In this issue of Blood, te Raa et al report that cytomegalovirus (CMV)-specific CD8+ T-cell function is preserved in chronic lymphocytic leukemia (CLL), on a background of global T-cell dysfunction.1
CMV is known to have a profound influence on the distribution of lymphocyte subsets in otherwise healthy individuals, with seropositivity for this virus leading to the development of oligoclonal populations of CMV-specific cytotoxic T cells that can constitute up to one-quarter of the total CD8+ T-cell population in the elderly.2 This expansion is even more pronounced in CLL where increased numbers of both CD4+ and CD8+ CMV-specific T cells have been described.3,4 This led to suggestions that these CMV-specific T cells are responsible for the global T-cell defect in CLL, either by a direct effect or by constriction of the total T-cell repertoire.5 However, subsequent work identified that an abnormal phenotype and defective function were present irrespective of CMV serostatus.6 Despite this, studies focusing in on the phenotypic and functional characteristics CMV-specific T cells in CLL have been lacking.
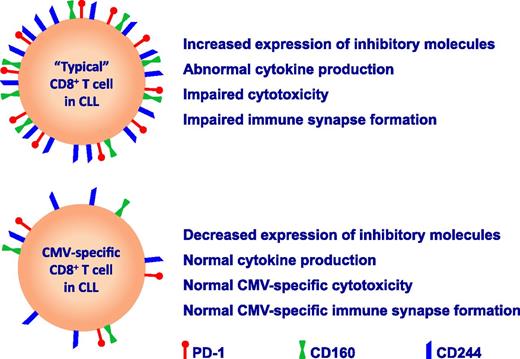
Despite the apparent defects in global T-cell function, clinically relevant reactivations of CMV are uncommon in untreated CLL patients.4,7 In a logical and detailed series of experiments, te Raa and colleagues analyzed the phenotype and function of CMV-specific CD8+ T cells in CLL patients as defined by CMV-tetramer staining.1 In agreement with previous reports, they found evidence of global CD8+ T-cell dysfunction in CLL with increased expression of the inhibitory receptors PD1, CD160, and CD244 on CD8+ T cells and increased production of interferon-γ and tumor necrosis factor-α.6 However, importantly, the expression levels of these markers were decreased on CMV-tetramer+CD8+ T cells, and these T cells also had intact cytokine production, whether they were stimulated by phorbol-12-myristate-13-acetate/ionomycin or with CMV-peptide–loaded antigen-presenting cells (see figure). Further functional work demonstrated that CMV-tetramer+CD8+ T cells from CLL patients were equally effective in killing CMV-peptide–loaded target cells as compared with CMV-specific T cells from healthy age-matched controls and showed equivalent immunologic synapse formation with CMV-peptide–loaded B cells. These observations further counter the hypothesis that chronic viral stimulation by CMV is a major contributor to the overall exhausted T-cell phenotype observed in CLL.
These findings also challenge the paradigm that there is global inhibition of T-cell function in CLL, and instead suggest that the antigenic identity and/or the differentiation state of T cells may be a critical factor in their susceptibility to the immunosuppressive effects of the malignant clone. This indicates that the interaction of CLL cells with the adaptive immune system may be quite specific, with the tumor cells just suppressing certain subpopulations of T cells. However, CMV is known to be uniquely immunodominant, even within the healthy immune system, and so caution needs to be exercised when extrapolating these findings to other populations of T cells, antiviral or otherwise. Furthermore, if a model of antigen-specific immunosuppression is the case, then it would remain unclear why global T-cell changes are observable, given that tumor-specific T cells, the most obvious type for the CLL cells to target, are likely to be low frequency due to their specificity for “self” antigen. An alternative hypothesis follows from evidence implicating T cells as an important component of the protumor microenviroment.8 In this case, the subversion of T cells to proliferate and produce protumoral factors may not be antigen-specific and could result in the observed global changes.
A further question remains as to why there are increased numbers of CMV-specific CD8+ T cells in CLL, if CMV-specific CD8+ T-cell function is not impaired. The authors have provided potential explanations for this observation, including that the expanded numbers of CMV-specific CD8+ T cells could be compensating for defects in other aspects of anti-CMV immune function such as natural killer cell–mediated or humoral immunity. They have also suggested that lower expression of inhibitory molecules may allow for enhanced T-cell proliferative capacity or an extended lifespan. The link between lower expression of inhibitory molecules such as PD-1 and improved functionality provides a further rationale for targeting “immune checkpoints” in CLL. Indeed, perhaps one of the most important aspects of this observation is that an understanding of the mechanism underlying this preservation of CMV-specific T-cell function may offer novel insights into how to repair the functionality of other T cells in CLL. For example, high expression of the transcription factor T-bet has been recently demonstrated to repress expression of PD-1 and to sustain virus-specific CD8+ T-cell responses during chronic infection, representing another potential immunotherapeutic target.9 Thus, the significance of the findings of this report by te Raa et al could extend well beyond CMV in CLL.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal