Key Points
IAPs are required for survival and expansion of activated T cells.
IAP antagonists sensitize to tumor necrosis factor (TNF)-induced cell death of activated T cells during viral infection.
Abstract
Inhibitors of apoptosis proteins (IAPs) were originally described as regulating apoptosis by direct binding to caspases. More recently, IAPs have been identified as important modulators of canonical and noncanonical nuclear factor κB signaling via their ubiquitin-E3 ligase activity. IAPs are therefore, not only gatekeepers of cell death, but are probably also involved in the regulation of inflammation, as well as innate and adaptive immunity. In this study, we analyzed the role of IAPs in T-cell immunity during lymphocytic choriomeningitis virus (LCMV) infection by pharmacological targeting with an IAP antagonist/second mitochondria-derived activator of caspase-mimetic. Expansion of virus-specific CD8 T cells was drastically reduced in LCMV-infected mice exposed to IAP antagonists. Accordingly, virus control was substantially impaired, indicated by high virus titres in the spleen and the spread of LCMV to peripheral organs. The profound negative effect of IAP antagonists on T-cell immunity was partially linked to tumor necrosis factor–mediated cell death of activated T cells and required inhibition of X-linked inhibitor of apoptosis, as well as cellular IAP-1. Thus, IAPs play an important role in T-cell expansion and survival in the context of a highly inflammatory environment such as a virus infection, indicating that IAP antagonists may interfere with immune responses.
Introduction
Inhibitors of apoptosis proteins (IAPs) were originally identified as apoptosis inhibitors1 and as components of the signaling complex of tumor necrosis factor receptor 2 (TNF-R2).2 Based on their homology to Drosophila IAPs and their caspase-binding properties, mammalian IAPs were initially considered broad-spectrum apoptosis inhibitors. However, only X-linked inhibitor of apoptosis (XIAP) has direct caspase-inhibiting activity at physiological levels.3 Recent work has shown that IAPs regulate signaling through TNF-family receptors, where they are involved in the activation of nuclear factor κB (NF-κB), which regulates the expression of genes important in inflammation, innate and adaptive immunity, and cell survival.4
The structurally related IAPs, cellular IAP-1 (cIAP1), cellular IAP-2 (cIAP2), and XIAP, contain three baculovirus IAP-like repeat domains and one really interesting new gene-finger domain that functions as an ubiquitin-E3 ligase.5 cIAP1 and cIAP2 also contain caspase activation and recruitment domains. IAPs regulate the activity of large signaling complexes involved in NF-κB regulation. This has been shown for the TNF-R1-signaling complex, whose activity is regulated through IAP-mediated ubiquitylation of receptor-interacting protein kinase 1 (RIPK1) and other targets, thereby determining the activity of the canonical NF-κB pathway,6,7 and preventing caspase-dependent and -independent cell death.8,9 IAPs also regulate noncanonical NF-κB activation, together with TNF receptor associated factors 2 and 3 via ubiquitylation and proteasomal degradation of NF-κB–inducing kinase.10-12
High levels of IAPs are seen in a number of human tumor cells and are sometimes associated with a poor prognosis.13,14 Because of this correlation, pharmaceutical companies have developed IAP antagonists. The development of these compounds has been guided by the IAP-binding and inhibiting activity of the mitochondrial protein second mitochondria-derived activator of caspase/direct IAP binding protein with low pI.15,16 IAP antagonists/second mitochondria-derived activator of caspase-mimetics cause the ubiquitin-mediated degradation of cIAP1 and cIAP217,18 ; and although they don’t cause its degradation, they inhibit XIAP with varying efficiencies.
IAP antagonists sensitize tumor cells to death induced by TNF in vitro. Some cancer cells die in response to IAP antagonists due to autocrine TNF production as a result of NF-κB activation.10,11,19 Some of these substances have entered clinical trials as cancer therapeutics.20 However, IAPs are also widely expressed in cells of the immune system, and targeting of IAPs is therefore likely to affect immune functions. A number of pathways critically involved in immune defense are regulated by IAPs, most notably NF-κB pathways. IAPs are also involved in the control of signaling downstream of the toll-like receptor-adapter, toll-receptor-associated activator of interferon,21 and the pattern recognition receptors (PRR), nucleotide oligomerization domain 1 and 2.22,23 IAP antagonists have further been shown to kill isolated human monocytes and to have some NF-κB–activating and maturation-inducing capacity in isolated dendritic cells.24
In B cells, signaling through the TNFR-family receptors, CD40 and B-cell–activating factor-receptor, is critical for activation and survival. These receptors activate NF-κB via the noncanonical pathway.12,25 A recent study showed that genetic loss of cIAP1 and cIAP2 removes B-cell–activating factor-dependency for survival, and causes the accumulation of B cells in mice.26 In activated T cells, a costimulatory effect of IAP-loss has been reported.27 In vitro, mouse and human T cells secreted higher levels of IL-2 during stimulation with CD3/CD28 antibodies when an IAP antagonist was present. In vivo, coadministration of an IAP antagonist with a cellular tumor vaccine enhanced the immune response and reduced tumor growth.27
These observations suggest that IAPs play a significant role during T-cell– mediated immunity, and the loss of IAP function may have profound effects on complex immune reactions such as during antimicrobial host defense. When treatment of cancer patients, many of whom may already be immune compromised, with IAP antagonists is considered, potential alterations of the immune response are a major issue. To test the role of IAPs during antiviral immunity and any negative effects of IAP antagonist treatment on the immune system, we analyzed the T-cell–immunity of mice treated with LBW242 in the context of lymphocytic choriomeningitis virus (LCMV) infection. Our results showed a drastic reduction of CD8 T-cell expansion and impaired virus control when mice were exposed to an IAP antagonist. The results indicated an important role for IAPs in T-cell activation and survival, and consequently a strong immunomodulatory function of IAP antagonists, which may be linked to TNF-mediated induction of cell death in activated T cells.
Materials and methods
Mice and viruses
C57BL/6J mice were obtained from Janvier (Le Genest Saint Isle, France), kept under specific pathogen-free conditions, and analyzed between 8 to 14 weeks of age. XIAP−/− mice were obtained from David Vaux (Melbourne, Australia).28 TNF−/− mice were obtained from Andreas Diefenbach (Freiburg, Germany) who received them from Prof J. Sedgwick (Sydney, Australia).29 Mouse experiments were approved by the Regierungspraesidium, Freiburg. The LCMV-WE was originally obtained from Dr F. Lehmann-Grube (Hamburg, Germany), and quantified in organs using a standard focus-forming assay. Mice were treated daily with 5, 10, or 50 mg/kg body weight LBW242 (provided by Dr Brant Firestone, Novartis) dissolved in phosphate-buffered saline.
In vitro proliferation
Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled splenocytes were stimulated with plate-bound αCD3 antibody (clone 17A2; 1 μg/mL), and incubated with the indicated concentrations of LBW242 or solvent. For analysis, cultured cells were harvested completely, resuspended in an equal volume fluorescence-activated cell sorter (FACS) buffer, and counted for a defined time-interval on a FACSCalibur (BD Biosciences). Cell death was analyzed by staining cells for CD8 and CD4 populations in addition to Annexin V-FITC (BD Biosciences) and LIVE/DEAD Fixable Far Red stain (Life Technologies).
Antibodies and intracellular staining
Antibodies were purchased from eBioscience, BD Biosciences, BioLegend, or Bio X Cell. For intracellular cytokine staining, 106 lymphocytes were stimulated with 10−7 M LCMV GP33-peptide (KAVYNFATM) for 4 hours in the presence of Brefeldin A. Cells were then surface-stained with anti-CD8a antibody, followed by a fixation/permeabilization step using Cytofix/Cytoperm kit (BD Biosciences) and anti-IFNγ, or anti-TNF antibody staining. LCMV-specific CD8 T cells were detected by fluorochrome-labeled H-2Db tetramers complexed with GP33-peptide. Cell samples were analyzed using FACSCalibur and FlowJo software (Tree Star).
Chromium release assay
Cytolytic activity of T cells was analyzed in a standard 51Chromium-release assay using GP33-41, NP396-404, and adeno-peptide–loaded EL-4 target cells. Splenocytes from LCMV-infected mice and peptide-loaded EL-4 target cells were incubated for 5 hours at 37°C. Duplicate wells were assayed for each effector to target (E:T) ratio and percentages of specific lysis were calculated.
Results
In vitro activated CD4 and CD8 T cells are sensitive to IAP antagonists
To evaluate the impact of the IAP antagonist LBW242 on the proliferation and survival of T cells after in vitro stimulation, CFSE-labeled splenocytes were activated by plate-bound anti-CD3 antibodies and exposed to titrated concentrations of LBW242. For analysis, splenocyte cultures were harvested completely, and cells were resuspended and counted in the flow cytometer for a defined time interval. The area under each curve is a measure for T-cell expansion and the dilution of CFSE fluorescence represents the number of cell divisions. After 45 hours and 69 hours stimulation, CD4 and CD8 T cells exposed to 10 μM LBW242 failed to proliferate, when compared with the solvent control culture (Figure 1A), indicating a profound negative effect of the IAP antagonist on activated T cells. During exposure to 5 μM LBW242, T cells initially proliferated (after 45 hours), although slightly less than controls, but were drastically decreased in cell numbers when analyzed after 69 hours (Figure 1A). At 0.5 μM LBW242, T-cell proliferation and accumulation were comparable to the solvent control (Figure 1A). Importantly, 5 μM LBW242 during in vitro culture had no significant negative effect on unstimulated T cells (Figure 1A), indicating that the IAP antagonist mainly affects activated T cells. To further determine that LBW242 targets activated, proliferating T cells, we performed bromodeoxyuridine (BrdU) incorporation experiments. A clear dose-dependent reduction of BrdU-positive CD4 and CD8 T cells were detectable under LBW242 treatment. Only T cells that entered the cell cycle and stained positive for BrdU were LBW242-sensitive, thereby confirming the CFSE experiments results (see supplemental Figure 1, available on the Blood Web site). To demonstrate that the effect of IAP antagonists on T cells is independent of the mode of activation, we stimulated splenocytes with combinations of different stimuli. Expansion of CD4 and CD8 T cells was heavily impaired under LBW242 treatment with all the distinct stimulation protocols (supplemental Figure 2), indicating that activated T cells are sensitive to LBW242 treatment, independent of the mode of stimulation. Thus, LBW242 had a profound negative effect on the proliferation and survival of in vitro-stimulated T cells in a concentration range that did not affect resting T cells to the same extent.
