In this issue of Blood, Percopo et al provide intriguing new evidence supporting a role for eosinophils in protecting mice against the lethal effects of respiratory virus infection.1
Two distinct models of Th2-driven inflammation resulting in eosinophilia suggest that eosinophil degranulation is implicated in protection of the host against the lethal effects of infection with PVM.
Two distinct models of Th2-driven inflammation resulting in eosinophilia suggest that eosinophil degranulation is implicated in protection of the host against the lethal effects of infection with PVM.
Eosinophils are enigmatic cells of the innate immune system, and defining their role in immunity is still under intense scrutiny. They are produced in many vertebrates, including fish, reptiles, amphibians, and birds, as well as all mammalians thus far investigated,2 suggesting an important and highly conserved evolutionary role. For many years, the immune function of eosinophils was thought to be associated with protection against invasive helminthic parasite infections. However, recent findings have suggested otherwise. Mouse models of eosinophil depletion have not shown any significant impact on helminthic infection, and paradoxically, some helminth larval forms actually depend on eosinophils for their propagation and survival.2 This has put researchers back to the drawing board in trying to define a coherent evolutionarily conserved immunological role for these cells. Against this backdrop, eosinophils are largely known in the clinical realm for their contribution to the pathology of allergic diseases and are prominent in eosinophilic asthma, which is responsive to treatment with anti–interleukin (IL)-5 (mepolizumab).3
Several reports over the last 2 decades have hinted at an antiviral role for eosinophils and their granule proteins. Eosinophils express highly cationic granule proteins, such as eosinophil-derived neurotoxin (EDN), which have potent antiviral activities in vitro against single-stranded RNA viruses such as respiratory syncytial virus (RSV) and HIV.4,5 Furthermore, eosinophils have been shown to promote respiratory virus clearance in animal models.6 However, a potential role for eosinophils in maintaining survival and preventing significant morbidity in response to respiratory viral infection has not been explored before.
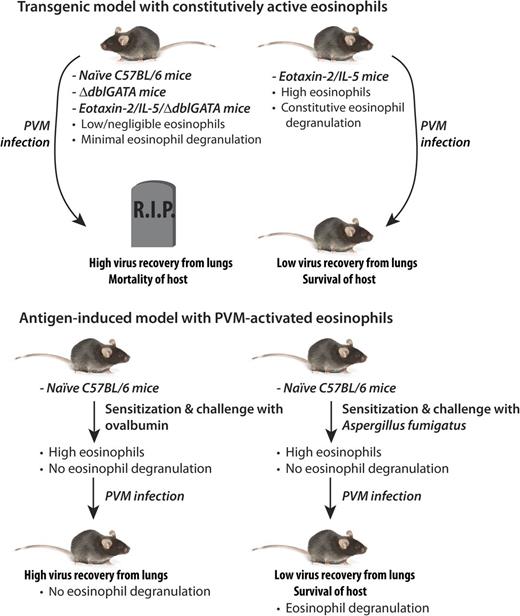
Here, the authors used 2 distinct Th2-driven models of inflammation to determine the effects of eosinophils on lethal inoculation with a respiratory virus. The virus used in their study was pneumonia virus of mice (PVM), a mouse pathogen that is closely related to human RSV. Because human RSV is not a natural pathogen for mice, PVM can be considered a more appropriate alternative model of severe RSV infection in this species. The highly eosinophilic double transgenic eotaxin-2/IL-5 mouse strain,7 which possesses constitutively activated and degranulating eosinophils, showed a substantial twofold log reduction in virus recovery 4 days after PVM inoculation compared with the control strain. When the eosinophil-deficient ΔdblGATA strain was crossed with double transgenic eotaxin-2/IL-5 mice, creating a phenotype expressing high levels of eosinophil-specific cytokines but negligible numbers of eosinophils, virus recovery returned to baseline levels. Thus, decreased virus recovery was related to eosinophils alone and not the high cytokine levels present in the double transgenic eotaxin-2/IL-5 strain. These elegantly conducted experiments confirm earlier studies showing that eosinophils are important in respiratory virus clearance.
However, what was striking was that when survival curves were evaluated in each of these strains following PVM infection, the eosinophil-enriched double transgenic eotaxin-2/IL-5 strain emerged as the only one completely protected against the lethal effects of PVM infection (see figure). All other strains tested—the C57BL/6, the ΔdblGATA, and the eotaxin-2/IL-5/ΔdblGATA strains—succumbed to the fatal effects of virus infection by day 12 of inoculation. This remarkable finding suggests that eosinophils may have a critical role in protection against lethal respiratory virus infection beyond simply virus clearance.
However, these findings were obtained using transgenic mouse strains that may not reflect the pathology of viral infection. What are the effects of antigen-induced eosinophil recruitment to the airways, as would occur in eosinophilic asthma, on subsequent PVM infection?
To address this, the authors tested the effect of sensitization and challenge of mice with the fungus Aspergillus fumigatus to induce eosinophil recruitment to the airways, followed by PVM infection. There was no evidence of eosinophil degranulation in mice treated with A fumigatus alone, even as large numbers of eosinophils accumulated in the lungs. It was only after A fumigatus–treated animals were infected with PVM that high levels of eosinophil granule proteins appeared in the airways (in bronchoalveolar lavage samples).
Eosinophils isolated from animals treated with A fumigatus and PVM showed evidence of piecemeal degranulation, a major mechanism for the release of granule proteins from these cells.8 The presence of granule proteins and evidence of piecemeal degranulation also correlated with a 1.6 log decrease in virus recovery compared with control mice. Finally, and perhaps most importantly, sensitization and challenge with A fumigatus, promoting eosinophil recruitment and activation, led to full protection from an otherwise lethal inoculum of PVM.
In contrast, when animals were sensitized and challenged with ovalbumin, there was no evidence of eosinophil degranulation even when the animals were subsequently infected with PVM. Consequently, there was no reduction in virus recovery in ovalbumin-treated, PVM-infected animals compared with those infected with PVM alone. The reasons for why eosinophils in ovalbumin-treated, PVM-infected mice did not respond in an analogous fashion to that of the A fumigatus model are not clear. The authors speculate that this may be related to differential effects by A fumigatus and ovalbumin on cytokine profiles and that the A fumigatus extract is more complex than ovalbumin, the latter of which is an otherwise inert substance.
In summary, these findings signify several key events associated with eosinophils in virus-induced mortality using two distinct models. First, eosinophil degranulation correlated with virus clearance and increased survival in PVM-infected mice. Second, piecemeal degranulation appeared to be a prominent mechanism by which eosinophils release their cationic granule products in viral infection. Third and finally, antigen sensitization and challenge of otherwise naïve mice is simply insufficient for evoking degranulation responses from eosinophils; degranulation only occurred on subsequent activation of A fumigatus–treated animals with PVM. This would support an important antiviral and prosurvival role for eosinophils and would argue against the concept that eosinophil degranulation may be a vestigial function of this granulocyte lineage.9
For over 2 decades, eosinophil degranulation has been characterized in children infected with RSV.10 If the apparently beneficial effects of eosinophils in respiratory virus infection prove to be similar in humans, then caution would be urged in the application of current therapeutic strategies aimed at profound depletion of eosinophils, particularly in pediatric cases of asthma and allergy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal