new light on the complexity of interactions between T lymphocytes and CLL cells. The reported changes in total CD8+ T cells in CLL should be extrapolated to specific T-cell populations with caution. A thorough understanding of the mechanisms underlying the (differing) functionality of specific T-cell subsets may provide clues for the development and improvement of T-cell–mediated immunotherapy in CLL.17,35
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
A.P.K. is funded by a personal Dutch Cancer Society Clinical Fellowship grant.
Authorship
Contribution: G.D.t.R. and M.F.P. designed and performed experiments, analyzed data, and wrote the paper; J.J.G.-V. performed imaging flow cytometry, analyzed data, and reviewed the manuscript; E.R. performed experiments; E.B.M.R. designed flow cytometry analyses and performed experiments; I.J.M.t.B., R.A.W.v.L., E.E., and M.H.J.v.O. contributed to the design of experiments and reviewed the manuscript; and S.H.T. and A.P.K. designed the study, performed data analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arnon P. Kater, Department of Hematology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands; e-mail: a.p.kater@amc.nl.
References
Author notes
S.H.T. and A.P.K. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal