Abstract
Several risk factors are associated with increased mortality in patients with chronic graft-versus-host disease (cGVHD), but there is considerable variability in the reported factors. Therefore, we evaluated patient, transplantation, and cGVHD characteristics to develop a risk score in 5343 patients with cGVHD. Ten variables were identified as being significant in multivariate analysis of overall survival and nonrelapse mortality (NRM): age, prior acute GVHD, time from transplantation to cGVHD, donor type, disease status at transplantation, GVHD prophylaxis, gender mismatch, serum bilirubin, Karnofsky score, and platelet count. These 10 variables were used to build a cGVHD risk score, and 6 risk groups (RGs) were identified. The 5-year NRM was 5% (1%-9%) in RG1, 20% (19%-23%) in RG2, 33% (29%-37%) in RG3, 43% (40%-46%) in RG4, 63% (53%-74%) in RG5, and 72% (59%-85%) in RG6. The 5-year overall survival was highest at 91% (95% confidence interval [CI]:85%-97%) in RG1, followed by 67% (65%-69%) in RG2, 51% (46%-55%) in RG3, 40% (37%-43%) in RG4, 21% (12%-30%) in RG5, and 4% (0%-9%) in RG6 (all P < .01). This analysis demonstrates the usefulness of data from a large registry to develop risk-score categories for major transplantation outcomes. Validation of this cGVHD risk score is needed in a different population to ensure its broad applicability.
Introduction
Chronic graft-versus-host disease (cGVHD) is the leading cause of late morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT).1,2 Several risk factors predicting survival in patients with cGVHD have been reported.3-11 These include recipient and donor age,11 lichenoid skin changes,10 > 50% body surface area of skin involvement,4 elevated bilirubin,10 progressive onset of cGVHD,10 thrombocytopenia,4,5,9 gut involvement,5,7 Karnofsky performance status (KPS)/Lansky status,7 still receiving corticosteroids at the time of cGVHD diagnosis,11 HLA mismatch,11 and absence of early response to immunosuppression.5 Of these, the most consistent factors across studies remains thrombocytopenia at diagnosis of cGVHD and progressive onset of disease, with studies reporting variability in the other factors reported. We proposed to identify a simple model using readily available patient, disease, and HCT characteristics in conjunction with objective variables at diagnosis of cGVHD (thrombocytopenia, serum bilirubin, KPS, time to onset of cGVHD, and type of onset of cGVHD) to develop a risk score in a cohort of 5343 patients with cGVHD who registered with the Center for International Blood and Marrow Transplant Research (CIBMTR). We hypothesized that the development of a simple cGVHD risk score using the largest available multicenter cohort could lead to better generalizability and easier identification of high-risk populations for therapeutic trials or clinical use. Therefore, in the current study, we evaluated this cohort of patients with cGVHD for overall survival and nonrelapse mortality (NRM) and incorporated multiple variables to develop a new risk-score model that predicts overall survival and NRM for patients with cGVHD.
Methods
The CIBMTR is a research organization formed in 2004 as an affiliation of the International Bone Marrow Transplant Registry (IBMTR), the Autologous Blood and Marrow Transplant Registry (ABMTR), and the National Marrow Donor Program (NMDP). The CIBMTR is a voluntary organization involving more than 500 transplantation centers that have collaborated to share patient data and conduct scientific studies. The quality and compliance of data submission are monitored by computerized checks for errors, physician reviews, and on-site audits.
Patient selection
Our study population included all patients who received an allogeneic hematopoietic cell transplantation (HCT) from a related or unrelated donor (URD) including umbilical cord blood for acute myelogenous leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia, or myelodysplastic disorders between 1995 and 2004; were diagnosed with cGVHD within 1 year of transplantation; and were registered with CIBMTR. All surviving recipients who received transplantations from URDs included in this analysis were retrospectively contacted and provided informed consent for participation in the NMDP research program. Informed consent for retrospective data analysis was waived by the NMDP institutional review board for all deceased patients. Surviving patients who did not provide signed informed consent to allow analysis of their clinical data were excluded. To adjust for potential bias introduced by the exclusion of nonconsenting surviving patients, a corrective action plan modeling process randomly excluded approximately the same percentage of deceased patients using a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of data in survivors. The study included a total of 5343 HCT recipients with cGVHD.
Study end points and definitions
The primary end points of the study were overall survival and NRM. Predictive factors significant in the model for overall survival were used to develop a risk score for recipients with cGVHD. Overall survival was estimated from the onset of cGVHD. Death from any cause was treated as the event. Surviving patients were censored at the date of last contact. NRM was defined as death in continuous remission. The event was summarized by the cumulative incidence estimate with relapse as the competing risk. Grade II-IV acute GVHD was graded according to IBMTR criteria based on the pattern of severity of abnormalities in the skin, gastrointestinal tract, and liver.12 cGVHD was diagnosed according to standard CIBMTR criteria,7,13 which includes all patients with clinical criteria of cGVHD13 with or without positive histology, irrespective of time of onset of symptoms. Data required to generate an National Institutes of Health (NIH) score were not prospectively collected by the CIBMTR during the study period.14 We defined reduced intensity/nonmyeloablative regimens as follows: busulfan dose < 9 mg/kg, melphalan dose < 150 mg/m2, and total body irradiation dose < 500 cGy (single or fractionated) or 500-800 cGy (fractionated).15
Statistical analysis
Variables related to patient, disease, and transplantation characteristics were described using descriptive statistics. Cumulative incidence for NRM was calculated treating disease progression/relapse as the competing risk.16 Overall survival was calculated using Kaplan-Meier estimates, and 95% confidence intervals (CIs) were calculated using the variance derived from the formula of Greenwood.17 We used the log-rank test to compare the differences between groups in the time-to-event analyses. All P values were 2-sided.
Patient-, disease-, transplant-, and cGVHD-related variables were included in the multivariate analyses using a stepwise forward selection technique. P ≤ .01 was the criterion for inclusion in the final models.18 Patient and transplantation-related variables included were: recipient age, donor type (HLA-matched sibling donor vs other related donor vs well-matched URD vs partially matched URD vs mismatched URD), graft type (bone marrow, peripheral blood, or umbilical cord blood), donor-recipient sex mismatch, donor-recipient cytomegalovirus serology, conditioning regimen (ablative vs reduced intensity conditioning/nonmyeloablative), GVHD prophylaxis, presence of prior acute GVHD, and grade and year of transplantation. Disease-related variables included diagnosis and disease status before transplantation. cGVHD-related variables included time from HCT to cGVHD onset, platelet count at cGVHD diagnosis (< 100 × 109/L vs > 100 × 109/L), serum bilirubin at cGVHD onset (< 1 mg/dL, 1-2 mg/dL, or > 2 mg/dL), type of onset of cGVHD (progressive, quiescent, or de novo), and KPS at diagnosis of cGVHD. These cGVHD-related variables were chosen because they are objective, simple to use, and available at the time of diagnosis. Organ involvement was not considered because cGVHD may progress to involve more organs. The CIBMTR collects cGVHD data over a time period of 100 days, 6 months, and 1 year after transplantation for patients registered with the NMDP, and at 6 months and 1 year for patients registered with the IBMTR. Forms report the maximum organ involvement in the preceding time interval, which may represent disease that had progressed since diagnosis. Analysis methodology for development of risk score is discussed with the results.
Results
Patient characteristics
The study cohort included 5343 recipients with cGVHD. Table 1 demonstrates the patient-, disease-, transplantation-, and cGVHD-related variables for these recipients. The median age at HCT was 36 years (range < 1-72). The majority (55%) received HCT for acute leukemia, 34% for chronic myelogenous leukemia, and 11% for myelodysplastic disorders. The majority received transplantation in early disease status (55%). Thirty (7%) received HCT from an HLA-identical sibling donor, 4% from other related donors, and 54% from a URD (including 127 recipients of unrelated umbilical cord blood). Among the cGVHD-related variables, the median time from transplantation to cGVHD onset was 5 months (range 1-11 months), 43% had progressive onset of disease, and 36% had a platelet count of < 100 × 109/L at diagnosis.
Patient and transplantation characteristics
| Characteristics . | n (%) . |
|---|---|
| Number of patients | 5343 |
| Number of centers | 286 |
| Age at transplantation, y | |
| Median (range) | 36 (< 1-72) |
| 0-9 | 451 (8) |
| 10-19 | 665 (12) |
| 20-29 | 906 (17) |
| 30-39 | 1207 (23) |
| 40-49 | 1212 (23) |
| 50-59 | 742 (14) |
| ≥ 60 | 160 (3) |
| Male sex | 3182 (60) |
| Disease | |
| AML | 1802 (34) |
| ALL | 1140 (21) |
| CML | 1813 (34) |
| MDS | 588 (11) |
| Disease status before transplantation* | |
| Early | 2956 (55) |
| Intermediate | 1367 (26) |
| Advanced | 1020 (19) |
| Graft source | |
| Bone marrow | 3302 (62) |
| Peripheral blood | 1914 (36) |
| Umbilical cord blood | 127 (2) |
| HLA-matching status† | |
| HLA-identical sibling | 1968 (37) |
| Other related | 226 (4) |
| Well-matched unrelated | 1261 (24) |
| Partially matched unrelated | 1060 (20) |
| Mismatched unrelated | 532 (10) |
| Missing HLA data | 296 (6) |
| Conditioning regimen | |
| Myeloablative | 4749 (89) |
| Reduced intensity/nonmyeloablative | 594 (11) |
| Donor-recipient gender match | |
| Male donor → male recipient | 1804 (34) |
| Male donor → female recipient | 1105 (21) |
| Female donor → male recipient | 1378 (26) |
| Female donor → female recipient | 1056 (20) |
| Donor-recipient CMV match | |
| Donor(−)/recipient(−) | 1561 (29) |
| Donor(−)/recipient(+) | 1140 (21) |
| Donor(+)/recipient(−) | 697 (13) |
| Donor(+)/recipient(+) | 1688 (32) |
| Unknown | 257 (5) |
| GVHD prophylaxis | |
| T-cell depletion | 1242 (23) |
| CSA ± MTX ± other | 3341 (63) |
| FK506 ± MTX ± other | 760 (14) |
| Year of transplantation | |
| 1995-1999 | 2742 (51) |
| 2000-2004 | 2601 (49) |
| Grade of acute GHVD | |
| None | 1433 (27) |
| I-II | 2258 (42) |
| III-IV | 1652 (31) |
| Time from transplantation to cGHVD, mo (range) | 5 (1-11) |
| Onset of cGVHD | |
| Progressive | 2301 (43) |
| Quiescent | 1430 (27) |
| De novo | 1433 (27) |
| Missing | 179 (3) |
| Maximum grade of cGVHD | |
| Limited | 1707 (32) |
| Extensive | 3636 (68) |
| Karnofsky score at diagnosis of cGVHD | |
| < 80 | 1570 (29) |
| 80-100 | 3007 (56) |
| Unknown | 766 (14) |
| Platelet count at cGVHD, × 109/L | |
| Median (range) | 117 (< 1-718) |
| < 100 | 1921 (36) |
| ≥ 100 | 2650 (50) |
| Missing | 772 (14) |
| Serum bilirubin at cGVHD, mg/dL | |
| Median (range) | 1 (< 1-98) |
| < 1 | 2928 (55) |
| 1-2 | 982 (18) |
| > 2 | 925 (17) |
| Missing | 508 (10) |
| Duration of immunosuppression | |
| < 1 y | 1953 (37) |
| 1-2 y | 650 (12) |
| 2-3 y | 404 (8) |
| 3-4 y | 371 (7) |
| > 4 y | 1007 (19) |
| Missing | 958 (18) |
| Median follow-up of survivors, mo | 73 (3-168) |
| Characteristics . | n (%) . |
|---|---|
| Number of patients | 5343 |
| Number of centers | 286 |
| Age at transplantation, y | |
| Median (range) | 36 (< 1-72) |
| 0-9 | 451 (8) |
| 10-19 | 665 (12) |
| 20-29 | 906 (17) |
| 30-39 | 1207 (23) |
| 40-49 | 1212 (23) |
| 50-59 | 742 (14) |
| ≥ 60 | 160 (3) |
| Male sex | 3182 (60) |
| Disease | |
| AML | 1802 (34) |
| ALL | 1140 (21) |
| CML | 1813 (34) |
| MDS | 588 (11) |
| Disease status before transplantation* | |
| Early | 2956 (55) |
| Intermediate | 1367 (26) |
| Advanced | 1020 (19) |
| Graft source | |
| Bone marrow | 3302 (62) |
| Peripheral blood | 1914 (36) |
| Umbilical cord blood | 127 (2) |
| HLA-matching status† | |
| HLA-identical sibling | 1968 (37) |
| Other related | 226 (4) |
| Well-matched unrelated | 1261 (24) |
| Partially matched unrelated | 1060 (20) |
| Mismatched unrelated | 532 (10) |
| Missing HLA data | 296 (6) |
| Conditioning regimen | |
| Myeloablative | 4749 (89) |
| Reduced intensity/nonmyeloablative | 594 (11) |
| Donor-recipient gender match | |
| Male donor → male recipient | 1804 (34) |
| Male donor → female recipient | 1105 (21) |
| Female donor → male recipient | 1378 (26) |
| Female donor → female recipient | 1056 (20) |
| Donor-recipient CMV match | |
| Donor(−)/recipient(−) | 1561 (29) |
| Donor(−)/recipient(+) | 1140 (21) |
| Donor(+)/recipient(−) | 697 (13) |
| Donor(+)/recipient(+) | 1688 (32) |
| Unknown | 257 (5) |
| GVHD prophylaxis | |
| T-cell depletion | 1242 (23) |
| CSA ± MTX ± other | 3341 (63) |
| FK506 ± MTX ± other | 760 (14) |
| Year of transplantation | |
| 1995-1999 | 2742 (51) |
| 2000-2004 | 2601 (49) |
| Grade of acute GHVD | |
| None | 1433 (27) |
| I-II | 2258 (42) |
| III-IV | 1652 (31) |
| Time from transplantation to cGHVD, mo (range) | 5 (1-11) |
| Onset of cGVHD | |
| Progressive | 2301 (43) |
| Quiescent | 1430 (27) |
| De novo | 1433 (27) |
| Missing | 179 (3) |
| Maximum grade of cGVHD | |
| Limited | 1707 (32) |
| Extensive | 3636 (68) |
| Karnofsky score at diagnosis of cGVHD | |
| < 80 | 1570 (29) |
| 80-100 | 3007 (56) |
| Unknown | 766 (14) |
| Platelet count at cGVHD, × 109/L | |
| Median (range) | 117 (< 1-718) |
| < 100 | 1921 (36) |
| ≥ 100 | 2650 (50) |
| Missing | 772 (14) |
| Serum bilirubin at cGVHD, mg/dL | |
| Median (range) | 1 (< 1-98) |
| < 1 | 2928 (55) |
| 1-2 | 982 (18) |
| > 2 | 925 (17) |
| Missing | 508 (10) |
| Duration of immunosuppression | |
| < 1 y | 1953 (37) |
| 1-2 y | 650 (12) |
| 2-3 y | 404 (8) |
| 3-4 y | 371 (7) |
| > 4 y | 1007 (19) |
| Missing | 958 (18) |
| Median follow-up of survivors, mo | 73 (3-168) |
AML indicates acute myeloid leukemia; ALL, acute lymphoid leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; MDS, myelodysplastic syndrome; CSA, cyclosporine, and MTX, methotrexate, FK506:tacrolimus.
Disease status before transplantation was defined as follows: early disease included patients undergoing HCT in first remission (acute leukemia) or first chronic phase (CML) or MDS with refractory anemia or refractory anemia with ringed sideroblasts (RA, RARS); intermediate disease was defined as second or later complete remission (ALL), second or later chronic phase/accelerated phase (CML); and advanced disease included patients in relapse or primary induction failure (acute leukemia) or blast crisis (CML) or MDS with refractory anemia with excess blasts or excess blasts in transformation
HLA-matching criteria used were as defined by Weisdorf et al.20 : well-matched, no defined mismatches and no untested HLA locus; partially matched, only one untested or mismatched locus; and mismatched, 2 or more known or mismatched or untested HLA loci.
Overall survival
The probability of overall survival was 72% (95% CI 71%-73%) at 1 year and 55% (95% CI 54%-57%) at 5 years. In the multiple-regression model, increasing recipient age, the presence of and higher grade of prior acute GVHD, early onset of cGVHD (< 5 months), higher serum bilirubin at cGVHD onset, lower KPS at cGVHD onset, presence of thrombocytopenia at cGVHD onset (platelet count of < 100 × 109/L), transplantation from a mismatched URD or other related donor versus an HLA-identical sibling donor, disease status at transplantation (intermediate or advanced versus early), GVHD prophylaxis, and gender mismatch (female donor to male recipient versus male donor to male recipient) were significantly associated with a higher risk of mortality (Table 2).
Variables independently predictive of overall survival and NRM in multivariate analysis
| Variable . | n . | Overall survival . | NRM . | ||
|---|---|---|---|---|---|
| Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | ||
| Age, y | < .0001 | < .0001 | |||
| 0-9 | 451 | 1.00 | 1.00 | ||
| 10-19 | 666 | 1.35 (1.11-1.37) | .0026 | 1.41 (1.09-1.82) | .0080 |
| 20-29 | 905 | 1.31 (1.08-1.58) | .0057 | 1.46 (1.14-1.86) | .0030 |
| 30-39 | 1207 | 1.41 (1.17-1.69) | .0003 | 1.66 (1.31-2.11) | < .0001 |
| 40-49 | 1212 | 1.53 (1.27-1.84) | < .0001 | 1.90 (1.50-2.41) | < .0001 |
| 50-59 | 742 | 1.73 (1.43-2.10) | < .0001 | 2.17 (1.69-2.79) | < .0001 |
| > 60 | 160 | 2.18 (1.66-2.85) | < .0001 | 2.75 (1.96-3.87) | < .0001 |
| Acute GVHD | < .0001 | < .0001 | |||
| None | 1483 | 1.00 | 1.00 | ||
| Grade I-II | 2259 | 1.23 (1.10-1.37) | .0003 | 1.31 (1.14-1.51) | .0001 |
| Grade III-IV | 1651 | 1.55 (1.38-1.74) | < .0001 | 1.71 (1.48-1.97) | < .0001 |
| Time from transplantation to cGVHD | |||||
| < 5 mo | 3026 | 1.00 | 1.00 | ||
| > 5 mo | 2317 | 0.79 (0.72-0.86) | < .0001 | 0.84 (0.75-0.94) | .0016 |
| Serum bilirubin at cGVHD, mg/dL | < .0001 | < .0001 | |||
| 1 | 2927 | 1.00 | 1.00 | ||
| 1-2 | 982 | 1.11 (1.00-1.24) | .0557 | 1.22 (1.07-1.32) | .0035 |
| > 2 | 926 | 1.65 (1.48-1.84) | < .0001 | 1.88 (1.65-2.15) | < .0001 |
| Missing | 508 | 1.05 (0.90-1.23) | .5486 | 1.09 (0.90-1.32) | .3747 |
| KPS at cGVHD onset | < .0001 | < .0001 | |||
| < 80 | 1570 | 1.00 | 1.00 | ||
| 80-100 | 3008 | 0.57 (0.52-0.63) | < .0001 | 0.47 (0.42-0.53) | < .0001 |
| Unknown | 765 | 0.67 (0.59-0.76) | < .0001 | 0.60 (0.52-0.71) | < .0001 |
| Platelet count at cGVHD onset | < .0001 | < .0001 | |||
| < 100 × 109/L | 1920 | 1.00 | 1.00 | ||
| > 100 × 109/L | 2651 | 0.64 (0.58-0.71) | < .0001 | 0.53 (0.47-0.59) | < .0001 |
| Unknown | 772 | 0.81 (0.70-0.92) | .0014 | 0.76 (0.65-0.89) | .0006 |
| HLA group | < .0001 | < .0001 | |||
| HLA sibling | 1967 | 1.00 | 1.00 | ||
| Other related | 226 | 1.38 (1.13-1.70) | .0019 | 1.37 (1.07-1.77) | .0137 |
| Well-matched unrelated | 1261 | 1.03 (0.91-1.17) | .6405 | 1.00 (0.86-1.16) | .9863 |
| Partially matched unrelated | 1060 | 1.11 (0.98-1.26) | .0931 | 1.17 (1.01-1.36) | .0360 |
| Mismatched unrelated | 532 | 1.37 (1.18-1.59) | < .0001 | 1.51 (1.26-1.80) | < .0001 |
| Missing HLA data | 297 | 0.93 (0.75-1.15) | .4831 | 1.02 (0.79-1.30) | .8950 |
| Disease status at BMT | < .0001 | ||||
| Early | 2957 | 1.00 | 1.00 | ||
| Intermediate | 1366 | 1.22 (1.10-1.36) | .0001 | 0.99 (0.88-1.13) | .0137 |
| Advanced | 1020 | 1.86 (1.68-2.05) | < .0001 | 1.83 (1.22-1.57) | < .0001 |
| GVHD prophylaxis | < .0001 | ||||
| T-cell depletion | 1243 | 1.00 | 1.00 | ||
| CSA ± MTX ± other | 3340 | 0.81 (0.73-0.90) | < .0001 | 0.77 (0.68-0.87) | < .0001 |
| FK506 ± MTX ± other | 760 | 1.12 (0.98-1.28) | .1108 | 1.07 (0.91-1.27) | .4099 |
| Sex match | < .0001 | < .0001 | |||
| Male donor → male recipient | 1805 | 1.00 | 1.00 | ||
| Male donor → female recipient | 1104 | 0.91 (0.81-1.02) | .1096 | 0.89 (0.77-1.03) | .1087 |
| Female donor → male recipient | 1378 | 1.94 (1.08-1.33) | .0009 | 1.25 (1.10-1.42) | .0006 |
| Female donor → female recipient | 1056 | 1.10 (0.98-1.23) | .1203 | 1.15 (1.01-1.32) | .0428 |
| Variable . | n . | Overall survival . | NRM . | ||
|---|---|---|---|---|---|
| Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | ||
| Age, y | < .0001 | < .0001 | |||
| 0-9 | 451 | 1.00 | 1.00 | ||
| 10-19 | 666 | 1.35 (1.11-1.37) | .0026 | 1.41 (1.09-1.82) | .0080 |
| 20-29 | 905 | 1.31 (1.08-1.58) | .0057 | 1.46 (1.14-1.86) | .0030 |
| 30-39 | 1207 | 1.41 (1.17-1.69) | .0003 | 1.66 (1.31-2.11) | < .0001 |
| 40-49 | 1212 | 1.53 (1.27-1.84) | < .0001 | 1.90 (1.50-2.41) | < .0001 |
| 50-59 | 742 | 1.73 (1.43-2.10) | < .0001 | 2.17 (1.69-2.79) | < .0001 |
| > 60 | 160 | 2.18 (1.66-2.85) | < .0001 | 2.75 (1.96-3.87) | < .0001 |
| Acute GVHD | < .0001 | < .0001 | |||
| None | 1483 | 1.00 | 1.00 | ||
| Grade I-II | 2259 | 1.23 (1.10-1.37) | .0003 | 1.31 (1.14-1.51) | .0001 |
| Grade III-IV | 1651 | 1.55 (1.38-1.74) | < .0001 | 1.71 (1.48-1.97) | < .0001 |
| Time from transplantation to cGVHD | |||||
| < 5 mo | 3026 | 1.00 | 1.00 | ||
| > 5 mo | 2317 | 0.79 (0.72-0.86) | < .0001 | 0.84 (0.75-0.94) | .0016 |
| Serum bilirubin at cGVHD, mg/dL | < .0001 | < .0001 | |||
| 1 | 2927 | 1.00 | 1.00 | ||
| 1-2 | 982 | 1.11 (1.00-1.24) | .0557 | 1.22 (1.07-1.32) | .0035 |
| > 2 | 926 | 1.65 (1.48-1.84) | < .0001 | 1.88 (1.65-2.15) | < .0001 |
| Missing | 508 | 1.05 (0.90-1.23) | .5486 | 1.09 (0.90-1.32) | .3747 |
| KPS at cGVHD onset | < .0001 | < .0001 | |||
| < 80 | 1570 | 1.00 | 1.00 | ||
| 80-100 | 3008 | 0.57 (0.52-0.63) | < .0001 | 0.47 (0.42-0.53) | < .0001 |
| Unknown | 765 | 0.67 (0.59-0.76) | < .0001 | 0.60 (0.52-0.71) | < .0001 |
| Platelet count at cGVHD onset | < .0001 | < .0001 | |||
| < 100 × 109/L | 1920 | 1.00 | 1.00 | ||
| > 100 × 109/L | 2651 | 0.64 (0.58-0.71) | < .0001 | 0.53 (0.47-0.59) | < .0001 |
| Unknown | 772 | 0.81 (0.70-0.92) | .0014 | 0.76 (0.65-0.89) | .0006 |
| HLA group | < .0001 | < .0001 | |||
| HLA sibling | 1967 | 1.00 | 1.00 | ||
| Other related | 226 | 1.38 (1.13-1.70) | .0019 | 1.37 (1.07-1.77) | .0137 |
| Well-matched unrelated | 1261 | 1.03 (0.91-1.17) | .6405 | 1.00 (0.86-1.16) | .9863 |
| Partially matched unrelated | 1060 | 1.11 (0.98-1.26) | .0931 | 1.17 (1.01-1.36) | .0360 |
| Mismatched unrelated | 532 | 1.37 (1.18-1.59) | < .0001 | 1.51 (1.26-1.80) | < .0001 |
| Missing HLA data | 297 | 0.93 (0.75-1.15) | .4831 | 1.02 (0.79-1.30) | .8950 |
| Disease status at BMT | < .0001 | ||||
| Early | 2957 | 1.00 | 1.00 | ||
| Intermediate | 1366 | 1.22 (1.10-1.36) | .0001 | 0.99 (0.88-1.13) | .0137 |
| Advanced | 1020 | 1.86 (1.68-2.05) | < .0001 | 1.83 (1.22-1.57) | < .0001 |
| GVHD prophylaxis | < .0001 | ||||
| T-cell depletion | 1243 | 1.00 | 1.00 | ||
| CSA ± MTX ± other | 3340 | 0.81 (0.73-0.90) | < .0001 | 0.77 (0.68-0.87) | < .0001 |
| FK506 ± MTX ± other | 760 | 1.12 (0.98-1.28) | .1108 | 1.07 (0.91-1.27) | .4099 |
| Sex match | < .0001 | < .0001 | |||
| Male donor → male recipient | 1805 | 1.00 | 1.00 | ||
| Male donor → female recipient | 1104 | 0.91 (0.81-1.02) | .1096 | 0.89 (0.77-1.03) | .1087 |
| Female donor → male recipient | 1378 | 1.94 (1.08-1.33) | .0009 | 1.25 (1.10-1.42) | .0006 |
| Female donor → female recipient | 1056 | 1.10 (0.98-1.23) | .1203 | 1.15 (1.01-1.32) | .0428 |
CSA indicates cyclosporine; MTX, methotrexate; FK506:tacrolimus; and BMT, bone marrow transplantation.
NRM
The cumulative incidence of NRM was 21% (95% CI 20%-22%) at 1 year and 31% (95% CI 29%-32%) at 5 years. In the multiple-regression model, the same factors identified for overall survival were also identified to be predictive of NRM (Table 2). Because patients with progressive onset of cGVHD were identified to have an earlier time to onset (median 3.6 months) compared with de novo (median 5.2 months) and quiescent (median 5.5 months) cGVHD, the relationship between time to onset and type of onset was evaluated and found to be nonsignificant.
Development of the cGVHD risk score
Using the predictive variables identified in the preceding two paragraphs, we next developed a cGVHD risk score for overall survival or NRM. This was done in a dataset (n = 3550) in which all missing or unknown categories were deleted. Ten variables identified as significant in multivariate analysis of overall survival and NRM were used to build the cGVHD risk score. Variable-specific risk scores were constructed for each variable based on the relative risk of each category of the variable, as detailed in Table 3.
Assigned variable-specific risk scores for each category of the variables selected based on relative risk
| Variable . | Risk score . |
|---|---|
| Recipient age at transplantation | |
| < 29 y | 0 |
| 30-59 y | 1 |
| > 60 y | 2 |
| Prior acute GVHD | |
| None | 0 |
| Present | 1 |
| Time from transplantation to cGVHD | |
| > 5 mo | 0 |
| < 5mo | 1 |
| Serum bilirubin at cGVHD onset | |
| < 2 mg/dL | 0 |
| > 2 mg/dL | 2 |
| KPS at cGVHD onset | |
| > 80 | 0 |
| < 80 | 1 |
| Platelet count at cGVHD onset | |
| < 100 × 109/L | 0 |
| > 100 × 109/L | 1 |
| Type of donor | |
| HLA-identical sibling/well-matched or partially matched URD | 0 |
| Other related/mismatched URD | 1 |
| Disease status at transplantation | |
| Early | 0 |
| Intermediate | 1 |
| Advanced | 2 |
| Sex mismatch (donor/recipient) | |
| Male/ male, male/female, female/female | 0 |
| Female/male | 1 |
| GVHD prophylaxis | |
| CSA + MTX + other | 0 |
| Tacrolimus + MTX + other or T-cell depletion | 1 |
| Variable . | Risk score . |
|---|---|
| Recipient age at transplantation | |
| < 29 y | 0 |
| 30-59 y | 1 |
| > 60 y | 2 |
| Prior acute GVHD | |
| None | 0 |
| Present | 1 |
| Time from transplantation to cGVHD | |
| > 5 mo | 0 |
| < 5mo | 1 |
| Serum bilirubin at cGVHD onset | |
| < 2 mg/dL | 0 |
| > 2 mg/dL | 2 |
| KPS at cGVHD onset | |
| > 80 | 0 |
| < 80 | 1 |
| Platelet count at cGVHD onset | |
| < 100 × 109/L | 0 |
| > 100 × 109/L | 1 |
| Type of donor | |
| HLA-identical sibling/well-matched or partially matched URD | 0 |
| Other related/mismatched URD | 1 |
| Disease status at transplantation | |
| Early | 0 |
| Intermediate | 1 |
| Advanced | 2 |
| Sex mismatch (donor/recipient) | |
| Male/ male, male/female, female/female | 0 |
| Female/male | 1 |
| GVHD prophylaxis | |
| CSA + MTX + other | 0 |
| Tacrolimus + MTX + other or T-cell depletion | 1 |
CSA indicates cyclosporine; and MTX, methotrexate.
Variable-specific risk scores were summed for each patient to assign an overall risk score. Risk groups (RGs) were assigned based on the overall risk score as follows: RG1: 0-2; RG2: 3-6; RG 3: 7-8; RG 4: 9-10; RG 5: 11; and RG6: > 12. Table 4 gives the 5-year overall survival and NRM estimates for each group.
Five-year overall survival and NRM by cGVHD risk score*
| Risk group . | Overall risk score . | n . | Overall survival (95% CI)† . | NRM (95% CI)‡ . |
|---|---|---|---|---|
| RG1 | 0-2 | 90 | 90.8 (84.6-96.9) | 4.6 (0,2-9.1) |
| RG2 | 3-6 | 1712 | 67.1 (69.4-69.4) | 20.4 (18.5-22.6) |
| RG3 | 7-8 | 627 | 50.5 (46.4-54.6) | 33.2 (29.1-36.8) |
| RG4 | 9-10 | 984 | 40.2 (37.0-43.3) | 43.2 (40.1-46.4) |
| RG5 | 11 | 87 | 21.0 (12.2-29.8) | 63.4 (53.3-73.6) |
| RG6 | > 12 | 50 | 4 (0-9.4) | 72.0 (59.4-84.6) |
| RG5 and RG6 | > 11 | 137 | 14.5 (8.5-20.6) | 66.9 (58.9-74.9) |
| Risk group . | Overall risk score . | n . | Overall survival (95% CI)† . | NRM (95% CI)‡ . |
|---|---|---|---|---|
| RG1 | 0-2 | 90 | 90.8 (84.6-96.9) | 4.6 (0,2-9.1) |
| RG2 | 3-6 | 1712 | 67.1 (69.4-69.4) | 20.4 (18.5-22.6) |
| RG3 | 7-8 | 627 | 50.5 (46.4-54.6) | 33.2 (29.1-36.8) |
| RG4 | 9-10 | 984 | 40.2 (37.0-43.3) | 43.2 (40.1-46.4) |
| RG5 | 11 | 87 | 21.0 (12.2-29.8) | 63.4 (53.3-73.6) |
| RG6 | > 12 | 50 | 4 (0-9.4) | 72.0 (59.4-84.6) |
| RG5 and RG6 | > 11 | 137 | 14.5 (8.5-20.6) | 66.9 (58.9-74.9) |
Shown is the probability of overall survival (95% CI) at 5 years and the cumulative incidence of NRM (95% CI) at 5 years.
P < .01 for all comparisons of overall survival (RG1 vs RG2 vs RG3 vs RG4 vs RG5 vs RG6 and RG4 vs RG5 + 6).
P < .01 for the following comparisons of NRM: RG1 vs RG2 vs RG3 vs RG4 vs RG5. RG5 vs RG6 was not significant (P = 0.2984). For RG4 vs RG5 and RG6, P < .0001.
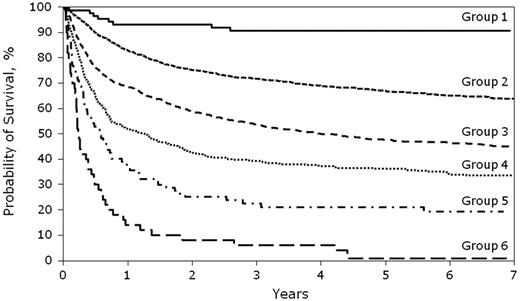
The results of risk stratification by cGVHD risk score are also given in Figure 1 (overall survival) and Figure 2 (NRM). The probability of overall survival at 5 years was 91% (95% CI:85%-97%) in RG1, followed by 67% (95% CI:65%-69%) in RG2, 51% (95% CI:46%-55%) in RG3, 40% (95% CI: 37%-43%) in RG4, 21% (95% CI: 12%-30%) in RG5, and 4% (95% CI: 0%-9%) in RG6.
Overall survival by cGVHD risk score. Shown is the probability of overall survival by cGVHD risk score. P < .01 for all comparisons of overall survival (RG1 vs RG2 vs RG3 vs RG4 vs RG5 vs RG6).
Overall survival by cGVHD risk score. Shown is the probability of overall survival by cGVHD risk score. P < .01 for all comparisons of overall survival (RG1 vs RG2 vs RG3 vs RG4 vs RG5 vs RG6).
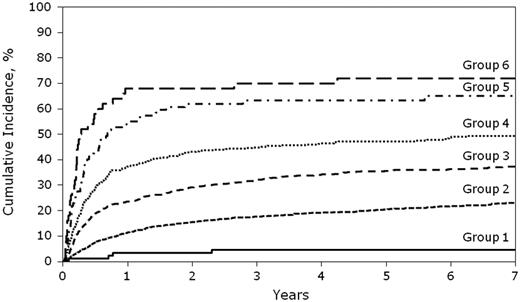
NRM by cGVHD risk score. Shown is the cumulative incidence of NRM by cGVHD risk score. P < .01 for the following comparisons for NRM: RG1 vs RG2 vs RG3 vs RG4 vs RG5 (P for RG5 vs RG6 was not significant at .2984).
NRM by cGVHD risk score. Shown is the cumulative incidence of NRM by cGVHD risk score. P < .01 for the following comparisons for NRM: RG1 vs RG2 vs RG3 vs RG4 vs RG5 (P for RG5 vs RG6 was not significant at .2984).
The cumulative incidence of NRM at 5 years was 5% (95% CI: 1%-9%) in RG1, 20% (95% CI: 19%-23%) in RG2, 33% (95% CI: 29%-37%) in RG 3, 43% (95% CI: 40%-46%) in RG4, 63% (95% CI: 53%-74%) in RG5, and 72% (95% CI: 59%-85%) in RG6.
Discussion
In this analysis of the largest cohort of cGVHD patients to date, we evaluated patient- and HCT-related factors, as well as objective, cGVHD-specific variables to develop a cGVHD risk score. We identified 6 different RGs based on the sum of risk factors that demonstrated a difference in overall survival and NRM.
Prior studies have identified several variables pertaining to organ involvement at the onset of cGVHD as being significant for higher mortality. For example, in a study evaluating a clinical grading system for cGVHD,4 extensive skininvolvement, thrombocytopenia, and progressive onset of cGVHD were identified to be significant predictors of disease-free survival. In the present study, disease-free survival in the 4 RGs was 82%, 68%, 34%, and 3% at 10 years. The performance of this prognostic score was tested in 1105 patients from 4 cohorts.3 The extent of skin involvement was quantified in 3 cohorts using available data. In this dataset, the mortality hazard associated with extensive skin involvement was lower in each of these test samples compared with the learning sample.
In a study by Lee et al,7 KPS, mouth and skin involvement, diarrhea, and weight loss were found to be important, with high KPS and mouth involvement being favorable prognostic signs, and skin involvement, diarrhea, and weight loss being unfavorable factors. Lee et al also evaluated 2 other grading schemes (limited/extensive and mild/moderate/severe) for predicting survival. Three prognostic groups were devised in which survival was statistically different in a training dataset. Low-risk patients had a high KPS (> 80%) and no diarrhea or weight loss. High-risk patients had high KPS, diarrhea, and weight loss or low KPS with either no oral involvement or all 3 poor prognostic signs: diarrhea, weight loss, and skin involvement. This was validated in 2 separate datasets. In the first validation dataset, low-risk patients had better survival than the intermediate- and high-risk groups and the intermediate- and high-risk groups were similar. In the NMDP validation set, patients with low-, intermediate-, and high-risk cGVHD had statistically different survival times. Our analysis similarly found KPS and platelet count at onset of cGVHD to be significant for survival. However, we incorporated other HCT and patient characteristics that are readily available at the time of cGVHD diagnosis into our model while developing the cGVHD risk score.
In a study by Stewart et al,11 751 patients with cGVHD were evaluated to determine factors associated with discontinuation of immunosuppression and NRM. Similar to our study, NRM was increased in patients with HLA mismatch, hyperbilirubinemia, increased age, thrombocytopenia at cGVHD onset, and progressive onset of disease. Stewart et al also reported increased NRM among recipients with older donors and those receiving a higher dose of prednisone immediately before the onset of cGVHD.
In a more recent analysis19 evaluating prognostic criteria for NRM in a cGVHD cohort as defined by NIH consensus criteria, the same 6 variables reported in their prior analysis (as given in the preceding paragraph) were found to be significant except donor age at transplantation. However, both of these studies included recipients of myeloablative transplantations with bone marrow as the source of stem cells in approximately 80% of recipients.
With recent changes in transplantation strategies, there is increasing use of alternative donor sources including umbilical cord blood, more frequent use of peripheral blood stem cells and reduced intensity or nonmyeloablative conditioning, and older recipient age at transplantation. Therefore, our study included a large, multicenter cohort of all donor sources, all graft sources, and all ages. We included patients who had undergone a nonmyeloablative or reduced intensity transplantation, but this constituted only 11% of our cohort. Similar to the recent analysis, we did not find an impact of graft source on NRM or overall survival. In the study by Stewart et al,11 the use of peripheral blood stem cells was associated with the need for prolonged immunosuppression but this did not affect NRM. This was postulated to be related to higher CD4 T-cell counts and improved immunofunction among peripheral blood stem cell recipients compared with bone marrow recipients with chronic GVHD.11
With recent improvements in supportive care, a lower NRM in the coming years is anticipated. However, we did not find an impact of the year of transplantation on NRM or overall survival. This may be related to changing patient populations over the years, with more older patients undergoing transplantation, which could account for the higher mortality.11,19
Our patient population is selected from a time period when NIH consensus criteria were not applicable and therefore we could not test the impact of NIH staging and classification on survival and NRM. Nevertheless, the variables identified in this scoring schema can be easily obtained and applied in patients at the time of cGVHD presentation. In cGVHD trials specifically, this system could be used as a complement to the new NIH staging criteria for the purposes of obtaining better patient population descriptions and concurrent validation relative to survival and NRM.
We also recognize that persistent acute GVHD may have many features similar to progressive onset cGVHD, so we repeated the analysis after excluding patients with early progressive onset cGVHD (< 130 days from HCT). The risk score remained similarly applicable for both overall survival and NRM in this population.
The present study used the largest available multicenter cohort of cGVHD patients to develop a cGVHD risk score using patient characteristics that are easily available at the time of cGVHD diagnosis. The score demonstrates high significance in predicting both overall survival and NRM. Our scoring system, if validated, will provide a practical tool to help identify high-risk patients for further therapy and enrollment in clinical trials of HCT.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos Inc; Amgen Inc; an anonymous donation to the Medical College of Wisconsin; Astellas Pharma US Inc; Be the Match Foundation; Biogen Idec Inc; BioMarin Pharmaceutical Inc; Biovitrum AB; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix GmbH; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai Inc; Genentech Inc; Genzyme Corporation; Histogenetics Inc; HKS Medical Information Systems; Hospira Inc; Kirin Brewery Co Ltd; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics Inc; Otsuka America Pharmaceutical Inc; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix Inc; StemCyte Inc; StemSoft Software Inc; Sysmex America Inc; Therakos Inc; Vidacare Corporation; ViraCor Laboratories; ViroPharma Inc; and Wellpoint Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
National Institutes of Health
Authorship
Contribution: M.A., J.P.K., D.J.W., D.A.J., M.E.D.F., S.S., M.M.H., and S.Z.P designed research; M.A. and A.H. collected data; M.A., J.P.K., and A.H. performed statistical analysis; M.A., J.P.K., D.J.W., A.H., D.A.J., M.E.D.F., S.S., M.M.H., and S.Z.P. interpreted data; M.A. drafted the manuscript; and C.S.C, A.U.I., J.H.A., M.B., J.-Y.C., M.S.C., L.I., M.J., T.R.K., S.J.L., E.W.P., R.P.G., A.C.S., and J.R.W. critically reviewed and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mukta Arora, MMC 480, 420 Delaware St SE, Minneapolis MN 55455; e-mail: arora005@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal