Key Points
FcγRIIb-dependent internalization of therapeutic mAbs is dependent on antibody specificity.
FcγRIIb can be activated in both cis and trans configurations.
Abstract
A major feature that distinguishes type I from type II anti-CD20 monoclonal antibodies (mAbs) and reduces their therapeutic efficacy is the tendency to internalize from the cell surface. We have shown previously that the extent of internalization correlates with the capacity of type I mAb to simultaneously engage both CD20 and the inhibitory Fcγ receptor, FcγRIIb, in a bipolar configuration. Here, we investigated whether mAbs directed at other B-cell surface receptors also engaged FcγRIIb and whether this interaction promoted internalization. Most mAbs engaged and activated FcγRIIb, with the strength of activation related to the level of mAb bound to the cell surface. However, engagement did not affect internalization of most mAb-ligated receptors, either in cell lines or primary chronic lymphocytic leukemia cells with the exception of CD19 and CD38. Furthermore, at high cell concentrations/density both cis and trans interactions between cell-surface bound mAb and FcγRIIb were evident, but trans interactions did not inhibit type I anti-CD20 mAb-mediated internalization. These data identify that FcγRIIb is engaged by many mAbs in both cis and trans configurations, triggering its activation, but that internalization via FcγRIIb occurs for only a select subset. These findings have implications when designing new antibody-based therapeutics.
Introduction
Anti-CD20 monoclonal antibodies (mAbs) are classified as type I (rituximab [RTX]-like) or type II (tositumomab-like) based on functional differences that they mediate on B cells in vitro.1 We have recently observed that type I mAbs induce appreciable internalization of CD20 from the surface of B cells, in contrast to type II mAbs that remain largely cell-surface localized.2 The rate of internalization directly correlated with expression of the inhibitory Fcγ receptor IIb (FcγRIIb) (CD32b), and was largely abrogated when target cells were treated with a F(ab′)2 fragment of RTX or coincubated with an anti-FcγRII blocking mAb.3 We demonstrated that type I anti-CD20 mAbs were able to engage and activate FcγRIIb on the surface of B cells via a process termed antibody bipolar bridging4 where CD20 and FcγRIIb were engaged by the antibody in a cis configuration which was sufficient to induce internalization of the mAb:CD20:FcγRIIb complex.3
Despite the widespread success of RTX in the treatment of follicular lymphoma and diffuse large B-cell lymphoma,5 it has proven less successful in the treatment of mantle cell lymphoma (MCL)6 and chronic lymphocytic leukemia (CLL)7 in which larger doses are required.8 Reduced responses in MCL and CLL appear to correspond to a higher expression of FcγRIIb and a faster rate of type I anti-CD20 mAb-mediated internalization of CD20. Furthermore, in 2 separate retrospective studies, higher expression of FcγRIIb on tumor cells was associated with reduced survival or response in patients treated with RTX therapy.3,9 These observations, together with the lower efficacy observed when using type I anti-CD20 mAb in murine models of B-cell depletion,2,10 have led us to propose that type I anti-CD20 mAb-mediated internalization may contribute to treatment failure of mAb therapy in the clinic3,11 supporting the use of noninternalizing type II mAb for more refractory tumor types.
Given the difficulty of finding effective mAb targets on cancer cells, including malignant B cells, we embarked on a study of how FcγRIIb might influence internalization of various target:mAb complexes from the cell surface. It is now established that certain B-cell targets such as CD22 and the B-cell receptor (BCR) are prone to rapid and extensive internalization either as a result of constitutive turnover12 or crosslinking-induced reorganization13 and such reagents are best suited for toxin delivery. In contrast, the utility of other molecules, such as CD19 and CD40 which continue to be explored as targets for mAbs that recruit natural effectors, might also be influenced by FcγRIIB.
Methods
Patient samples and cell lines
CLL or MCL samples were stored in the University of Southampton Cancer Sciences Unit Tumor Bank under Human Tissue Authority licensing.3 Cells were thawed, resuspended in complete media (RPMI 1640, 4mM l-glutamine, 1mM sodium pyruvate, and 10% [v/v (volume to volume)] fetal calf serum; all from Life Technologies), and used on the same day. Cell lines were obtained from the European Collection of Cell Cultures and maintained in complete media. This study was conducted in accordance with the Declaration of Helsinki.
Generation of human FcγRIIb1 and truncated FcγRIIb plasmid vectors
Human FcγRIIb1 complementary DNA (cDNA) was amplified from Daudi cells using specific primers and cloned into the pCDNA3.1 expression vector (Life Technologies). To generate a truncated (Tr) mutant version of FcγRIIb lacking the intracellular domain, a stop mutation at residue 250 was introduced using the QuickChange Multi Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer’s instructions.
Cell transfection and generation of stable transfectants
Cells were transfected by electroporation using Nucleofector kit V on the Nucleofector I device according to the manufacturer’s instructions (Lonza). Ramos and RPMI 8226 cells were transfected using programs O-06 and G-15, respectively. Stable transfectants were selected with 1 mg/mL G418 (Geneticin; Life Technologies) for Ramos cells or 0.5 mg/mL G418 for RPMI 8226 cells. Colonies were screened for cell-surface FcγRIIb expression using AT10-PE (AbD Serotec) by flow cytometry. FcγRIIb2-transfected Ramos cells were generated previously.3
Reverse transcription-PCR of FcγRIIb isoforms
Total RNA was isolated using the RNeasy minikit (Qiagen); cDNA was prepared using the Superscript III first-strand synthesis system (Life Technologies) before polymerase chain reaction (PCR) using primers specific to FcγRIIb1/b2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control.
mAb production and labeling
GA101-gly (anti-CD20, human immunoglobulin G1 [hIgG1], and mouse IgG2a [mIgG2a]) are the nonglycoengineered parent versions of GA101 and were produced in-house from patent-published sequences. RTX was gifted by Southampton General Hospital oncology pharmacy. AT13/5h (anti-CD38, chimeric hIgG114 ), RFB9 (anti-CD19, mIgG115 ), LOB 7-4 (anti-CD40, mIgG1), LOB 7-6 (anti-CD40, mIgG1), F3.3 (anti–major histocompatibility complex class II [MHCII], mIgG116 ), M15/8 (anti-µ, mIgG115 ), AT10 (anti-FcγRII, mIgG117 ), MB1/7 (anti-CD37, mIgG118 ), and 4KB128 (anti-CD22, mIgG119 ) were produced in-house. Antibodies were labeled with Alexa Fluor 488 (A488)–tetrafluorophenyl ester according to the manufacturer’s instructions (Life Technologies).
Antibody internalization assay
Internalization of A488-labeled mAb was quantified as reported previously2,3 using the following formula: % cell-surface mAb remaining on B cells = (mean fluorescence intensity [MFI] of unquenched − MFI of quenched)/MFI of unquenched × 100. The MFI of unstained cells was subtracted as background.
To investigate the effect of cell-surface FcγRIIb on mAb internalization, FcγRIIb was blocked with AT10 (50 μg/mL) for 30 minutes at 37°C as previously described.3 In coculture experiments, Ramos FcγRIIb1 high transfectants were prestained with PKH26 (Sigma-Aldrich) and mixed 1:1 with transfected RPMI 8226 cells.
Western blotting
Cells plated at 4 × 106/mL were preincubated at 37°C for 30 minutes before the addition of A488-labeled mAb (5 µg/mL) for 30 minutes, then washed in ice-cold phosphate-buffered saline (PBS), resuspended in lysis buffer, and processed as described previously.20 Membranes were probed with EP926Y (rabbit anti-human FcγRIIb [phospho-specific]; Cambridge Bioscience) in Tris-buffered saline–Tween 0.05%/5% bovine serum albumin/0.05% sodium azide at 4°C overnight, then with donkey anti-rabbit IgG horseradish peroxidase–linked F(ab′)2 for 1 hour at room temperature, washed, and the signal visualized using enhanced chemiluminescence reagents and light-sensitive film (all GE Healthcare Lifesciences). In coculture experiments, cells were stained with A488-labeled mAb (5 µg/mL) for 30 minutes at 4°C, washed 3 times with ice-cold PBS, then mixed 1:1 with unlabeled cells, incubated at 37°C for 30 minutes, and processed for western blotting.
Statistical analysis
Analyses were performed using nonparametric tests with a 2-tailed hypothesis. Unpaired samples were analyzed using the Mann-Whitney U test and paired samples were analyzed using the Wilcoxon signed-ranks test using Graphpad Prism version 6.00 for Windows (Graphpad Software).
Results
FcγRIIb1 and FcγRIIb2 augment the internalization of mAb on the surface of B cells
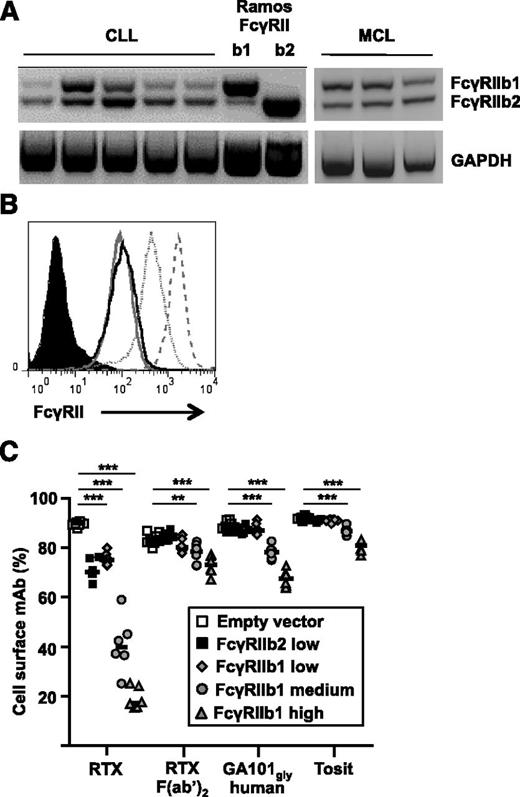
In a previous study with a small number of CLL samples, we showed that the predominant isoform of FcγRIIb expressed was b23 and that transfection of Ramos cells with the b2 isoform augmented the internalization of type I anti-CD20 mAbs.3 However, the predominant isoform expressed on nonmalignant B cells is b1 which has an extra 19 amino acids in its intracellular tail that prevent its internalization when coligated with the BCR.21 Subsequent analysis of a further 5 CLL samples and 3 MCL samples revealed that FcγRIIb1 was also expressed at substantial levels on malignant cells, albeit variably between patients (Figure 1A).
Expression and activity of FcγRIIb1 and b2 isoforms in regulating the rate of internalization of anti-CD20 mAbs. (A) mRNA from CLL and MCL samples alongside Ramos-FcγRIIb1 or -FcγRIIb2 transfectants as controls was converted to cDNA and analyzed by PCR for the expression of FcγRIIb1 and FcγRIIb2. (B) Ramos cells were transfected with empty vector, FcγRIIb2, or FcγRIIb1 and stable transfectants selected expressing different levels of FcγRIIb. Control cells (filled histogram), FcγRIIb2 low (solid black line), FcγRIIb1 low (solid gray line), FcγRIIb1 medium (dotted line), and FcγRIIb1 high (dashed line) were labeled with AT10-PE and assessed by flow cytometry. (C) Ramos transfectants were cultured with 5 µg/mL A488-labeled anti-CD20 mAb for 1 hour. The proportion of total mAb remaining on the cell surface was assessed by flow cytometry after treatment of cells with anti-A488 to quench cell-surface fluorescence. Transfectants were compared with control cells using the Mann-Whitney U test. **P < .01, ***P < .001, n = 6-8. Horizontal bars represent the median. mRNA, messenger RNA.
Expression and activity of FcγRIIb1 and b2 isoforms in regulating the rate of internalization of anti-CD20 mAbs. (A) mRNA from CLL and MCL samples alongside Ramos-FcγRIIb1 or -FcγRIIb2 transfectants as controls was converted to cDNA and analyzed by PCR for the expression of FcγRIIb1 and FcγRIIb2. (B) Ramos cells were transfected with empty vector, FcγRIIb2, or FcγRIIb1 and stable transfectants selected expressing different levels of FcγRIIb. Control cells (filled histogram), FcγRIIb2 low (solid black line), FcγRIIb1 low (solid gray line), FcγRIIb1 medium (dotted line), and FcγRIIb1 high (dashed line) were labeled with AT10-PE and assessed by flow cytometry. (C) Ramos transfectants were cultured with 5 µg/mL A488-labeled anti-CD20 mAb for 1 hour. The proportion of total mAb remaining on the cell surface was assessed by flow cytometry after treatment of cells with anti-A488 to quench cell-surface fluorescence. Transfectants were compared with control cells using the Mann-Whitney U test. **P < .01, ***P < .001, n = 6-8. Horizontal bars represent the median. mRNA, messenger RNA.
To investigate whether the b1 isoform could also promote type I anti-CD20 mAb-mediated internalization, FcγRIIb-negative Ramos cells were transfected with human FcγRIIb1. Colonies expressing low, medium, or high levels of the receptor were selected (Figure 1B), reflecting the expression of FcγRIIb1 on normal human B cells, primary lymphoma cells overexpressing the receptor, and an even higher (likely nonphysiological) level of FcγRIIb1, respectively. Empty vector–transfected cells were used as controls. Cells were cultured with A488-labeled anti-CD20 mAbs and the proportion of total mAb remaining on the cell surface was quantified after 1 and 6 hours (Figure 1C and supplemental Figure 1, respectively, available on the Blood Web site).
Expression of FcγRIIb1 at a physiological level (FcγRIIb1-low) resulted in a significant increase in internalized RTX compared with controls at both time points. Internalization was confirmed by both confocal microscopy and reduced detection of human IgG at the cell surface by flow cytometry (supplemental Figure 2). Neither CD20 shedding nor shaving appeared to occur (data not shown). Using transfectants expressing almost identical physiological levels of the b1 and b2 isoforms (MFI 152; b2 as used previously3 ), we were also able to confirm that both accelerate internalization of surface-bound mAb to a similar extent (Figure 1C). There was no significant internalization of the type II anti-CD20 mAb tositumomab (Tosit) or nonglycomodified forms of GA101 (GA101-gly) of hIgG1 or mIgG2 (m2a) isotypes (Figure 1C and supplemental Figure 1). As before with FcγRIIb2 transfectants,3 there was a dose- and time-dependent increase in the internalization of RTX in cells expressing higher levels of FcγRIIb1. There was also increased internalization of type II anti-CD20 mAbs with high levels of FcγRIIb1, but to a far lesser extent than RTX, with tositumomab being the most resistant to internalization (supplemental Figure 1A). As before,3 internalization was also related to isotype, with mouse 2a promoting less internalization than hIgG1 and mIgG1 (supplemental Figure 1B). Equivalent activity was also observed with mouse FcγRIIb1 and FcγRIIb2 isoforms transfected into Ramos cells (supplemental Figure 3).
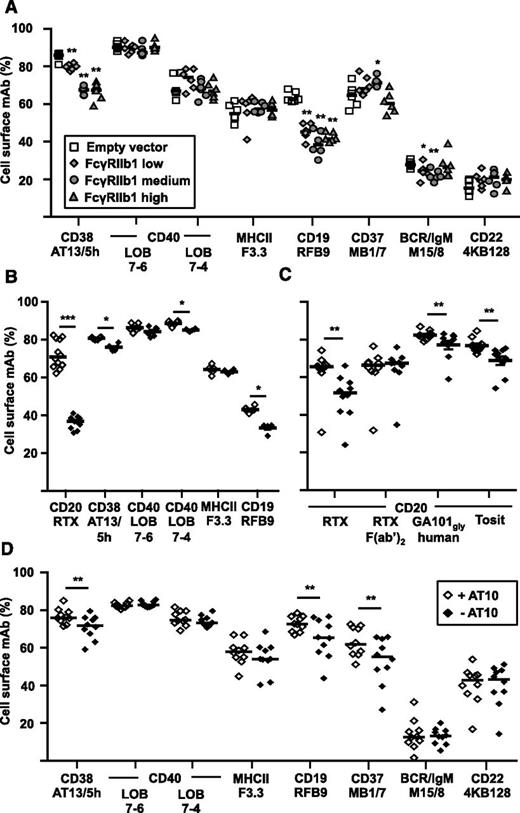
We next asked whether FcγRIIb expression affected the internalization of mAbs recognizing other targets (CD19, CD22, CD37, CD38, CD40, MHCII, and the BCR [IgM]). As we have observed differences in the ability of different IgG isotypes to activate FcγRIIb (supplemental Figure 4), all of the mAbs we chose were either human or mouse IgG1, which give equivalent activity in internalization assays with anti-CD20 mAb (supplemental Figure 1B), to minimize this effect. Using the low, medium, and high FcγRIIb1 transfectants, there was a significant increase in internalization of anti-CD19 and anti-CD38 mAb in the presence of FcγRIIb1, albeit to a lesser extent than with RTX (Figure 2A). The anti-CD19 mAb (RFB9) showed increased internalization with cells expressing FcγRIIb1 but, unlike RTX, an obvious dose response to the different levels of expression was not observed. The anti-MHCII, anti-CD37, and anti-CD40 mAb remained largely on the cell surface although a slow rate of internalization of anti-MHCII and anti-CD37 in particular was observed. However, all 3 specificities were unaffected by overexpression of FcγRIIb1. As expected,12,13 the anti-BCR and anti-CD22 mAbs were rapidly internalized independently of FcγRIIb1 expression. The results suggest that the effect of FcγRIIb expression on internalization is confined to a subset of mAbs, most prominently, the type I anti-CD20 mAbs.
Effect of FcγRIIb1 on the rate of internalization of other mAb-ligated receptors. (A) Ramos control, FcγRIIb1-low, FcγRIIb1-medium, and FcγRIIb1-high transfectants were cultured with 5 µg/mL A488-labeled mAbs for 6 hours and the proportion of total mAb remaining on the cell surface assessed as in Figure 1. *P < .05, **P < .01. (B) Ramos FcγRIIb1 low transfectants or (C-D) primary CLL cells were incubated with 50 µg/mL AT10 for 30 minutes to block the Fc binding site of FcγRIIb, or left untreated, then cultured as in panel A and the proportion of surface mAb was assessed. AT10-treated cells were compared with untreated cells using the Wilcoxon signed-ranks test. *P < .05, **P < .01, ***P < .001; n = 6-12 (B) and 10 (C-D). Horizontal bars represent the median.
Effect of FcγRIIb1 on the rate of internalization of other mAb-ligated receptors. (A) Ramos control, FcγRIIb1-low, FcγRIIb1-medium, and FcγRIIb1-high transfectants were cultured with 5 µg/mL A488-labeled mAbs for 6 hours and the proportion of total mAb remaining on the cell surface assessed as in Figure 1. *P < .05, **P < .01. (B) Ramos FcγRIIb1 low transfectants or (C-D) primary CLL cells were incubated with 50 µg/mL AT10 for 30 minutes to block the Fc binding site of FcγRIIb, or left untreated, then cultured as in panel A and the proportion of surface mAb was assessed. AT10-treated cells were compared with untreated cells using the Wilcoxon signed-ranks test. *P < .05, **P < .01, ***P < .001; n = 6-12 (B) and 10 (C-D). Horizontal bars represent the median.
Having established proof of concept using transfectants, we extended our study to primary tumor cells using a cohort of 10 CLL samples. For this analysis, we used a blocking anti-FcγRII mAb (AT10) which we have previously shown capable of impairing the internalization of RTX from FcγRIIb2-transfected cell lines and CLL cells.3 However, prior to our experiments on CLL cells, we also assessed the ability of AT10 to block the internalization of mAb targets from Ramos-FcγRIIb1 transfectants (Figure 2B).
As expected, the data broadly paralleled that in Figure 1C and Figure 2A and confirmed the ability of AT10 to impair FcγRIIb1-mediated internalization of RTX, CD19, and CD38 mAbs. We then compared the internalization of mAbs on CLL cells with and without AT10 (Figure 2C-D). In agreement with previous observations3 and despite the highly variable levels of RTX and AT10 binding observed between patients (supplemental Table 1), AT10 treatment significantly reduced the RTX internalization (Figure 2C) but had a much smaller, though still significant, effect on the internalization of both type II anti-CD20 mAb. There was no effect on internalization of RTX F(ab)2.
Consistent with the results from FcγRIIb1 transfectants (Figure 2A), AT10 treatment significantly decreased internalization of CD19 and CD38 mAb, suggesting that FcγRIIb promotes their internalization in primary CLL cells (Figure 2D). Of note, the internalization of the anti-CD37 mAb MB1/7 by CLL cells was also significantly inhibited by AT10 even though expression of FcγRIIb1 had no effect on its internalization by Ramos transfectants. Internalization of mAbs specific for CD19, CD22, CD40, MHCII, and the BCR (IgM) were unaffected by AT10.
mAb ligation of B-cell surface receptors activates FcγRIIb
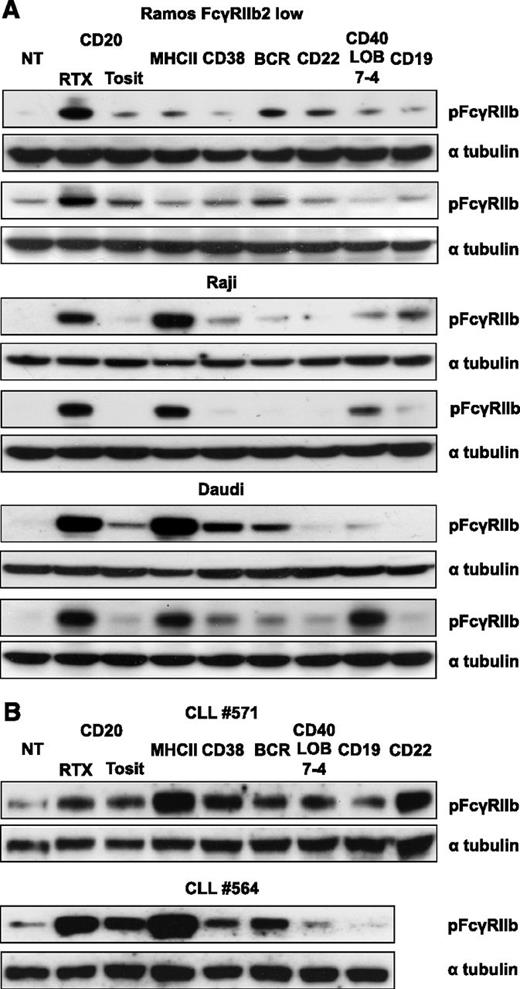
The failure of FcγRIIb to affect the internalization of most mAbs and the subtle effect on the internalization of others led us to speculate whether type I anti-CD20 mAbs were unique in their ability to engage and activate FcγRIIb. We have previously demonstrated that in Daudi cells RTX, but not tositumomab, induces phosphorylation of FcγRIIb.3 To investigate whether ligation of other receptors results in FcγRIIb activation, Raji, Daudi, and Ramos-FcγRIIb–transfected cells were treated with A488-labeled mAb and probed for phosphorylated FcγRIIb (pFcγRIIb) (Figure 3A). Most of the mAbs displayed some ability to phosphorylate FcγRIIb but at lower levels than RTX. The anti-MHCII mAb induced pronounced phosphorylation in Raji and Daudi cells but not Ramos-FcγRIIb transfectants. We have previously observed that Ramos cells express low levels of MHCII and so explored a possible link between expression and ability to phosphorylate FcγRIIb. In general, the level of phosphorylation corresponded to the level of surface-bound mAb (Table 1), with RTX and MHCII mAb binding at high levels (except for MHCII on Ramos cells) and inducing most phosphorylation, and the CD22 mAb the least. Similar results were obtained with primary CLL cells (Figure 3B).
Phosphorylation of FcγRIIb after mAb ligation of B-cell surface receptors. The Burkitt lymphoma cell lines (A) Ramos FcγRIIb2-low transfectants, Raji, and Daudi, and (B) primary CLL cells were treated with 5 µg/mL A488-labeled mAb for 30 minutes. Lysates were then blotted for pFcγRIIb and α tubulin as a loading control. Two representative examples for each cell line are shown.
Phosphorylation of FcγRIIb after mAb ligation of B-cell surface receptors. The Burkitt lymphoma cell lines (A) Ramos FcγRIIb2-low transfectants, Raji, and Daudi, and (B) primary CLL cells were treated with 5 µg/mL A488-labeled mAb for 30 minutes. Lysates were then blotted for pFcγRIIb and α tubulin as a loading control. Two representative examples for each cell line are shown.
Level of binding of fluorochrome-labeled mAbs on the surface of B cells (MFI)
| mAb . | Ramos . | Raji . | Daudi . | CLL #571 . | CLL #564 . |
|---|---|---|---|---|---|
| RTX A488 | 500 | 786 | 1017 | 185 | 2037 |
| Tosit A488 | 226 | 313 | 527 | 80 | 1624 |
| MHCII (F3.3) A488 | 290 | 2142 | 1983 | 3504 | 4314 |
| CD38 (AT13/5h) A488 | 129 | 289 | 346 | 145 | 134 |
| BCR/IgM (M15/8) A488 | 358 | 80 | 107 | 127 | 1189 |
| CD22 (4KB128) A488 | 52 | 51 | 93 | 55 | 99 |
| CD40 (LOB 7-4) A488 | 208 | 323 | 402 | 130 | 140 |
| CD19 (RFB9) A488 | 92 | 298 | 365 | 354 | 524 |
| CD32 (AT10) PE | 104 | 97 | 93 | 128 | 172 |
| mAb . | Ramos . | Raji . | Daudi . | CLL #571 . | CLL #564 . |
|---|---|---|---|---|---|
| RTX A488 | 500 | 786 | 1017 | 185 | 2037 |
| Tosit A488 | 226 | 313 | 527 | 80 | 1624 |
| MHCII (F3.3) A488 | 290 | 2142 | 1983 | 3504 | 4314 |
| CD38 (AT13/5h) A488 | 129 | 289 | 346 | 145 | 134 |
| BCR/IgM (M15/8) A488 | 358 | 80 | 107 | 127 | 1189 |
| CD22 (4KB128) A488 | 52 | 51 | 93 | 55 | 99 |
| CD40 (LOB 7-4) A488 | 208 | 323 | 402 | 130 | 140 |
| CD19 (RFB9) A488 | 92 | 298 | 365 | 354 | 524 |
| CD32 (AT10) PE | 104 | 97 | 93 | 128 | 172 |
FcγRIIb activation is associated with the level of cell-surface mAb binding
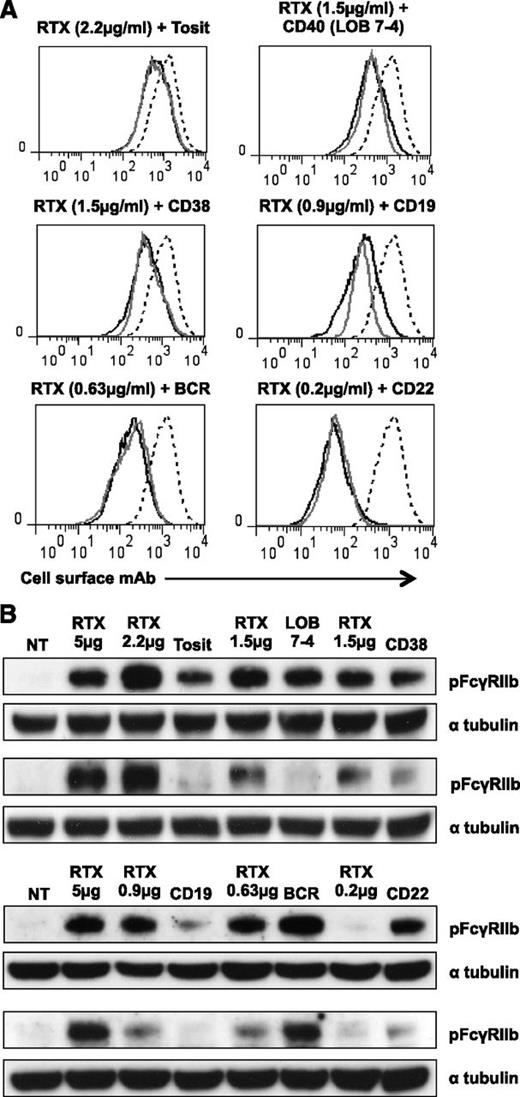
Although RTX was not unique in its ability to activate FcγRIIb, the response was greater and more consistent than with other mAb. This may be due to more mAb bound to the cell surface (Table 1), or alternatively, RTX may be more potent at activating FcγRIIb. To investigate this, we titrated RTX to provide a comparable level of cell-bound mAb to a range of other mAbs (Figure 4A) and repeated probing for pFcγRIIb (Figure 4B). RTX consistently induced more phosphorylation than tositumomab and CD19 mAb, even with the same level of surface staining. LOB 7-4 (CD40) and AT13/5h (CD38) did not always induce phosphorylation of FcγRIIb (Figures 3 and 4), but when they did, the levels were similar to those seen with RTX (Figure 4). In contrast, IgM and to a lesser extent CD22 mAb induced equivalent or more phosphorylation than RTX, suggesting that type I mAbs are not inherently more potent at activating FcγRIIb but that their effect largely reflects surface binding.
Phosphorylation of FcγRIIb after mAb binding to B-cell surface receptors at the same level. (A) A488-labeled RTX (solid black line) was titrated on Daudi cells to give the same binding as A488-labeled mAbs (5 μg/mL) specific for a range of other receptors (solid gray line). The binding of a saturating concentration of RTX (5 µg/mL) is included for comparison (dashed line). (B) Daudi cells were treated with A488-labeled RTX (RTX at concentration shown, others at 5 μg/mL) for 30 minutes and lysates were then blotted for pFcγRIIb and α tubulin as a loading control. Two representative examples are shown.
Phosphorylation of FcγRIIb after mAb binding to B-cell surface receptors at the same level. (A) A488-labeled RTX (solid black line) was titrated on Daudi cells to give the same binding as A488-labeled mAbs (5 μg/mL) specific for a range of other receptors (solid gray line). The binding of a saturating concentration of RTX (5 µg/mL) is included for comparison (dashed line). (B) Daudi cells were treated with A488-labeled RTX (RTX at concentration shown, others at 5 μg/mL) for 30 minutes and lysates were then blotted for pFcγRIIb and α tubulin as a loading control. Two representative examples are shown.
Cell-surface–specific mAbs interact with FcγRIIb in cis via bipolar antibody bridging
At the high cell concentrations used in Figures 3 and 4 (4 × 106/mL), we have previously shown that cell-cell contact between adjacent cells may occur3 allowing both cis and trans interactions between surface mAbs and FcγRIIb. We considered whether the susceptibility of type I anti-CD20 mAbs to FcγRIIb-mediated internalization may be explained by a unique ability to engage FcγRIIb in a cis fashion. Daudi cells (natively expressing FcγRIIb) were labeled with a panel of A488-labeled mAbs at an 80 times lower density (5 × 104 cells/mL). After extensive washing, these were mixed 1:1 with unlabeled FcγRIIb-negative Ramos cells at a final concentration of 1 × 105/mL to minimize cell-cell contact.3 When probed for pFcγRIIb, we found a similar pattern to that observed at high density (compare Figure 5A left panel with Figure 3A), suggesting that all mAbs could engage FcγRIIb in a cis fashion. In the reverse experiment mixing mAb-labeled FcγRIIb-negative Ramos cells with unlabeled Daudi cells, none of the mAbs induced FcγRIIb phosphorylation (Figure 5A, right panel), confirming that at low cell density they interact with (and phosphorylate) FcγRIIb through cis interactions on the cell surface.
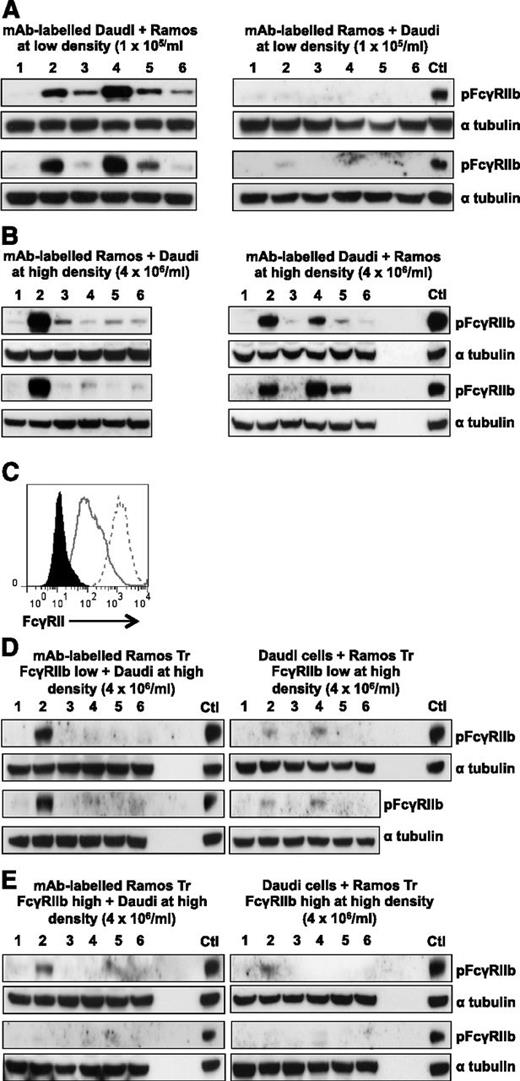
B-cell surface receptor–specific mAbs interact with and activate FcγRIIb in a cis and trans fashion. (A) FcγRIIb-positive Daudi cells (left) or FcγRIIb-negative Ramos cells (right) were incubated with 5 µg/mL A488-labeled mAb at 5 × 104 cells/mL at 4°C. After extensive washing, the cells were mixed 1:1 with FcγRIIb-negative Ramos cells (left) or FcγRIIb-positive Daudi cells (right) at a final concentration of 1 × 105 cells/mL for 30 minutes at 37°C. Lysates were blotted for pFcγRIIb and α tubulin as a loading control. Sample lanes: (1) NT, (2) RTX, (3) Tosit, (4) F3.3 (MHC II), (5) AT13/5h (CD38), (6) M15/8 (BCR). (B) A488-mAb labeled FcγRIIb-negative Ramos cells (left) or FcγRIIb-positive Daudi cells (right) were mixed 1:1 with FcγRIIb-positive Daudi cells (left) or FcγRIIb-negative Ramos cells (right) at a final concentration of 4 × 106 cells/mL then lysates were blotted as described in panel A. (C) Ramos cells B-cell surface receptors were transfected with empty vector, or Tr FcγRIIb and assessed for expression by flow cytometry using AT10-PE. Clones expressing low (solid gray line) or high (dotted line) were compared with control cells (filled histogram). (D) Ramos Tr FcγRIIb-low (left) or FcγRIIb-positive Daudi cells (right) were incubated with 5 µg/mL A488-labeled mAb at 4 × 106 cells/mL at 4°C. After washing, the cells were mixed 1:1 with untransfected Daudi cells (left) or Ramos Tr FcγRIIb-low (right) cells at 4 × 106 cells/mL for 30 minutes at 37°C. Lysates were then blotted as in panel A. (E) As in panel D but using Ramos Tr FcγRIIb-high cells. In all blots, a positive control of A488-RTX–labeled FcγRIIb-positive Daudi cells mixed 1:1 with FcγRIIb-negative Ramos cells at a final concentration of 4 × 106/mL was included. Two representative blots are shown for each condition. Ctl, control.
B-cell surface receptor–specific mAbs interact with and activate FcγRIIb in a cis and trans fashion. (A) FcγRIIb-positive Daudi cells (left) or FcγRIIb-negative Ramos cells (right) were incubated with 5 µg/mL A488-labeled mAb at 5 × 104 cells/mL at 4°C. After extensive washing, the cells were mixed 1:1 with FcγRIIb-negative Ramos cells (left) or FcγRIIb-positive Daudi cells (right) at a final concentration of 1 × 105 cells/mL for 30 minutes at 37°C. Lysates were blotted for pFcγRIIb and α tubulin as a loading control. Sample lanes: (1) NT, (2) RTX, (3) Tosit, (4) F3.3 (MHC II), (5) AT13/5h (CD38), (6) M15/8 (BCR). (B) A488-mAb labeled FcγRIIb-negative Ramos cells (left) or FcγRIIb-positive Daudi cells (right) were mixed 1:1 with FcγRIIb-positive Daudi cells (left) or FcγRIIb-negative Ramos cells (right) at a final concentration of 4 × 106 cells/mL then lysates were blotted as described in panel A. (C) Ramos cells B-cell surface receptors were transfected with empty vector, or Tr FcγRIIb and assessed for expression by flow cytometry using AT10-PE. Clones expressing low (solid gray line) or high (dotted line) were compared with control cells (filled histogram). (D) Ramos Tr FcγRIIb-low (left) or FcγRIIb-positive Daudi cells (right) were incubated with 5 µg/mL A488-labeled mAb at 4 × 106 cells/mL at 4°C. After washing, the cells were mixed 1:1 with untransfected Daudi cells (left) or Ramos Tr FcγRIIb-low (right) cells at 4 × 106 cells/mL for 30 minutes at 37°C. Lysates were then blotted as in panel A. (E) As in panel D but using Ramos Tr FcγRIIb-high cells. In all blots, a positive control of A488-RTX–labeled FcγRIIb-positive Daudi cells mixed 1:1 with FcγRIIb-negative Ramos cells at a final concentration of 4 × 106/mL was included. Two representative blots are shown for each condition. Ctl, control.
Cell-surface–specific mAbs interact with FcγRIIb in cis and trans at high cell concentrations
We then determined whether mAbs can also interact with adjacent cells in trans. FcγRIIb-negative Ramos cells were labeled with A488 mAb then washed and mixed with Daudi cells at a final concentration of 4 × 106/mL. In this system, phosphorylation is induced by trans interactions between surface mAb on Ramos and FcγRIIb on Daudi cells. Only RTX induced robust phosphorylation (Figure 5B, left panel) reflecting the level of mAb binding on Ramos cells. The reverse experiment with A488-labeled Daudi mixed with Ramos was performed as a control (Figure 5B, right panel).
In Figure 5A-B, B cells were at low density or FcγRIIb was only present on a single cell type, preventing trans and cis interactions from occurring, respectively. Next, we wished to investigate whether cis or trans interactions predominate at high density when FcγRIIb is present on both populations. To examine whether cis interactions predominate we used Ramos cells transfected with a Tr version of FcγRIIb which can bind to IgG Fc but cannot be phosphorylated as it lacks the intracellular domain. Transfectants expressing Tr FcγRIIb at approximately the same level as Daudi cells (Ramos Tr FcγRIIb low) or expressing a much higher level (Ramos Tr FcγRIIb high) (Figure 5C) were used to repeat the experiments in Figure 5B but here, pFcγRIIb represents a trans interaction between mAbs bound to the Ramos transfectants and FcγRIIb on Daudi cells. With the exception of RTX, pFcγRIIb was lower with A488-mAb–labeled Ramos Tr FcγRIIb low cells. With the Ramos Tr FcγRIIb high cells, pFcγRIIb was also lower in response to RTX, compared with FcγRIIb-negative Ramos cells (Figure 5B left panel). These results suggest that cis interactions between Tr FcγRIIb and surface mAbs on Ramos cells were able to compete with trans interactions of FcγRIIb on Daudi cells. The strong signal in response to RTX in Figure 5D (left panel) may be due to some RTX Fc still being available for trans interaction with Daudi FcγRIIb after saturation of the low level of surface Tr FcγRIIb; this is supported by the reduced signal with the Tr FcγRIIb high cells (Figure 5E, left panel).
The level of pFcγRIIb was also lower when mAb-labeled Daudi cells were mixed with Ramos Tr FcγRIIb cells (Figure 5D-E, right panel) suggesting that trans binding of the Fc with Tr FcγRIIb on the transfectants was able to compete with cis binding on the Daudi cell.
Trans interactions do not inhibit type I anti-CD20 mAb-mediated internalization
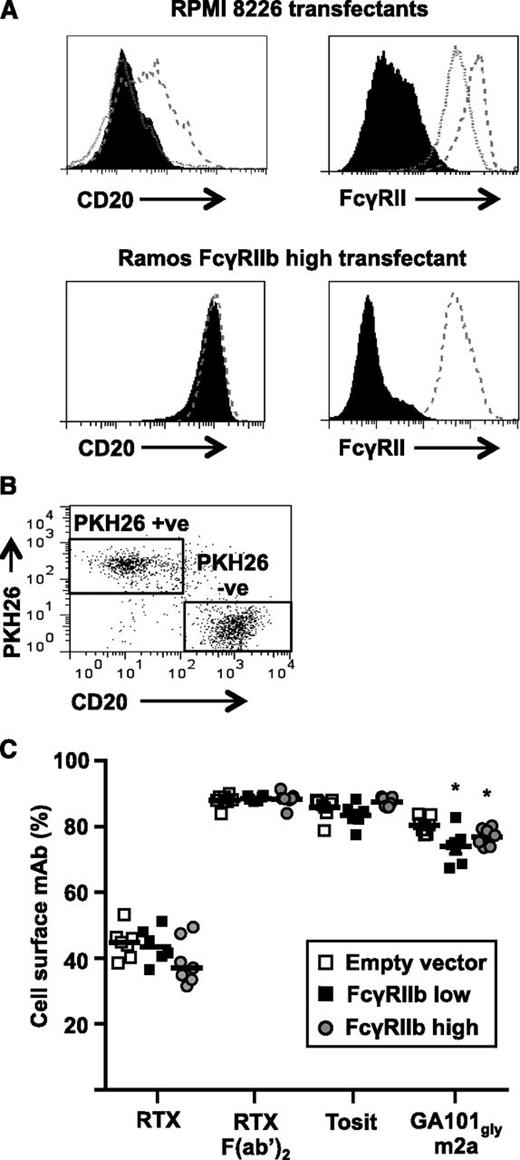
Having established that competition from FcγRIIb expressed in trans impairs cis-mediated FcγRIIb phosphorylation, we hypothesized that it may affect the FcγRIIb-mediated internalization of CD20 mAbs. To investigate this we transfected CD20-negative FcγRIIb-negative RPMI 8226 cells with FcγRIIb1 and selected colonies expressing low or high levels (Figure 6A). Cells transfected with empty vector were used as controls. The transfectants were stained with PKH26 and mixed 1:1 with Ramos-FcγRIIb1-high cells and cultured with A488-labeled anti-CD20 mAbs for 1 hour (Figure 6C). The proportion of mAb remaining on the cell surface of Ramos-FcγRIIb1 high cells (PKH26-negative; Figure 6B) was compared. RTX internalization was the same in Ramos cells cultured with RPMI 8226 FcγRIIb1-high cells and FcγRIIb-negative controls, inferring that FcγRIIb1 expressed on adjacent cells (with the potential for trans interaction) does not impair internalization even when expressed at high levels. Furthermore, this lack of effect was seen over a range of RTX concentrations and levels of FcγRIIb1.
FcγRIIb1 in trans does not inhibit the rate of anti-CD20 mAb internalization. (A) CD20-negative FcγRIIb-negative RPMI 8226 cells were transfected with empty vector or human FcγRIIb1 and stable transfectants were selected expressing different levels of FcγRIIb1 as assessed by flow cytometry. Control cells (filled histogram), FcγRIIb1 low cells (dotted line), and FcγRIIb1 high cells (dashed line) were stained with RTX F(ab′)2-A488 (top left panel) and AT10-PE (top right panel). Equivalent staining of Ramos control cells transfected with empty vector (solid histogram) and Ramos FcγRIIb1 high cells (dashed line) stained with RTX F(ab′)2-A488 (bottom left panel) and AT10-PE (bottom right panel) is included for comparison. (B-C) Unlabeled Ramos FcγRIIb1-high cells were mixed 1:1 with PKH26-labeled RPMI 8226 FcγRIIb-negative control, RPMI 8226 FcγRIIb1-low or RPMI 8226-high cells and cultured with 5 µg/mL A488-labeled anti-CD20 mAb for 1 hour. The proportion of total mAb remaining on the cell surface of PKH26-negative Ramos FcγRIIb1 high cells was assessed by flow cytometry after treatment of cells with anti-A488 to quench cell-surface fluorescence. (B) Dot plot showing the gating strategy used to discriminate PKH26-positive RPMI 8226 transfectants from PKH26-negative Ramos FcγRIIb1-high transfectants. (C) Ramos FcγRIIb1-high transfectants cocultured with FcγRIIb-expressing RPMI 8226 and RPMI 8226 FcγRIIb-negative control cells were compared using the Mann-Whitney U test. Horizontal bars represent the median. *P < .05; n = 6.
FcγRIIb1 in trans does not inhibit the rate of anti-CD20 mAb internalization. (A) CD20-negative FcγRIIb-negative RPMI 8226 cells were transfected with empty vector or human FcγRIIb1 and stable transfectants were selected expressing different levels of FcγRIIb1 as assessed by flow cytometry. Control cells (filled histogram), FcγRIIb1 low cells (dotted line), and FcγRIIb1 high cells (dashed line) were stained with RTX F(ab′)2-A488 (top left panel) and AT10-PE (top right panel). Equivalent staining of Ramos control cells transfected with empty vector (solid histogram) and Ramos FcγRIIb1 high cells (dashed line) stained with RTX F(ab′)2-A488 (bottom left panel) and AT10-PE (bottom right panel) is included for comparison. (B-C) Unlabeled Ramos FcγRIIb1-high cells were mixed 1:1 with PKH26-labeled RPMI 8226 FcγRIIb-negative control, RPMI 8226 FcγRIIb1-low or RPMI 8226-high cells and cultured with 5 µg/mL A488-labeled anti-CD20 mAb for 1 hour. The proportion of total mAb remaining on the cell surface of PKH26-negative Ramos FcγRIIb1 high cells was assessed by flow cytometry after treatment of cells with anti-A488 to quench cell-surface fluorescence. (B) Dot plot showing the gating strategy used to discriminate PKH26-positive RPMI 8226 transfectants from PKH26-negative Ramos FcγRIIb1-high transfectants. (C) Ramos FcγRIIb1-high transfectants cocultured with FcγRIIb-expressing RPMI 8226 and RPMI 8226 FcγRIIb-negative control cells were compared using the Mann-Whitney U test. Horizontal bars represent the median. *P < .05; n = 6.
Discussion
We have previously demonstrated that type I anti-CD20 mAbs engage FcγRIIb by bipolar antibody bridging, resulting in internalization of the mAb:CD20:FcγRIIb complex.2,3 Here, we confirmed that both b1 and b2 isoforms of FcγRIIb are expressed in malignant B cells (CLL and MCL) and demonstrated that the native B-cell b1 isoform of FcγRIIb augments the internalization of RTX in the same manner and to an equivalent extent to FcγRIIb2. Given that only FcγRIIb2 has previously been shown to elicit efficient internalization21,22 this is perhaps surprising. Previous studies examined immune complex internalization whereas here we investigated internalization of mAb:target:FcγRIIb complexes. These findings indicate that alternative trafficking mechanisms, perhaps not based upon coated pits are required for mAb:target:FcγRIIb internalization. We are currently studying these mechanisms in further detail. Overexpression of either isoform is therefore able to potentiate the internalization of type I anti-CD20 mAbs, rendering them less effective. As previously observed,2,3 type II anti-CD20 mAbs were less efficiently internalized than type I. Interestingly, at very high levels of FcγRIIb1 expression (far greater than typically expressed on normal B cells), internalization of certain type II mAbs was induced. The degree of internalization appeared related to both the epitope specificity and isotype of the mAb as GA101 m2a was less readily internalized than GA101 hIgG1 (which interacts more strongly with human FcγRIIb23 ) but more readily internalized than tositumomab (m2a), which was only very modestly affected by FcγRIIb expression. These observations may have implications for further refinements of anti-CD20 mAbs.
We also investigated the effect of FcγRIIb on other mAb specificities. With most mAbs, expression of FcγRIIb had no effect on the rate of internalization. The 2 exceptions were anti-CD19 and anti-CD38 mAb which showed small but statistically significant increases in internalization with physiological levels of FcγRIIb. A second anti-CD19 mAb had similar activity (data not shown) indicating that therapeutic mAbs directed at this target may also be reduced in efficacy through this mechanism, particularly in tumors expressing a high level of FcγRIIb. Of interest, 2 anti-CD40 mAbs (LOB 7-4 and LOB 7-6) remained almost entirely at the cell surface in the presence or absence of FcγRIIb on both CLL cells and Ramos transfectants, suggesting that anti-CD40 mAbs may hold promise for directly targeting CD40-expressing tumors as suggested previously.24 One of these, LOB 7-4 has recently been chimerized and is completing phase 1 trials.25
The lack of internalization was not due to an inability of bound mAbs to engage FcγRIIb because most mAbs were able to phosphorylate FcγRIIb in cis, similar to RTX. In contrast to our previous observations,3 we also saw activation of FcγRIIb in response to tositumomab, albeit at a reduced level than by RTX. Type II mAbs bind at approximately half the surface density of type I mAbs,26 possibly explaining their weaker signaling. To address this, we titrated RTX to achieve equivalent cell-surface binding and then repeated our signaling experiments. The results revealed a clear dose-response effect with less phosphorylation of FcγRIIb at lower doses of RTX, perhaps explaining why some targets (eg, CD22) expressed at low levels do not elicit extensive phosphorylation. Certain mAbs such as LOB 7-4 and AT13/5h evoked equivalent levels of phosphorylation to RTX, but others including tositumomab, did not. This may be due to the m2a Fc being less able to activate FcγRIIb than hIgG1 Fc (supplemental Figure 3). Alternatively, type I and II anti-CD20 mAbs have been shown to have different orientations when bound to CD20,27 possibly affecting their ability to interact with and activate FcγRIIb.
The finding that the internalization of most anti–B-cell mAbs was not increased by FcγRIIb, whereas most were able to phosphorylate FcγRIIb, suggests that the ability to activate FcγRIIb will not predict whether a new therapeutic candidate will remain cell-surface localized. Furthermore, the lack of correlation between internalization and phosphorylation of FcγRIIb may indicate that activation of the receptor is not required for internalization.
At high cell density there was competition for mAb Fc region binding between FcγRIIb expressed in cis and trans, which may have implications for the success of mAb therapeutics in vivo. Antibody bipolar bridging between mAbs bound to herpes simplex virus I antigens and the virally encoded FcγR protects infected cells from antibody-dependent cellular cytotoxicity.28,29 Thus, cis FcγRIIb may compete for mAbs with activatory FcγR expressed on NK cells and macrophages in trans, reducing effector activity. Also, cis interactions with FcγRIIb may reduce complement fixation and complement-dependent cytotoxicity by competing with C3b for Fc binding as has been demonstrated for FcγRIII.30,31 As some mAbs, including the type I anti-CD20 mAb, efficiently fix complement,32 inhibition of this activity may further reduce their therapeutic efficacy. Conversely, although our results indicate that trans competition for anti-CD20 mAb by inhibitory FcγR has no effect on the rate of CD20 internalization, it is possible that trans competition by cells expressing higher affinity activatory FcγR may inhibit internalization. However, reduced internalization as a result of trans engagement between type I anti-CD20 mAb on malignant B cells and high-affinity activatory FcγR on cells such as monocytes could promote loss of the mAb:CD20 complex via trogocytosis as discussed by Beum et al33 and may not improve therapeutic efficacy.
There are also other potential downstream effects of FcγRIIb activation. By virtue of its role as an inhibitory immunoreceptor tyrosine-based inhibitory motif–bearing receptor, coligation with FcγRIIb often results in termination of downstream signaling,34,35 so in situations where agonistic mAbs have been chosen for their ability to activate cell-surface receptors, coligation with FcγRIIb may be detrimental. Conversely, we36 and others37 have shown that FcγRIIb can transmit apoptotic signals. It is possible, therefore, that cis engagement of FcγRIIb by cell-surface–targeted mAb may contribute to therapy.
Indirect interactions between the BCR and FcγRIIb may also play a role in determining the efficacy of type I anti-CD20 mAb therapy. We have previously shown that type I mAb ligation causes CD20 to become physically associated with the BCR, resulting in activation.20 BCR activation is a prerequisite for antigen processing and loading of peptides onto MHCII38 and so may promote adaptive antitumor immune responses after type I mAb treatment.39 However, FcγRIIb expression inhibits BCR activation20,40 reducing antigen presentation,41 potentially impairing the development of adaptive immune responses upon treatment with RTX.
The data presented here provide evidence that preventing FcγRIIb engagement has the potential to improve the efficacy of direct-targeting mAb. Thus, engineering the Fc region to reduce binding or blocking FcγRIIb with anti-FcγRIIb mAb should be considered. mAb-mediated blocking of FcγRIIb is a particularly promising approach, as it could reduce internalization, increase both antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity, and potentially augment vaccine effects. In addition, mAb ligation of FcγRIIb has been considered as a potential therapy for treating B-cell malignancies in its own right.42
One area in which FcγRIIb-dependent internalization might have a positive effect on therapeutic efficacy is in the treatment of autoimmune disease. It has been proposed that type I anti-CD20 mAbs promote a regulatory B-cell response that can suppress autoimmunity.43 FcγRIIb is downregulated on B cells in patients with systemic lupus erythematosis,44 but is upregulated on a subset of regulatory B cells.45 Therefore, FcγRIIb-mediated internalization of CD20 in response to type I mAb ligation may result in preferential clearance of pathogenic FcγRIIb-low cells in systemic lupus erythematosis, while sparing FcγRIIb-high regulatory B cells, leading to amelioration of autoimmune symptoms. In addition, the negative signaling transduced by FcγRIIb itself may also be beneficial in the context of autoimmunity.
In summary, we have shown that FcγRIIb expressed in cis and trans can compete for binding to cell-surface–bound mAbs. Although competition from FcγRIIb expressed in trans does not affect the rate of CD20 internalization, cis-trans competition for Fc binding by FcγRIIb and other FcγR may affect therapeutic efficacy via other mechanisms.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to I. Henderson, A. Tilbury, and K. N. Potter for provision of and assistance with clinical material, and R. R. French for critical review of the manuscript. We are also grateful to Esther Porée, Giusi Manfredi, Robert Oldham, and Sonya James for technical assistance. We thank the Experimental Cancer Medicine Centre-funded University of Southampton Faculty of Medicine Human Tissue Bank (Human Tissue Authority licence 12009) for tissue collection and preparation. We thank David Johnston (Biomedical Imaging Unit, Southampton, United Kingdom) for assistance with confocal microscopy.
This work was supported by Leukaemia and Lymphoma Research grants 09009 and 12050, Cancer Research UK grants C328/A2738 and C328/A2737, and a research grant from BioInvent International.
Authorship
Contribution: A.T.V. performed research, analyzed and interpreted data, and wrote the manuscript; C.I., S.H.L., E.L.W., V.S., and A.R. performed research; S.A.B. and C.H.T.C. contributed vital new reagents; B.F. designed research; M.J.G. designed research, analyzed and interpreted data, and edited the manuscript; and M.S.C. designed research, analyzed and interpreted data, and wrote the manuscript with A.T.V.
Conflict-of-interest disclosure: B.F. is a paid employee of BioInvent International. M.J.G. acts as a consultant to a number of biotech companies to write general antibody expert reports and receives institutional payments and royalties from antibody patents and licenses. M.S.C. serves as a consultant for and received grant funding from BioInvent International, and has previously served as an ad hoc consultant for Roche. The remaining authors declare no competing financial interests.
Correspondence: Mark S. Cragg, Antibody and Vaccine Group, Cancer Sciences Unit, University of Southampton, Faculty of Medicine, General Hospital, Southampton, SO16 6YD, United Kingdom; e-mail: msc@soton.ac.uk.
References
Author notes
M.J.G. and M.S.C. are the senior authors and contributed equally to the study.