IAP antagonist inhibits T cell proliferation in vitro. (A) A total of 3 × 105 CFSE-labeled wild-type (WT) splenocytes were stimulated with plate-bound anti-CD3 antibody. Splenocytes were exposed to LBW242 (red line) or solvent (green line). After 45 hours (left column) and 69 hours (right column), cultures were harvested completely and stained with anti-CD4 and anti-CD8 antibodies. Cells were resuspended in equal volumes of FACS buffer and counted in the flow cytometer for a defined time interval. The area under each curve indicates the total number of cells collected and the dilution steps of CFSE fluorescence represent the number of cell divisions. Red numbers represent a statistical analysis of the proportion of total T cells exposed to IAP antagonist relative to solvent control from 5 experiments. (B) Assessment of cell death in ConA/IL-2 stimulated CD8 T cells. Splenocytes were stimulated with 2 μg/ml ConA and 10 IU/ml IL-2 for 4 days in the presence of LBW242 and/or 10 µM necrostatin-1. CD8 T cells were analyzed for Annexin V exposure and Live/Dead FACS staining. Annexin V positive cells were classified as apoptotic and cells single positive for Live/Dead stain were classified as necrotic. Dot plots show a representative experiment and column graph shows the mean of 2 experiments.
IAP antagonist inhibits T cell proliferation in vitro. (A) A total of 3 × 105 CFSE-labeled wild-type (WT) splenocytes were stimulated with plate-bound anti-CD3 antibody. Splenocytes were exposed to LBW242 (red line) or solvent (green line). After 45 hours (left column) and 69 hours (right column), cultures were harvested completely and stained with anti-CD4 and anti-CD8 antibodies. Cells were resuspended in equal volumes of FACS buffer and counted in the flow cytometer for a defined time interval. The area under each curve indicates the total number of cells collected and the dilution steps of CFSE fluorescence represent the number of cell divisions. Red numbers represent a statistical analysis of the proportion of total T cells exposed to IAP antagonist relative to solvent control from 5 experiments. (B) Assessment of cell death in ConA/IL-2 stimulated CD8 T cells. Splenocytes were stimulated with 2 μg/ml ConA and 10 IU/ml IL-2 for 4 days in the presence of LBW242 and/or 10 µM necrostatin-1. CD8 T cells were analyzed for Annexin V exposure and Live/Dead FACS staining. Annexin V positive cells were classified as apoptotic and cells single positive for Live/Dead stain were classified as necrotic. Dot plots show a representative experiment and column graph shows the mean of 2 experiments.
IAPs regulate TNF-signaling, and IAP antagonists are able to alter signaling from TNF-R1 to a pro-death signal.11 Enhanced TNF-killing seen by IAP antagonist sensitization can be blocked by necrostatin, an inhibitor of RIPK1 activity.30 To analyze whether LBW242-induced loss of activated CD8 T cells is due to a RIPK1-dependant mechanism, we pretreated mouse splenocytes with necrostatin prior to the addition of LBW242. T cells were stimulated with concanavalin A (ConA)/IL-2, followed by assessing cell death with Annexin V and Live/Dead stain. Necrostatin substantially protected CD8 T cells treated with low-to-mid range doses of LBW242 from cell death, but was less effective at higher doses (Figure 1B). Cell death induced under these conditions showed an apoptotic signature with positive Annexin V staining. Of note, CFSE dilution revealed that those T cells rescued by necrostatin treatment, cycled largely normally and proliferated to the same extent as solvent-treated T cells, supporting the idea that the phenotype is due to excessive T-cell death, rather than blockage of proliferation (data not shown).
IAP-antagonism inhibits virus-specific T cell responses and impairs virus control in vivo
To investigate the impact of an IAP antagonist on antiviral T-cell responses, we used the LCMV infection model. C57BL/6 mice were treated intraperitoneally with 50 mg/kg LBW242 daily, a dose successfully administered in mouse tumor models.31,32 Some studies have also reported an oral application of LBW242 using similar doses.19,27 To confirm this, 50 mg/kg LBW242 were administered either intraperitoneally or orally (supplemental Figure 3). Targeting of cIAPs by LBW242 was demonstrated on whole cell lysates from spleens and thymi by immunoblotting for cIAP1 and XIAP expression (supplemental Figure 3A; cIAP2 is not detectable by immunoblotting in mouse cells). The results demonstrated the loss of cIAP1 after treatment of mice with titrated doses of LBW242 either intraperitoneally or orally, indicating that the IAP antagonist is active over a wide concentration range in vivo. There was no loss of XIAP at these concentrations, as expected.33
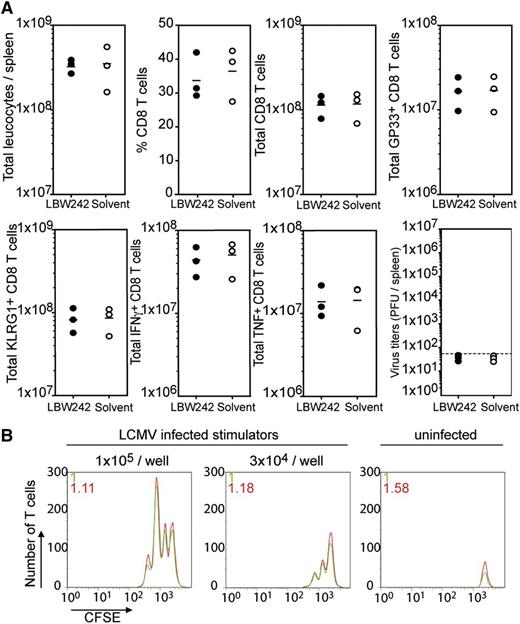
Analysis of the immune response on day 8 after LCMV infection showed a reduction of spleen cellularity in LBW242-treated mice when compared with solvent controls (Figure 2A). The proportions of CD8 T cells were about 20% in the LBW242 group compared with ∼60% in the solvent group. In absolute numbers, CD8 T cells were reduced 5- to 10-fold in the spleen of LBW242-treated mice compared with control mice (Figure 2A). Lower absolute numbers of CD8 T cells specific for the immunodominant LCMV epitopes GP33 (Figure 2A) and NP396 (data not shown) were detectable in LBW242-treated mice. Thus, in the presence of an IAP antagonist, CD8 T cells are profoundly limited in their expansion during an antiviral immune response.
Impact of IAP antagonist on T cell immunity during LCMV infection. WT mice were infected intravenously with 200 PFU LCMV and treated daily with 50 mg/kg LBW242 or solvent intraperitoneally, or were left untreated. (A) At day 8 post-infection, total leukocyte counts, percent and total counts of CD8 T cells, and GP33-specific T cells were determined in the spleen. (B-C) Differentiation of effector T cell subpopulations were analyzed by FACS staining with anti-KLRG-1/anti-CD127 or anti-CD44/anti-CD62L antibodies on gated CD8 T cells. (D-E) Percentages and total numbers of INFγ- and TNF-expressing CD8 T cells are given. (F) Cytolytic activity of splenocytes was determined on GP33-, NP396-, or irrelevant adenovirus peptide-loaded EL-4 target cells in a 51Cr-release assay. (G) LCMV titres were analyzed in the spleen, liver, lung, and kidney using a focus forming assay. Dotted line represents detection levels. Symbols represent individual mice. The horizontal lines represent mean values. *P < .05; **P < .005; ***P < .001. NS, not significant (Student unpaired t test). Data are representative of 3 independent experiments.
Impact of IAP antagonist on T cell immunity during LCMV infection. WT mice were infected intravenously with 200 PFU LCMV and treated daily with 50 mg/kg LBW242 or solvent intraperitoneally, or were left untreated. (A) At day 8 post-infection, total leukocyte counts, percent and total counts of CD8 T cells, and GP33-specific T cells were determined in the spleen. (B-C) Differentiation of effector T cell subpopulations were analyzed by FACS staining with anti-KLRG-1/anti-CD127 or anti-CD44/anti-CD62L antibodies on gated CD8 T cells. (D-E) Percentages and total numbers of INFγ- and TNF-expressing CD8 T cells are given. (F) Cytolytic activity of splenocytes was determined on GP33-, NP396-, or irrelevant adenovirus peptide-loaded EL-4 target cells in a 51Cr-release assay. (G) LCMV titres were analyzed in the spleen, liver, lung, and kidney using a focus forming assay. Dotted line represents detection levels. Symbols represent individual mice. The horizontal lines represent mean values. *P < .05; **P < .005; ***P < .001. NS, not significant (Student unpaired t test). Data are representative of 3 independent experiments.
The impact of IAP-antagonist treatment on differentiation of CD8 effector T cells into short-lived effector cells (KLRG-1hi/CD127lo) and memory precursor effector cells (KLRG-1lo/CD127hi)34 was analyzed. CD8 T cells from LBW242-treated mice were severely impaired in KLRG-1 upregulation, as shown in relative and absolute numbers (a 20- to 30-fold reduction) (Figure 2B). Accordingly, the proportion of short-lived effector cells in the CD8 T-cell compartment was reduced in the LBW242 group (Figure 2B). Equal percentages of CD44hi/CD62Llo CD8 effector T cells were detectable in LBW242- and solvent-treated mice, although the total number of CD44hi/CD62Llo CD8 T cells was decreased in the LBW242 group due to the limited expansion of virus-specific T cells (Figure 2C). This indicates that under IAP antagonist exposure, the remaining LCMV-specific CD8 effector T cells exhibit a highly activated phenotype.
Effector T cells from LBW242-treated mice were assessed for function by analysis of cytokine expression after short-term re-stimulation. An equal proportion of CD8 T cells (30% to 40%) produced IFNγ in LBW242- and solvent-treated mice. Nevertheless, a drastic reduction in absolute numbers of IFNγ-expressing CD8 T cells was observed under LBW242 treatment, reflecting the limited T-cell expansion (Figure 2D). In contrast, TNF-expressing T cells were significantly reduced in relative numbers in LBW242-treated mice as well (Figure 2E). Ex vivo cytolytic activity assayed on GP33- and NP396-loaded EL-4 target cells was reduced in LBW242-treated animals (Figure 2F). To examine the effect of the observed impairment of T-cell activation on viral replication, virus titres from various organs were examined. Elevated virus titres were detectable in the spleens of treated mice compared with control mice. Additionally, the spread of LCMV in peripheral organs like the liver, kidney, and lung was observed under LBW242 treatment, indicating a loss of viral control (Figure 2G).
IAP antagonists do not affect naïve T cells and the antigen presenting cell (APC) function of the spleen in vivo
To analyze whether IAP antagonists affect naive T cells, WT mice were first treated with LBW242 for 7 days, treatment was then discontinued, and the mice were infected with LCMV. Total lymphocyte counts in the spleen were comparable in LBW242 and solvent pretreated groups. Expansion of total CD8 or GP33-specific CD8 T cells was not affected (Figure 3A). T-cell differentiation was identical in both experimental groups, and KLRG-1 upregulation was not impaired in mice pretreated with LBW242. No differences in the absolute numbers of IFNγ- and TNF-producing T cells could be detected, and complete virus elimination was observed by day 8 in mice of both experimental groups (Figure 3A).
Impact of IAP antagonist on naïve T cells and APC function of the spleen. (A) To evaluate the impact of IAP antagonist on naïve T cells, WT mice were pretreated intraperitoneally with LBW242 or solvent for 7 days. Three days after the last application, the mice were infected intravenously with 200 PFU LCMV. On day 8 post-infection, total leukocyte counts, percent and total counts of CD8 T cells, and GP33-specific T cells were determined in the spleen (upper panels). KLRG-1 upregulation, as well as total numbers of IFNγ- and TNF-expressing CD8 T cells were calculated. Viral titres in the spleens were determined in a virus focus forming assay (lower panels). (B) Antigen presenting function of splenocytes from day 4 LCMV-infected WT mice, treated during infection with LBW242 or solvent, was tested with CFSE-labeled P14 T cells in an in vitro proliferation assay. Data are representative of 2 to 3 independent experiments.
Impact of IAP antagonist on naïve T cells and APC function of the spleen. (A) To evaluate the impact of IAP antagonist on naïve T cells, WT mice were pretreated intraperitoneally with LBW242 or solvent for 7 days. Three days after the last application, the mice were infected intravenously with 200 PFU LCMV. On day 8 post-infection, total leukocyte counts, percent and total counts of CD8 T cells, and GP33-specific T cells were determined in the spleen (upper panels). KLRG-1 upregulation, as well as total numbers of IFNγ- and TNF-expressing CD8 T cells were calculated. Viral titres in the spleens were determined in a virus focus forming assay (lower panels). (B) Antigen presenting function of splenocytes from day 4 LCMV-infected WT mice, treated during infection with LBW242 or solvent, was tested with CFSE-labeled P14 T cells in an in vitro proliferation assay. Data are representative of 2 to 3 independent experiments.
One possible explanation for the limited antiviral immune response in LBW242-treated mice may be due to a defect in the function of APCs. To test for this, splenocytes of day 4 LCMV-infected mice treated with or without LBW242 were used as stimulator cells in an in vitro proliferation assay. CFSE-labeled GP33-specific P14 T cells were cultured with the two-stimulator cell preparations. Preliminary results indicated no significant differences in P14 T-cell proliferation or survival when titrated numbers of LCMV-infected splenocytes from LBW242- or solvent-treated mice were used as APCs (Figure 3B). Although we did not test other APC features such as expression of costimulatory molecules, this assay showed no indication of a rate-limiting function of APCs.
TNF is partially responsible for T-cell dysfunction during LCMV infection in LBW242-treated mice
Direct sensitization to TNF-induced cell death has been reported for tumor cells exposed to IAP antagonists. In some cells, IAP antagonists induce the production of TNF by activation of the noncanonical NF-κB signaling pathway; and autocrine TNF signaling via TNF-R1 leads to cell death. The loss of TNF-expressing T cells after LCMV infection in LBW242-treated mice also suggests a role for TNF in the phenotype. To test whether TNF-mediated killing of T cells is responsible for their reduced expansion in vitro, TNF−/− splenocytes were used in proliferation assays. Interestingly, there was a clear protection of TNF−/− T cells exposed to 5 μM IAP antagonist as compared with WT controls (Figure 4A). However, a reduction of dividing T cells was also observed in cultures of TNF−/− T cells at high concentrations of LBW242. Thus, the protection seen in TNF−/− T cells seems to be more reliable at lower doses of LBW242, reflecting the results seen with necrostatin, which also protects mainly at lower doses of LBW242.
Effect of IAP antagonist is reduced in TNF-deficient mice. (A) CFSE-labeled WT and TNF−/− splenocytes were stimulated with plate-bound anti-CD3 antibody and exposed to graded concentrations of LBW242 (red line) or solvent (green line). After 72 hours, the cultures were harvested and stained with anti-CD8 antibody. Cells were resuspended in equal volumes of FACS buffer and counted in the flow cytometer for a defined time interval. The area under each curve represents the total number of cells collected. WT or TNF−/− mice were infected intravenously with 200 PFU LCMV and treated daily with LBW242 or solvent intraperitoneally. At day 8 post-infection, (B) total counts of leukocytes, (C) CD8 T cells, and (D) GP33-specific CD8 T cells were determined in the spleen. (E) KLRG-1 upregulation was analyzed on gated CD8 T cells and total numbers are shown. (F) Expression of intracellular INFγ was quantified on CD8 T cells and total numbers are given. Numbers displayed in the panels indicate the ratio of the different cell populations from solvent and LBW242 treated animals for WT and TNF−/− mouse groups, respectively. (G) Serum was taken from day 8 LCMV-infected mice and analyzed for TNF levels using enzyme-linked immunosorbent assay. Data are representative of 3 independent experiments.
Effect of IAP antagonist is reduced in TNF-deficient mice. (A) CFSE-labeled WT and TNF−/− splenocytes were stimulated with plate-bound anti-CD3 antibody and exposed to graded concentrations of LBW242 (red line) or solvent (green line). After 72 hours, the cultures were harvested and stained with anti-CD8 antibody. Cells were resuspended in equal volumes of FACS buffer and counted in the flow cytometer for a defined time interval. The area under each curve represents the total number of cells collected. WT or TNF−/− mice were infected intravenously with 200 PFU LCMV and treated daily with LBW242 or solvent intraperitoneally. At day 8 post-infection, (B) total counts of leukocytes, (C) CD8 T cells, and (D) GP33-specific CD8 T cells were determined in the spleen. (E) KLRG-1 upregulation was analyzed on gated CD8 T cells and total numbers are shown. (F) Expression of intracellular INFγ was quantified on CD8 T cells and total numbers are given. Numbers displayed in the panels indicate the ratio of the different cell populations from solvent and LBW242 treated animals for WT and TNF−/− mouse groups, respectively. (G) Serum was taken from day 8 LCMV-infected mice and analyzed for TNF levels using enzyme-linked immunosorbent assay. Data are representative of 3 independent experiments.
To test whether this TNF-mediated cell death pathway is a mechanistic basis for the limited T-cell expansion observed in IAP antagonist-treated animals, we examined CD8 T cell responses in TNF−/− mice during LCMV infection. The impact of LBW242 treatment in relation to the solvent group was calculated as ratio for WT and TNF−/− mice, respectively. Total leukocyte counts in the spleens of LBW242-treated WT mice were reduced by a factor of ∼3.6 when compared with solvent controls. Interestingly, only a 1.7-fold reduction was observed in LBW242-treated TNF−/− mice (Figure 4B). Total numbers of CD8 T cells in WT mice under LBW242 treatment were reduced by a factor of ∼9.3, whereas the same treatment caused only a threefold reduction in TNF−/− mice (Figure 4C). Correspondingly, a 2.5-fold reduction in the number of GP33-specific T cells could be detected in TNF−/− mice compared with an eightfold reduction in LBW242-treated WT mice (Figure 4D). Thus, in the absence of TNF-signaling, treatment of mice with IAP antagonists had less severe negative effects on T-cell immunity during LCMV infection. The failure to upregulate KLRG-1 during T-cell effector differentiation was also less pronounced in LBW242-treated TNF−/− (4.6-fold reduction) compared with WT mice (∼18-fold reduction) (Figure 4E). The number of IFNγ-expressing CD8 T cells was reduced ∼eightfold in WT mice compared with only a 2.5-fold reduction in TNF-deficient mice (Figure 4F).
TNF is expressed during LCMV infection.35 To examine if there was a difference in the TNF serum levels under LBW242 treatment, TNF was measured on day 8 of infection in WT mice (Figure 4G). A significant increase in the production of TNF was detected in LBW242-treated mice when compared with solvent-treated mice. This demonstrates that LBW242 triggers an upregulation in TNF levels in LCMV-infected mice. This enhanced TNF production may, in turn, trigger cell death of activated T cells.
XIAP inhibition is required for IAP–antagonist-induced T-cell death
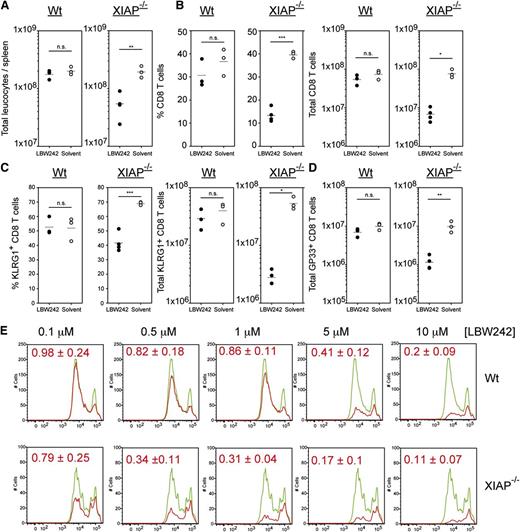
Mice treated with 10 mg/kg of LBW242 during LCMV infection showed no significant effect on T cell proliferation (supplemental Figure 3B) and virus control, despite exhibiting clear degradation of cIAP1 at this dose (supplemental Figure 3A). Thus, we observed a discrepancy between doses of LBW242 required to degrade cIAP1 and cIAP2, and doses required to induce a T-cell phenotype. cIAP2 has been reported to return after IAP antagonist treatment.17 XIAP was also not degraded by LBW242. One likely explanation for this discrepancy between cIAP1 degradation and the observed immune phenotype is a failure of LBW242 to fully inhibit either cIAP2 or XIAP. To confirm if cIAP2 has returned in activated T cells at both low and high doses of LBW242, human peripheral blood mononuclear cells were stimulated in vitro due to a lack of effective antibodies against mouse cIAP2. Stimulated human peripheral blood mononuclear cells showed detectable levels of cIAP2 24- and 48-hours after LBW242 treatment at all doses tested (supplemental Figure 4A). By contrast, cIAP1 was degraded at all doses, and XIAP as published previously, was not (supplemental Figure 4A-B). Consequently, both cIAP2 and XIAP may be driving resistance to low doses of LBW242. However, the inhibitory activity of LBW242 toward XIAP is quite low compared with cIAP1 and cIAP2.33,36 Therefore, the dose/phenotype discrepancy could be due to the lower LBW242 dose not effectively blocking XIAP function. To determine this and to test for off-target toxic effects of LBW242, XIAP−/− mice were treated with 5 mg/kg of LBW242 (a 10-fold lower dose than used previously) during LCMV infection. XIAP−/− mice treated with 5 mg/kg LBW242 showed a near identical phenotype to WT mice at 50 mg/kg with significantly reduced numbers of leukocytes, reduced CD8 T-cell populations, reduced KLRG1+ populations, as well as reduced numbers of GP33-specific T cells (Figure 5A-D). In all cases, this low dose of LBW242 had no significant effect on WT T cells (Figure 5). When splenocytes from XIAP−/− mice were stimulated with αCD3 antibodies in vitro, doses as low as 0.5 μM LBW242 impaired T-cell expansion, while only doses of 5 μM or above reduced WT T-cell populations (Figure 5E). This CD8 T-cell loss was due to apoptotic cell death as previously shown in Figure 1 and was also observed for CD4 T cells (data not shown). These results demonstrate that high doses of LBW242 do not exhibit an off-target toxic side effect, but are needed to successfully block XIAP function. Based on these results, cIAP2 is unlikely to be responsible for the discrepancy between LBW242 doses and the T-cell phenotype.
XIAP deficiency sensitizes mice to LBW242-induced T-cell death. C57BL/6 WT and XIAP−/− mice were infected intravenously with 200 PFU LCMV and treated daily with 5 mg/kg LBW242 or solvent intraperitoneally. At day 8 post-infection, (A) total leukocyte counts and (B) percent and total counts of CD8 T cells were determined in the spleen. (C) Differentiation of effector T-cell subpopulations was analyzed by staining with anti-KLRG-1. (D) At day 8 post-infection, GP33-specific T cells were determined in the spleen. (E) Proliferation of XIAP−/− CD8 T cells treated with LBW242. A total of 3 × 105 CFSE-labeled WT splenocytes were stimulated with plate-bound anti-CD3 antibody. Splenocytes were exposed to LBW242 (red line) or solvent (green line). After 72 hours, cultures were harvested completely and stained with anti-CD8 antibodies. Cells were resuspended in equal volumes of FACS buffer and counted in the flow cytometer for a defined time interval. The area under each curve indicates the total number of cells collected, and the dilution steps of CFSE fluorescence represent the number of cell divisions. Red numbers represent a statistical analysis of the proportion of total T cells exposed to IAP antagonists relative to solvent control from 4 experiments. Horizontal lines represent mean values. *P < .05; **P < .005; ***P < .001. NS, not significant (Student unpaired t test). Data are representative of 2 independent experiments for in vivo and 4 for CFSE in vitro experiments.
XIAP deficiency sensitizes mice to LBW242-induced T-cell death. C57BL/6 WT and XIAP−/− mice were infected intravenously with 200 PFU LCMV and treated daily with 5 mg/kg LBW242 or solvent intraperitoneally. At day 8 post-infection, (A) total leukocyte counts and (B) percent and total counts of CD8 T cells were determined in the spleen. (C) Differentiation of effector T-cell subpopulations was analyzed by staining with anti-KLRG-1. (D) At day 8 post-infection, GP33-specific T cells were determined in the spleen. (E) Proliferation of XIAP−/− CD8 T cells treated with LBW242. A total of 3 × 105 CFSE-labeled WT splenocytes were stimulated with plate-bound anti-CD3 antibody. Splenocytes were exposed to LBW242 (red line) or solvent (green line). After 72 hours, cultures were harvested completely and stained with anti-CD8 antibodies. Cells were resuspended in equal volumes of FACS buffer and counted in the flow cytometer for a defined time interval. The area under each curve indicates the total number of cells collected, and the dilution steps of CFSE fluorescence represent the number of cell divisions. Red numbers represent a statistical analysis of the proportion of total T cells exposed to IAP antagonists relative to solvent control from 4 experiments. Horizontal lines represent mean values. *P < .05; **P < .005; ***P < .001. NS, not significant (Student unpaired t test). Data are representative of 2 independent experiments for in vivo and 4 for CFSE in vitro experiments.
Discussion
A number of IAP antagonists are currently in clinical trials for the treatment of both solid tumors and lymphomas.4 Originally designed to antagonize the caspase-inhibiting function of IAPs, they are now known to regulate numerous immune signaling pathways such as PRR signaling, NF-κB, and inflammasome activation. Since T cells use many of the pathways regulated by IAPs during their activation, we assessed the impact of IAP antagonism in this situation. In vivo and in vitro IAP antagonism had no significant effect on resting T cells, as mice pretreated with LBW242 for several days before subsequent infection showed normal expansion of CD8 T cells and control of LCMV. This is consistent with previous findings that the systemic administration of IAP antagonists for 1 week did not alter the composition of the T- and NK-cell compartments in the spleen of mice.27 In contrast, LCMV-infected mice treated with IAP antagonist in parallel showed severe reductions in CD8 T-cell expansion, differentiation, and survival. Of note, the remaining CD8 T cells exhibited a highly activated phenotype. Although ex vivo cytolytic activity of these cells showed reduced killing activity, this reflected the lower number of virus-specific T cells in these mice, since on a per cell basis no differences in cytolytic activity and degranulation capacity could be observed between T cells from LBW242- or solvent-treated mice (data not shown). The impaired T-cell expansion and differentiation resulted in the loss of viral control, with increased LCMV titres in the spleen and the virus spreading to peripheral organs.
One possible reason for the reduced T-cell expansion could be a limited APC function in LBW242-treated mice since IAPs are known to be involved in PRR signaling. However, when APC function was assessed, LBW242 appeared to have no effect since APCs from infected mice receiving LBW242 showed normal T-cell stimulatory capacity. This approach does not analyze in detail a number of important APC functions; therefore, we cannot fully exclude an effect of LBW242 on APC function using this approach.
When comparing cytokine production as a marker for activation, the remaining live CD8 T cells from LBW242-treated mice showed a normal upregulation of INFγ expression during LCMV infection. Conversely, there was a severe reduction in TNF-expressing CD8 T cells. Under conditions of IAP-deficiency, TNF can induce both caspase-dependent and RIP kinase-dependent cell death.11,37 TNF, therefore, may limit CD8 T-cell expansion under IAP-antagonist exposure through the induction of cell death, and TNF-producing T cells may die in an autocrine fashion. Indeed, when in vitro–stimulated T cells were assessed for death, there was a strong increase in the number of apoptotic cells detected when the cells were stimulated in the presence of LBW242. This increase in cell death could be partially blocked by the addition of necrostatin, an inhibitor of RIPK1 activity. Inhibition of cell death by necrostatin is often interpreted as a sign of necroptosis; however, necrostatin may also block caspase-dependent cell death induced by TNF,38 and such a mechanism could be at play here. In line with this finding, αCD3-stimulated TNF−/− T cells exhibited improved in vitro proliferation in the presence of low-to-mid range doses of LBW242. This is similar to the effect of necrostatin and may suggest that TNF plays a major role in activated T-cell death. T-cell expansion during LCMV infection was improved in LBW242-treated TNF−/− mice relative to WT, but was still impaired relative to solvent controls, suggesting that TNF is not the only contributing factor. IAPs are also known to regulate signaling through other death receptors such as TRAIL19 and Fas,37 and both receptors have been reported to be engaged during T-cell responses.39,40 It is likely that death induced through several death receptors may contribute to the impaired T-cell expansion during LCMV infection in the absence of IAPs.
When LCMV-infected XIAP−/− mice were treated with only 5 mg/kg of LBW242, they showed a similar T-cell phenotype to WT mice treated at 50 mg/kg. This clearly indicates that XIAP plays a critical role in activated T-cell survival in the context of loss of cIAP1 activity. The observation that cIAP2 returns even at higher doses of LBW242, combined with the dramatic increase in LBW242 sensitivity in XIAP−/− mice, suggests that cIAP2 is still inhibited by low doses of LBW242 or that it plays a minor role in the observed phenotype. Thus, the poor targeting of XIAP by LBW242 likely explains the discrepancy between doses of LBW242 required to degrade cIAP1 and cIAP2, and doses required to induce T-cell death during viral infection. This also indicates that the T-cell death phenotype seen in WT mice treated with 50 mg/kg of LBW242 is not simply an off-target toxic effect. Higher doses of LBW242 have little or no effect on resting T cells, which argues against a nonspecific “toxic” effect.
The exact role performed by the IAPs during T-cell activation is not clear, but it may be linked to levels of cytokine production, as shown by the significant increase in TNF production in LCMV-infected mice treated with 50 mg/kg of LBW242. The combined loss of all three IAPs can have a stronger promoting effect on cytokine production in response to various stimuli, than the loss of only one or two41,42 ; and IAP antagonists that efficiently target all three IAPs are much more potent inducers of cell death than those that target cIAP1 and cIAP2.43 LBW242 may induce more potent cytokine upregulation at high doses due to combined inhibition of cIAP1 and XIAP, while at lower doses there is enough “active” XIAP to defy the antagonist.
A recent study has demonstrated that in vivo application of IAP antagonists can augment the potency of tumor vaccines. Mice treated with a combination of IAP antagonists and irradiated B16 melanoma cells exhibited better tumor protection compared with mice with single treatments.27 The immunostimulatory effect of IAP antagonists was correlated with an increase of tumor-specific T cells and IFNγ-producing NK cells in the spleen of these mice. The conflicting results may be explained by the different inflammatory milieu present during an antitumor response vs an antiviral reaction. LCMV induces a strong inflammatory milieu characterized by high levels of type I interferons, IFNγ, TNF, and other cytokines. Under these highly inflammatory conditions, IAP expression is likely required for T-cell survival.
Our data suggests that IAPs play a crucial role in the survival of activated T cells and that their function is to some extent redundant, as at least cIAP1 and XIAP, and probably cIAP2, must be inhibited to kill activated T cells. T-cell death due to the loss of IAP activity is at least partly due to TNF, and likely other TNF super-family cytokines. It is possible that IAP antagonists may be useful in the treatment of cancer patients; however, due to their ability to induce or enhance cytokine production in immune cells, inhibition of PRR signaling, sensitization to TNF super-family cytokines, as well as the reduced T-cell–mediated antiviral responses, it is clear that IAP antagonists can have a powerful immunomodulatory function as well. And, therefore, their effects on the immune system should be carefully considered.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Leigh Zawel and Dr Dale Porter (Novartis, Boston, MA) for providing LBW242. TNF−/− mice were a kind donation from Prof Andreas Diefenbach (Institute of Medical Microbiology and Hygiene, Freiburg, Germany). We also thank Associate Prof J. Silke for providing cIAP1 and cIAP2 antibodies and Prof D. David Vaux (both of Walter and Eliza Hall Institute, Melbourne Australia) for XIAP−/− mice.
This work was supported by grants from the German Federal Ministry of Education and Research (BMBF 01 EO 0803) (P.A.), the Deutsche Krebshilfe (G.H.), and EMBO LTF (Lt_781_2009) (I.E.G.).
Authorship
Contribution: I.E.G. designed and performed research, analyzed data, and wrote the paper; I.M., N.L., S.B., and S.V. performed research and analyzed data; and G.H. and P.A. designed research, analyzed data, wrote the paper, and provided joint senior authorship.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Aichele, Department of Medical Microbiology and Hygiene, Institute of Immunology, Hermann-Herder-Strasse 11, D-79104 Freiburg, Germany; e-mail: peter.aichele@uniklinik-freiburg.de; and Georg Häcker, Department for Medical Microbiology and Hygiene, Institute of Microbiology, Hermann-Herder-Strasse 11, D-79104 Freiburg, Germany; e-mail: georg.haecker@uniklinik-freiburg.de.
References
Author notes
I.E.G. and I.M. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal