In this issue of Blood, Vaughan et al demonstrate that certain antibodies that are used therapeutically in lymphoma treatment (eg, rituximab) undergo Fcγ receptor IIb (FcγRIIb)–mediated internalization from the B-cell surface in a manner that is independent of activation of FcγRIIb with important implications for the design of antibody-based therapeutics (see figure).1
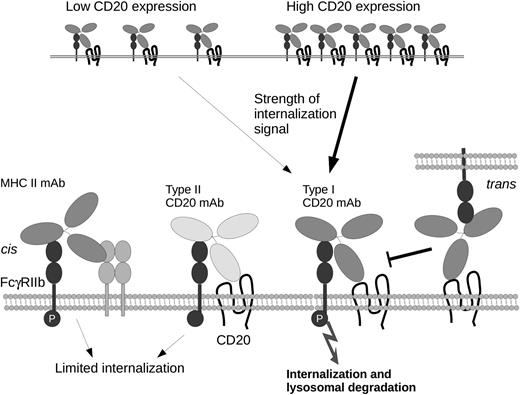
Schematic representation of mechanisms underlying the impact of FcγRIIb upon activation and internalization of BCRs. Internalization of type I CD20 mAbs correlates with levels of expression. major histocompatibility complex class II (MHCII) monoclonal antibodies (mAbs) or type II CD20 mAb binding in cis results in limited internalization, despite robust phosphorylation of FcγRIIb (P). In contrast, type I CD20 mAbs promote internalization/degradation. CD20 mAb binding to FcγRIIb in trans competes for binding in cis but does not affect internalization.
Schematic representation of mechanisms underlying the impact of FcγRIIb upon activation and internalization of BCRs. Internalization of type I CD20 mAbs correlates with levels of expression. major histocompatibility complex class II (MHCII) monoclonal antibodies (mAbs) or type II CD20 mAb binding in cis results in limited internalization, despite robust phosphorylation of FcγRIIb (P). In contrast, type I CD20 mAbs promote internalization/degradation. CD20 mAb binding to FcγRIIb in trans competes for binding in cis but does not affect internalization.
It is now clear that the balance of expression of activatory and inhibitory FcγR on target effector cells is a pivotal factor in determining the therapeutic efficacy of antibodies that are used in treatment of many types of cancer, including lymphoma.2 Vaughan et al have focused their investigation on the effects of CD20 mAbs such as rituximab or ofatumumab1 ; FcγR expression on target cells themselves is critical.3 Immune effector cell Fc-FcγR interactions in trans act to deplete the target cell, particularly when the antigen is expressed at high levels. Although the importance of activatory FcγR for the efficacy of rituximab in preventing growth of subcutaneous Raji xenografts was demonstrated some time ago, the inhibitory FcR, FcγRIIb, expressed on certain types of B-cell malignancies, also plays a critical role.4 FcγRIIb contains an immunoreceptor tyrosine-based inhibitory motif that recruits specific phosphatases and acts to oppose signaling by activatory FcγRs. Cragg and colleagues have previously demonstrated that FcγRIIb-mediated internalization of rituximab from the B-cell surface may act to limit effector cell engagement,5 an effect that may be particularly important in malignancies such as chronic lymphocytic leukemia and mantle cell lymphoma that express high levels of FcγRIIb and show reduced responses to CD20 mAb therapy.
CD20 mAbs can be broadly categorized into type I mAbs that induce potent cytotoxicity associated with redistribution of CD20 into Triton X-100–insoluble lipid rafts and type II mAbs that induce cell death. Both type I and type II CD20 mAbs are equally effective at binding opsonized targets to macrophages and eliciting antibody-dependent cellular cytotoxicity. However, type II mAbs were found to be more effective in xenograft tumor studies and syngeneic models of B-cell depletion.6 To provide insight into the differential efficacy of CD20 mAbs, Vaughan et al have investigated the interactions of type I and type II CD20 mAbs with FcγRIIb and their functional consequences.1 Using a panel of different mAbs over a range of FcγRIIb expression levels in transfected cell lines, the authors observed specific internalization of CD20, CD22, and B-cell receptor (BCR) mAbs, but not MHCII, CD37, or CD40 mAbs, an effect that was also seen in chronic lymphocytic leukemia patient cells. These mAb/molecule-specific effects on internalization were not observed with F(ab′)2 fragments of rituximab and could be impaired by treatment with the FcγRIIb function blocking antibody AT10, demonstrating involvement of FcγRIIb. So does this effect reflect the capacity of CD20 mAb to engage and subsequently activate FcγRIIb? Comparison of mAbs recognizing a panel of surface receptors revealed that rituximab was not unique in ability to activate FcγRIIb and that a number of different antibodies were capable of inducing equivalent or greater phosphorylation of FcγRIIb. The impact of FcγRIIb interactions in either cis or trans may also be important in terms of therapy because cis interactions may initiate transmission of apoptotic signaling or act to compete for FcγRIIb binding in trans and reduce effector activity. In experiments using Ramos cells transfected with a truncated (nonphosphorylatable) FcγRIIb, the authors were able to definitively identify contributions of cis or trans interactions of FcγRIIb upon activation. Although they found that FcγRIIb expressed in trans could compete with cis-FcγRIIb interactions, they failed to impair internalization of rituximab, an effect that was independent of concentration of rituximab used or levels of FcγRIIb expression.
One important conclusion from these findings is that screening the capacity for phosphorylation of FcγRIIb would not adequately predict the rate of CD20 mAb internalization. However, because the rate and extent of internalization and FcγRIIb expression were inversely related, levels of FcγRIIb expression may represent a potential indicator of response to rituximab and thus identify patients for which treatment with type II CD20 antibodies would be preferable. Furthermore, FcγRIIb-mediated internalization of type I CD20 mAbs may lead to preferential clearance of pathogenic cells in systemic lupus erythematosus that express low levels of FcγRIIb, while sparing regulatory B cells that have high levels of expression of CD20,7 leading to amelioration of autoimmune symptoms. In addition, the negative signaling transduced by FcγRIIb itself may also be beneficial in the context of autoimmunity.
More broadly, a critical role for FcγRIIb in mediating the efficacy of therapeutic antibodies directed against a range of tumor targets including tumor necrosis factor receptor (TNFR) superfamily members DR4, DR5, or CD40 has been established.8,9 It is likely that involvement of FcγRIIb reduces the potential for FcγR-mediated target cell depletion, enhancing T-cell priming, while minimizing depletion of receptor-bearing cells. Recently, agonistic anti–TNFR antibodies have also been definitively shown to require FcγRIIb for their effects and that this effect is independent of FcγRIIb-mediated signaling.10 One important question is whether FcγRIIb expressed on infiltrating innate immune cells serves as a more effective cross-linking scaffold for enhancing antibody-induced cross-linking and cellular effector function. Improved CD20 reagents have been developed in recent years that include fully humanized antibodies with an engineered Fc region to tailor engagement of effector functions and decrease affinity for binding to FcγR, thereby minimizing target cell depletion. Defining the mechanisms underlying the differential FcγR-mediated internalization response of type I and type II CD20 mAbs will allow selection/design of mAbs that show reduced internalization while retaining cellular cytotoxic effects that are critical for therapeutic efficacy.
Conflict-of-interest disclosure: The author declares no competing financial interests.