To the editor:
A new risk score to predict mortality in patients with chronic graft-versus-host disease (GVHD) was recently reported by Arora et al by analyzing a large amount of data between 1995 and 2004 from the Center for International Blood and Marrow Transplant Registry (CIBMTR).1 The risk score consists of 10 variables defined at transplantation or at onset of chronic GVHD that are objective and easy to evaluate at any transplant center. Because performance of this risk score has not been examined in contemporary patients with chronic GVHD defined by the National Institutes of Health (NIH) criteria2,3 and because experience with application of the score to patients at individual centers is limited, we examined performance of the risk score in 376 consecutive patients with leukemia or myelodysplastic syndrome who received initial systemic treatment of NIH chronic GVHD between 2006 and 2010 at 2 individual centers (Figure 1). Patients had given written consent allowing the use of medical records for research in accordance with the Declaration of Helsinki, and the institutional review boards at the Fred Hutchinson Cancer Research Center (FHCRC) and the Princess Margaret Hospital (PMH) approved the study.
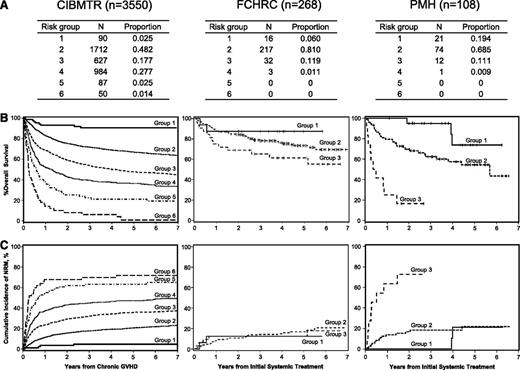
Application of the CIBMTR risk score to patients with systemically treated NIH chronic GVHD between 2006 and 2010 at 2 individual centers. Data represented includes (A) proportions of risk groups, (B) overall survival, and (C) non-relapse mortality. Diseases included acute myeloid leukemia (n = 215), acute lymphoblastic leukemia (n = 63), chronic myeloid leukemia (n = 28), and myelodysplastic syndrome (n = 70). Ages were ≤29 years in 54 patients (14%), 30 to 59 years in 237 patients (63%), and ≥60 years in 85 patients (23%); 279 patients (74%) had prior acute GVHD. Time from transplantation to chronic GVHD was <5 months in 124 patients (33%). Hyperbilirubinemia and thrombocytopenia were present in 17 (5%) and 109 (29%) patients, respectively, at the onset of systemic treatment. Karnofsky score was <80% in 117 patients (31%). Donor was a HLA identical sibling in 160 patients (43%), an HLA well-matched or partially matched unrelated donor in 171 patients (45%), and other related or HLA mismatched unrelated donor in 45 patients (12%). Disease status at transplantation was early in 225 patients (60%), intermediate in 84 patients (22%), and advanced in 67 patients (18%). Ninety-one male patients (24%) had transplantation from a female donor. GVHD prophylaxis was cyclosporine based in 146 patients (39%) and tacrolimus based or T-cell depletion in 230 patients (61%). Karnofsky score ≥80%, HLA identical sibling donor, early disease, and cyclosporine-based GVHD prophylaxis were more frequent at PMH than at FHCRC, and other characteristics were similar between the 2 centers. Curves for risk scores >3 are not shown due to a small numbers of patients.
Application of the CIBMTR risk score to patients with systemically treated NIH chronic GVHD between 2006 and 2010 at 2 individual centers. Data represented includes (A) proportions of risk groups, (B) overall survival, and (C) non-relapse mortality. Diseases included acute myeloid leukemia (n = 215), acute lymphoblastic leukemia (n = 63), chronic myeloid leukemia (n = 28), and myelodysplastic syndrome (n = 70). Ages were ≤29 years in 54 patients (14%), 30 to 59 years in 237 patients (63%), and ≥60 years in 85 patients (23%); 279 patients (74%) had prior acute GVHD. Time from transplantation to chronic GVHD was <5 months in 124 patients (33%). Hyperbilirubinemia and thrombocytopenia were present in 17 (5%) and 109 (29%) patients, respectively, at the onset of systemic treatment. Karnofsky score was <80% in 117 patients (31%). Donor was a HLA identical sibling in 160 patients (43%), an HLA well-matched or partially matched unrelated donor in 171 patients (45%), and other related or HLA mismatched unrelated donor in 45 patients (12%). Disease status at transplantation was early in 225 patients (60%), intermediate in 84 patients (22%), and advanced in 67 patients (18%). Ninety-one male patients (24%) had transplantation from a female donor. GVHD prophylaxis was cyclosporine based in 146 patients (39%) and tacrolimus based or T-cell depletion in 230 patients (61%). Karnofsky score ≥80%, HLA identical sibling donor, early disease, and cyclosporine-based GVHD prophylaxis were more frequent at PMH than at FHCRC, and other characteristics were similar between the 2 centers. Curves for risk scores >3 are not shown due to a small numbers of patients.
Compared with CIBMTR’s study, this contemporary cohort included higher proportions of patients ≥60 years of age and patients who received tacrolimus or T-cell depletion as GVHD prophylaxis and decreased proportions of patients with <5 months from transplantation to onset of chronic GVHD, hyperbilirubinemia, or thrombocytopenia. In contrast to the CIBMTR’s study, the current cohorts contained few patients in risk group 4 and no patients in risk group 5 or 6. In addition, most patients (70-80%) were classified as risk group 2 (Figure 1A). Overall survival (OS) was well stratified according to risk groups at both centers (Figure 1B), whereas nonrelapse mortality (NRM) was not well stratified at the FHCRC (Figure 1C). OS for risk group 1 was similar to CIBMTR results at both centers. Compared with CIBMTR results, OS for risk group 2 was slightly higher at FHCRC and slightly lower at PMH, and OS for risk group 3 was higher at FHCRC and much lower at PMH, despite favorable demographics at PMH. Compared with CIBMTR results, NRM for risk group 1 was slightly higher at FHCRC and similar at PMH, NRM for risk group 2 was similar at both centers, and NRM for risk group 3 was lower at FHCRC and much higher at PMH.
Our results confirm that the CIBMTR risk score performs well in predicting differences in OS in contemporary patients treated for NIH chronic GVHD. On the other hand, we identified at least 3 caveats in applying the risk score to our patients: (1) the proportion of patients with risk groups 4 to 6 was very low, (2) a finer separation of risk group 2 might be helpful, and (3) factors accounting for the center-specific difference in mortality, particularly for patients in risk group 3, remain to be determined. The dedicated long-term follow-up program at FHCRC could have contributed to the better survival of patients in this category.
Authorship
Acknowledgments: The authors thank Dr Mukta Arora for the courtesy in providing figures of CIBMTR’s study, which were adapted for this letter.
This work was supported in part by grant CA163438 from the National Institutes of Health, Department of Health and Human Services and the National Cancer Institute.
Contribution: Y.I., D.K., and M.E.D.F. designed the study, collected and analyzed data, and wrote the paper; B.E.S. performed the statistical analysis and wrote the paper; J.H.M., J.H.L., J.K., and P.J.M. interpreted data and wrote the paper; and all authors critically revised the manuscript for important intellectual content and approved the manuscript to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary E. D. Flowers, Fred Hutchinson Cancer Research Center, D5-290, PO Box 19024, Seattle, WA 98109-1024; e-mail: mflowers@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal