In this issue of Blood, Fish et al uncover how Epstein-Barr virus (EBV) enhances MYC-driven B-cell lymphoma by crossing EBV Eµ-EBV latent membrane protein 2A (LMP2A) transgenic mice with immunoglobulin-λ (Igλ)-MYC transgenic mice.1
Deregulated MYC function is a central driver in human cancer, cooperating with different cofactors to bring about tumor lineage-specific pathologies. MYC is the defining chromosomal translocation in Burkitt Lymphoma (BL). LMP2A accelerated cell cycle progression in the pretumor B cells by eliminating p27kip1 /CDKN1B post-transcriptionally, thus providing a permissive environment for MYC to act as a fully fledged oncogene. Normally, this would not happen, because deregulated MYC activates p53, which activates p27kip1 (among others), leading to MYC-induced apoptosis. Although p53 is mutated in most solid cancers with deregulated MYC, p53 is seldom mutated in EBV-positive, pediatric BL. P53 mutations tend to arise in EBV-negative, spontaneous BL or following cytotoxic chemotherapy.
In EBV-BL, MYC is not only translocated but also mutated.2 Mutations in MYC itself or alterations in other pathways such as Bcl-2 are essential to prevent MYC-induced apoptosis. LMP2A cooperates with MYC in transgenic mice at the premalignant stage. Like bcl-2 Eµ transgenic mice, LMP2A Eµ transgenic mice do not form lymphomas in the C57BL/6 background. Another EBV oncogene, LMP1, also modulates the premalignant B-cell state. This predisposes the mice to lymphomas at old age.3 The EBV LMP1 and LMP2A genes cooperate in reprogramming normal B-cell function, rather than driving lymphoma development. In fact, LMP2A counteracts LMP1’s hyperproliferative phenotype,4 leading to reduced lymphoma incidence in the double transgenic mice. This approximates the human pathology. Neither LMP1 nor LMP2 is consistently expressed in the fully malignant state of human BL; they are part of an EBV multigene driver set that promotes EBV infection in naïve B cells,5 initial expansion, reprogramming, and germinal center traversal before EBV establishes latency in memory B cells. Teleologically speaking, γ-herpesviruses in general are not intent on causing lymphomas; rather, they want to create a pseudo-germinal center environment that supports lifelong latency.6,7 The report by Fish et al suggests that this is the very same molecular environment that MYC needs to unfold its oncogenic potential.
What features does a premalignant B cell have to exhibit to become susceptible to MYC-dependent transformation? First, such a cell has to have activated B-cell receptor (BCR)8 and/or ligand-independent coreceptor signaling such as that generated by LMP1 or CD40. It is no coincidence that hyperendemic BL is associated with childhood EBV infection, as well as constant exposure to an environmental antigen, namely malaria. By contrast, spontaneous EBV-negative BL develops later in life or in immune-compromised patients, and we may surmise that somatic driver mutations substitute for hyperactive BCR signaling. Second, such a cell has to circumvent MYC-induced apoptosis, through bcl-2, through p52 mutation, through specific gain of function mutations in MYC itself, or, as Fish et al now introduce, through reducing the expression and activity of the p27Kip tumor suppressor protein. p27Kip is regulated at the protein level, signaled out by phosphorylation and then targeted for ubiquitin-mediated proteosomal degradation. LMP2A could overcome the tumor protective phenotype of wild-type p27Kip and even that of a p27Kip super-repressor allele (S10A), attesting to the importance of this molecule in premalignant B-cell hyperplasia.
Many types of MYC transgenic mice have been generated over the years. All develop B-cell lymphoma, but few of them are as potent and penetrant as the initial Eµ-MYC transgenic mice. Fish et al used the more accurate, Igλ-MYC transgenic model.9 Only for their LMP2A × p27Kip experiments did they have to move to C57BL/6 × 129. This allowed them to study the premalignant B-cell environment in the context of intermediate MYC activation. Perhaps it would be informative if one was to try and match MYC and EBV transgene expression even more closely, eg, by targeting MYC and viral protein expression to limited, more mature B-cell developmental stages. This would mimic more closely the preneoplastic state after human EBV infection. Unfortunately, the experience with EBV LMP1 has been sobering in this regard: CD19-CRE–directed tissue-specific expression of LMP1 in mature B cells led to rapid clearance of the LMP1 transgenic cells by the mouse T-cell response.10
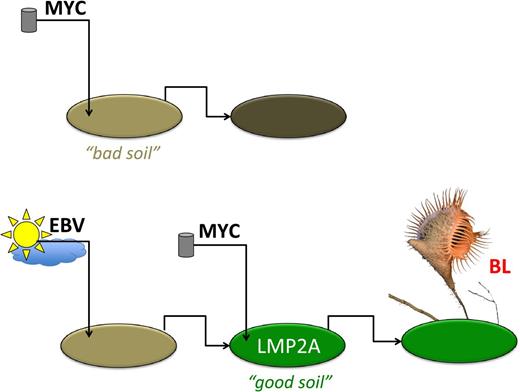
Fidler has popularized the “seed and soil” hypothesis in tumor metastasis. Metastasis only happens if tumor cells (the “seeds”) find a receptive tissue microenvironment (the “soil”). The same concept may help us understand the role of MYC activation (see figure). The MYC translocation/activation event has to happen in the right cellular environment at the right stage of B-cell differentiation to be transforming, otherwise apoptosis ensues. MYC needs a lot of help to function as an oncogene, and γ herpesviruses such as EBV are poised to lend a helping hand.
Conflict-of-interest disclosure: The author declares no competing financial interests.