Key Points
Platelet-specific lentiviral gene delivery to human hematopoietic stem cells can efficiently introduce FVIII expression in human platelets.
Human platelet–derived FVIII can ameliorate the hemophilic phenotype in an immunocompromised hemophilia A mouse model.
Abstract
Our previous studies have demonstrated that platelet FVIII (2bF8) gene therapy can improve hemostasis in hemophilia A mice, even in the presence of inhibitory antibodies, but none of our studies has targeted human cells. Here, we evaluated the feasibility for lentivirus (LV)-mediated human platelet gene therapy of hemophilia A. Human platelet FVIII expression was introduced by 2bF8LV-mediated transduction of human cord blood (hCB) CD34+ cells followed by xenotransplantation into immunocompromised NSG mice or NSG mice in an FVIIInull background (NSGF8KO). Platelet FVIII was detected in all recipients that received 2bF8LV-transduced hCB cells as long as human platelet chimerism persisted. All NSGF8KO recipients (n = 7) that received 2bF8LV-transduced hCB cells survived tail clipping if animals had greater than 2% of platelets derived from 2bF8LV-transduced hCB cells, whereas 5 of 7 survived when human platelets were 0.3% to 2%. Whole blood clotting time analysis confirmed that hemostasis was improved in NSGF8KO mice that received 2bF8LV-transduced hCB cells. We demonstrate, for the first time, the feasibility of 2bF8LV gene delivery to human hematopoietic stem cells to introduce FVIII expression in human platelets and that human platelet–derived FVIII can improve hemostasis in hemophilia A.
Introduction
Hemophilia A is a congenital bleeding disorder caused by a deficiency of factor VIII (FVIII). Protein replacement therapy using either plasma-derived or recombinant FVIII is effective for treating hemophilia A patients. However, it is expensive and requires frequent infusions owing to the short half-life of the protein. Furthermore, up to 35% of patients will develop anti-FVIII inhibitory antibodies, referred to as inhibitors, after exogenous FVIII replacement therapy.1-3 The clinical hallmark of inhibitor development in hemophilia A patients is failure to respond to routine replacement therapy for bleeding episodes.3-6
Gene therapy is an attractive strategy for treating hemophilia A. The goal of gene therapy is to introduce long-term expression of therapeutic levels of FVIII in vivo by genetically modifying the target cells resulting in a cure of the disease. Although substantial progress has been achieved in the past decade, potential development of an immune response to transgene product or vector remains a significant concern in genetic therapy.7-9 We have developed a novel clinically translatable platelet-targeted gene therapy approach using lentiviral gene delivery to hematopoietic stem cells (HSCs), in which FVIII expression is under the control of the platelet-specific glycoprotein IIb promoter (2bF8).10 Our previous studies have demonstrated that 2bF8 lentivirus (2bF8LV)-mediated platelet-specific gene therapy can efficiently introduce therapeutic levels of platelet FVIII in mice with hemophilia A that have no inhibitory or noninhibitory antibody development.10 Further studies have demonstrated that therapeutic levels of platelet FVIII are sustained while inhibitor titers decline with time after 2bF8 gene therapy in hemophilia A mice with preexisting anti-FVIII immunity.11 However, this approach has not been studied in human cells.
Since our ultimate goal is to express FVIII in the platelets of patients with hemophilia A, the questions we addressed in this study included (1) whether human HSCs (hHSCs) can be transduced by 2bF8LV, (2) whether 2bF8LV-transduced hHSCs can normally give rise to blood cells including the platelet lineage, (3) whether 2bF8LV-mediated gene transfer can efficiently introduce FVIII expression in human platelets, and (4) whether human platelet–derived FVIII can correct the hemophilic bleeding diathesis. We demonstrate, for the first time, the feasibility of 2bF8LV gene delivery to hHSCs to introduce FVIII expression in human platelets and that human platelet–derived FVIII can improve hemostasis in hemophilia A.
Materials and methods
Mice
Immunocompromised NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice, which combine the features of the NOD background, the severe combined immune deficiency mutation (scid), and IL2 receptor γ chain (IL2rg) deficiency,12 were purchased from the Jackson Laboratory (Bar Harbor, ME). The NSG mice have been shown to support greater engraftment of hHSCs than all other strains.12,13 The immunocompromised hemophilia A mouse model (NSGF8KO) was generated by our laboratory by crossing NSG mice into the FVIIInull background. The FVIIInull mice (in C57BL/6 × 129/SV background) were generated by targeted disruption of exon 17 of the F8 gene.14 All animals were kept in nonspecific-pathogen-free microisolator cages at the animal facilities operated by the Medical College of Wisconsin. Animal studies were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Virus production, purification, and titering
Lentivirus-mediated transduction of hCB CD34+ cells
Xenotransplantation
NSG or NSGF8KO mice 6 to 7 weeks old were conditioned for xenotransplantation with busulfan treatment. Busulfan (6 mg/mL) (DSM Pharmaceuticals, Inc., Greenville, NC) was diluted 1:4 in phosphate-buffered saline and administered intravenously at 25 mg/kg per day on days −2 and −1. Twenty-four hours after busulfan treatment, 1 to 2 × 106 2bF8LV-transduced hCB CD34+ cells were transplanted in a volume of 300 μL of QBSF-60 medium per mouse by retro-orbital vein injection. Untransduced cells were transplanted into controls. The animals that received hCB CD34+ cell transplantation (hCBT) are defined as a humanized model.
Human cell chimerism analysis
Blood samples from animals were collected by tail bleeds, and blood counts were performed by using the Scil Vet ABC Plus blood counter (Scil Animal Care Company, Gurnee, IL). The details are provided in the supplemental Data.
PCR and quantitative real-time PCR analysis
Immunogold electron microscopy
Electron microscopy studies were performed by Clive W. Wells in the Electron Microscopy Facility at the Medical College of Wisconsin. Isolated platelets were processed and stained for FVIII protein as previously described.16 The details are provided in the supplemental Data.
FVIII activity (FVIII:C) assays
Platelets and plasma were isolated as previously described.17 Functional FVIII:C in platelet lysates or plasma was quantitated by a modified FVIII chromogenic assay as previously described.16,17 Recombinant human B-domain–deleted FVIII (rhFVIII) (Xyntha; Wyeth Pharmaceuticals, Collegeville, PA) was used as the standard. Platelets and plasma from NSG mice, C57BL/6 mice, and humans were used as controls.
Assessment of phenotypic correction
To evaluate whether 2bF8LV-transduced human platelets could improve hemostasis in hemophilic mice, the tail clip survival test and rotational thromboelastometry (ROTEM) analysis were used. The tail clip tests were performed between 4 and 6 weeks after transplantation as previously reported.11,16,17 ROTEM analysis of whole blood clotting time (CT) was performed between 6 and 8 weeks after transplantation as described in our previous report.18
2bF8LV integration site analysis
2bF8LV integration site analysis was performed on genomic DNA extracted from peripheral blood by using nonrestrictive linear amplification-mediated PCR (nrLAM-PCR), which is faster and more cost-efficient than LAM-PCR as reported.19 The details are provided in the supplemental Data.
Statistical analysis
Data are presented as mean ± standard deviation (SD), and the significance of differences was evaluated by two-tailed Student t test. A value of P < .05 was considered statistically significant.
Results
Human cell chimerism
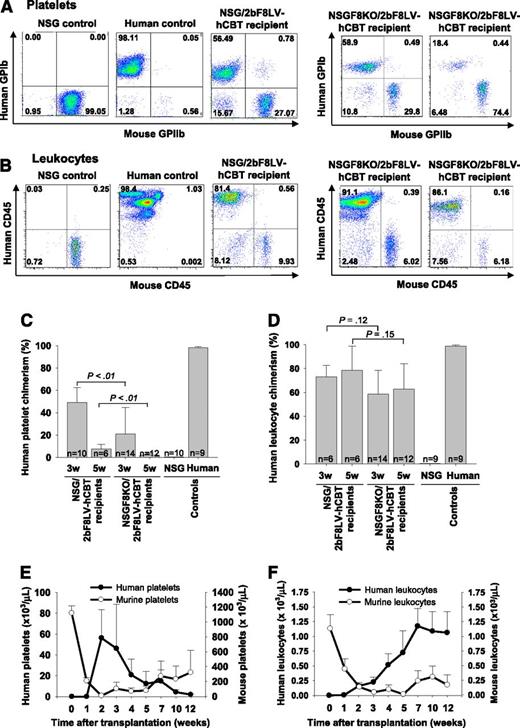
By using our culture conditions for in vitro transduction of hCB-derived hHSCs, we were able to maintain both a similar cell number and “stemness” (as indicated by CD34 expression) compared with the initial hCB sample. More than 90% of the cells were still CD34+ after 2bF8LV transduction. To determine whether 2bF8LV-transduced hCB CD34+ cells can differentiate into human platelets in our xenograft model, flow cytometry was used to analyze peripheral blood cells from transplanted recipients. No human cells could be detected 1 week after transplantation, but both human platelets and leukocytes were detected in NSG mice that received 2bF8LV-transduced hCB CD34+ cells at later time points. Three weeks after transplantation, 48.9% ± 13.5% (n = 10) of total platelets and 73.2% ± 9.6% (n = 6) of total leukocytes in peripheral blood were of human origin (Figure 1A-D). Human platelet chimerism dropped to 7.4% ± 4.3% (n = 6) at week 5 while leukocyte chimerism was still maintained at greater than 47% (Figure 1C-D).
Human cell chimerism in NSG and NSGF8KO mice that received 2bF8LV-transduced hCB CD34+cells. Blood samples were collected from tail bleeds. Human platelets were identified by a mouse anti-human glycoprotein Ib alpha chain (GPIbα) antibody AP1. Mouse platelets were stained with a rat anti-mouse CD41 (GPIIb) antibody. Human leukocytes were identified by mouse anti-human CD45 antibody, and mouse leukocytes were identified by rat anti-mouse CD45 antibody. (A) Representative flow cytometric analysis of platelets in peripheral blood of NSG and NSGF8KO recipients at 3 weeks after transplantation. (B) Representative flow cytometric analysis of leukocytes in peripheral blood of NSG and NSGF8KO recipients between 5 and 7 weeks after transplantation. (C) Human platelet chimerism in NSG and NSGF8KO recipients. Samples from NSG mice and normal human individuals were used as controls. Data are summarized from 10 trials of xenotransplants. (D) Human leukocyte chimerism in NSG and NSGF8KO recipients. Samples from NSG mice and normal human individuals were used as controls. Data are summarized from 10 trials of xenotransplants. (E) Time course of human platelet production in NSG recipients. Data are summarized from 2 trials of xenotransplants (n = 4 to 9 mice for each group). (F) Time course of human leukocyte production in NSG recipients. Data are summarized from 2 trials of xenotransplants (n = 4 to 7 mice for each group). w, weeks.
Human cell chimerism in NSG and NSGF8KO mice that received 2bF8LV-transduced hCB CD34+cells. Blood samples were collected from tail bleeds. Human platelets were identified by a mouse anti-human glycoprotein Ib alpha chain (GPIbα) antibody AP1. Mouse platelets were stained with a rat anti-mouse CD41 (GPIIb) antibody. Human leukocytes were identified by mouse anti-human CD45 antibody, and mouse leukocytes were identified by rat anti-mouse CD45 antibody. (A) Representative flow cytometric analysis of platelets in peripheral blood of NSG and NSGF8KO recipients at 3 weeks after transplantation. (B) Representative flow cytometric analysis of leukocytes in peripheral blood of NSG and NSGF8KO recipients between 5 and 7 weeks after transplantation. (C) Human platelet chimerism in NSG and NSGF8KO recipients. Samples from NSG mice and normal human individuals were used as controls. Data are summarized from 10 trials of xenotransplants. (D) Human leukocyte chimerism in NSG and NSGF8KO recipients. Samples from NSG mice and normal human individuals were used as controls. Data are summarized from 10 trials of xenotransplants. (E) Time course of human platelet production in NSG recipients. Data are summarized from 2 trials of xenotransplants (n = 4 to 9 mice for each group). (F) Time course of human leukocyte production in NSG recipients. Data are summarized from 2 trials of xenotransplants (n = 4 to 7 mice for each group). w, weeks.
Total human platelet number was calculated on the basis of the whole blood cell count, and the percent of chimerism was determined by flow cytometry analysis. The peak levels of human platelet chimerism in recipients occurred 2 to 3 weeks after transplantation, while the animals exhibited murine cell thrombocytopenia at that time point (Figure 1E). There were 56.3 ± 27.0 × 103 human platelets per microliter in the blood at 2 weeks but there was a drop to 12.4 ± 11.9 × 103 per microliter at 5 weeks and 1.9 ± 0.6 × 103 per microliter at 12 weeks after transplantation. Mouse platelet number was 9.3 ± 5.5 × 103/μL at 2 weeks after transplantation, which is only about 1% of the platelet number of NSG mice. The mouse platelet number started to increase at 3 weeks after transplantation but never recovered to pretransplantation levels during the study course (Figure 1E).
In contrast to platelets, the peak levels of human leukocyte chimerism occurred at 7 weeks after transplantation and were maintained around that level for the rest of the study course. When we looked at murine leukocytes, the animals had developed severe murine leukopenia at 3 to 5 weeks after transplantation. Compared with untreated NSG mice, there were only about 3% of mouse leukocytes remaining at 3 weeks after transplantation. Mouse leukocyte levels started to recover after 7 weeks. These levels were approximately 21% of normal in NSG mice and were maintained around that level onward during the study course (Figure 1F).
Analysis of 2bF8LV transduction in engrafted human cells
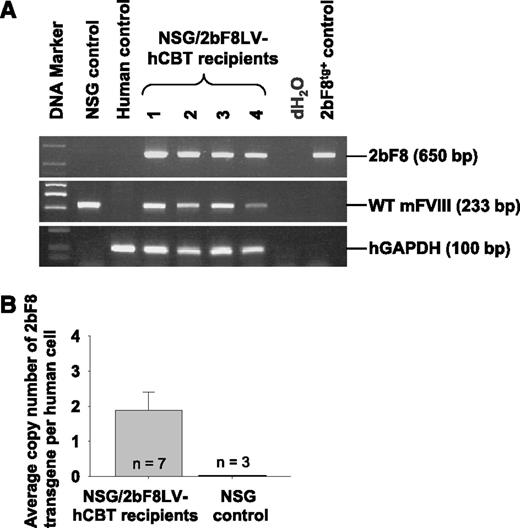
To investigate the transduction of viably engrafted 2bF8LV-transduced hCB-derived HSCs, PCR was used to detect the 2bF8 transgene in peripheral blood cell–derived DNA from NSG recipients. Representative results from recipients at least 3 weeks after transplantation are shown in Figure 2A. The 2bF8 transgene was detected in all NSG mice that received 2bF8LV-transduced hCB CD34+ cells. To determine the average copy number of 2bF8 proviral DNA in human cells, qPCR was used to quantify the 2bF8 expression cassette, normalized to the human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene. There were 1.88 ± 0.52 copies of the 2bF8 transgene per human cell in NSG mice that received 2bF8LV-transduced hCB CD34+ cells (Figure 2B). As expected, no 2bF8 proviral DNA was detected in NSG control mice.
Genetic analysis of 2bF8LV-transduced hCB transplantation recipients. (A) PCR analysis of the 2bF8 transgene. Genomic DNA was purified from peripheral leukocytes. Leukocytes from NSG mice and a normal human individual were used as controls. 2bF8 transgenic mouse DNA was used as a positive control for the 2bF8 transgene. Shown is one representative experiment that was performed 3 times. Wild-type (WT) mouse FVIII and human glyceraldehyde 3-phosphate dehydrogenase (hGAPDH) were used as internal controls. (B) qPCR quantified the average copy number of the 2bF8 transgene per cell in 2bF8LV-transduced hCB transplantation recipients. Peripheral leukocyte–derived genomic DNA was used to analyze 2bF8 proviral DNA with normalization to hGAPDH. Bars represent mean ± SD. For individual mice analyzed more than once over the course of study, the average copy number was calculated. Genomic DNA from NSG mice was used as a control. Data are summarized from 2 trials of xenotransplants.
Genetic analysis of 2bF8LV-transduced hCB transplantation recipients. (A) PCR analysis of the 2bF8 transgene. Genomic DNA was purified from peripheral leukocytes. Leukocytes from NSG mice and a normal human individual were used as controls. 2bF8 transgenic mouse DNA was used as a positive control for the 2bF8 transgene. Shown is one representative experiment that was performed 3 times. Wild-type (WT) mouse FVIII and human glyceraldehyde 3-phosphate dehydrogenase (hGAPDH) were used as internal controls. (B) qPCR quantified the average copy number of the 2bF8 transgene per cell in 2bF8LV-transduced hCB transplantation recipients. Peripheral leukocyte–derived genomic DNA was used to analyze 2bF8 proviral DNA with normalization to hGAPDH. Bars represent mean ± SD. For individual mice analyzed more than once over the course of study, the average copy number was calculated. Genomic DNA from NSG mice was used as a control. Data are summarized from 2 trials of xenotransplants.
Analysis of FVIII expression in 2bF8LV-transduced human platelets
The levels of platelet FVIII expression were determined by a chromogenic assay of platelet lysates from recipients at least 3 weeks after transplantation. Functional FVIII activity was detected in the NSG recipients that received 2bF8LV-transduced hCB CD34+ cells at an average of 0.76 ± 0.43 mU/108 total platelets (n = 6) (Figure 3A). This level of platelet FVIII corresponds to about 17.77 ± 10.36 mU/108 human platelets when calculated according to chimerism levels measured by flow cytometry (Figure 3B). As expected, no FVIII was detected in platelet lysates from control mice (NSG and C57BL/6) or from human control platelets.
Analysis of platelet FVIII expression in 2bF8LV-transduced xenotransplanted recipients. Platelets were lysed in 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). The levels of platelet-derived FVIII expression were determined by chromogenic FVIII activity (FVIII:C) assay of platelet lysates from recipients at least 3 weeks after transplantation. Bars represent mean ± SD. The average FVIII level was calculated for individual mice analyzed more than once over the course of study. Platelets from NSG mice, C57BL/6 mice, and normal human individuals were used as controls. (A) Quantitative evaluation of FVIII activity levels in platelet lysates from 2bF8LV-transduced hCBT NSG or NSGF8KO recipients. (B) Quantitative evaluation of FVIII activity levels in hCB-derived human platelets based on human platelet numbers as determined by flow cytometry. Data are summarized from 10 trials of xenotransplants. NA, not applicable.
Analysis of platelet FVIII expression in 2bF8LV-transduced xenotransplanted recipients. Platelets were lysed in 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). The levels of platelet-derived FVIII expression were determined by chromogenic FVIII activity (FVIII:C) assay of platelet lysates from recipients at least 3 weeks after transplantation. Bars represent mean ± SD. The average FVIII level was calculated for individual mice analyzed more than once over the course of study. Platelets from NSG mice, C57BL/6 mice, and normal human individuals were used as controls. (A) Quantitative evaluation of FVIII activity levels in platelet lysates from 2bF8LV-transduced hCBT NSG or NSGF8KO recipients. (B) Quantitative evaluation of FVIII activity levels in hCB-derived human platelets based on human platelet numbers as determined by flow cytometry. Data are summarized from 10 trials of xenotransplants. NA, not applicable.
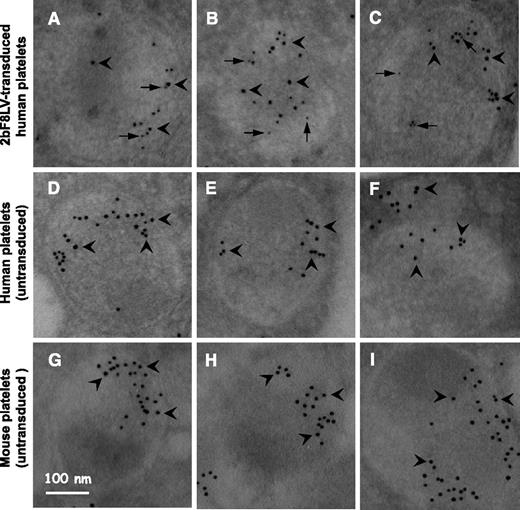
Localization of human FVIII transgene protein expression was determined by immunogold electron microscopy. Transgene hFVIII protein was labeled with small colloidal gold particles, and endogenous von Willibrand factor (VWF) was labeled with large colloidal gold particles. Transgene hFVIII protein was detected in 2bF8LV-transduced human platelets recovered from transplanted NSG mice and was colocalized with endogenous VWF in platelet alpha-granules (Figure 4A-C). No FVIII was detected in platelets from untransplanted NSG mice or normal human individuals, although a normal distribution of endogenous human VWF (Figure 4D-F) or murine VWF (Figure 4G-I) was observed.
Electron microscopy determines cellular location of 2bF8 transgene protein. Isolated platelets from (A-C) 2bF8LV-transduced xenotransplanted NSG recipients, (D-F) normal human individuals, and (G-I) untransplanted NSG control mice were immunostained for hFVIII and either endogenous human or mouse VWF. hFVIII was stained with mouse anti-human FVIII monoclonal antibody 103.3 and probed with goat anti-mouse colloidal gold probe (6 nm; some indicated by arrows). VWF was stained with rabbit anti-human VWF polyclonal antibody (Dako), which cross-reacts with mouse VWF, and were probed with goat anti-rabbit colloidal gold probe (10 nm; some indicated by arrowheads). The results show that hFVIII is stored together with human VWF in platelet alpha-granules of 2bF8LV-transduced human platelets (A-C).
Electron microscopy determines cellular location of 2bF8 transgene protein. Isolated platelets from (A-C) 2bF8LV-transduced xenotransplanted NSG recipients, (D-F) normal human individuals, and (G-I) untransplanted NSG control mice were immunostained for hFVIII and either endogenous human or mouse VWF. hFVIII was stained with mouse anti-human FVIII monoclonal antibody 103.3 and probed with goat anti-mouse colloidal gold probe (6 nm; some indicated by arrows). VWF was stained with rabbit anti-human VWF polyclonal antibody (Dako), which cross-reacts with mouse VWF, and were probed with goat anti-rabbit colloidal gold probe (10 nm; some indicated by arrowheads). The results show that hFVIII is stored together with human VWF in platelet alpha-granules of 2bF8LV-transduced human platelets (A-C).
Insertion site analysis
To investigate the chromosomal insertion sites of 2bF8LV in human cells, we used nrLAM-PCR. We identified 19 insertion sites in 5 of the NSG recipients and found 13 insertion sites in the introns of genes, 2 in the exons, and 4 in intergenic regions (Table 1). Similar to what we have found in transduced mouse cell studies,11 the integration of 2bF8LV into the first intron of human genes appears to have occurred at a higher frequency (27%) than other areas within genes. The frequency of sense versus antisense orientation of 2bF8 transgene within human genes appears to be approximately equal at 47% and 53%, respectively. No identified integration sites were located within known proto-oncogenes in human hematopoiesis.
Nonrestrictive linear amplification-mediated insertion sites of 2bF8 transgene determined by nrLAM-PCR
| No. . | Chromosome . | Direction of transgene . | Gene . |
|---|---|---|---|
| 1 | 1 | ↑ | ↓ DDI2 (DNA damage inducible protein 2) intron 1 |
| 2 | 1 | ↑ | ↑ ZSWIM5 (zinc finger, SWIM-domain-containing 5) intron 1 |
| 3 | 1 | ↑ | ↓ AHS1L (probable histone-lysine N-methyltransferase ASH1L) intron 14 |
| 4 | 3 | ↑ | ↓ CLASP2 (cytoplasmic linker-associated protein 2) intron 12 |
| 5 | 6 | ↑ | Intergenic (located downstream of hypothetical protein LOC100288169 and upstream of DUSP22 [dual-specificity phosphatase 22]) |
| 6 | 7 | ↓ | ↑ DGKB (diacylglyceral kinase β isoform 1, 2) intron 2 |
| 7 | 7 | ↑ | ↑ GTF2IRD2 (general transcription factor II-I repeat domain-containing protein 2A) intron 2 |
| 8 | 7 | ↓ | ↓ PILRB (paired immunoglobulin-like type 2 receptor β precursor) intron 3 |
| 9 | 8 | ↑ | Intergenic (located downstream of UNC5D [netrin receptor UNC5D precursor] and upstream of KCNUI [potassium channel subfamily U member]) |
| 10 | 11 | ↓ | ↓ RBM14-RBM41 (RBM14-RBM4 protein isoform 1, 2) intron 1 |
| 11 | 11 | ↓ | ↑ ARRB1 (β-arrestin-1 isoform A and B) intron 1 |
| 12 | 11 | ↓ | ↑ BSCL2 (Bernadinelli-Seip congenital lipidystrophy 2 [Seipin]) intron 5 |
| 13 | 11 | ↓ | Intergenic (located downstream of LTBP3 [latent transforming growth factor β binding protein 3] and upstream of SSSCA1 [Sjogren syndrome/scleroderma autoantigen 1]) |
| 14 | 12 | ↓ | Intergenic (located downstream of ATP2B1 [plasma membrane calcium-transporting ATPase 1 isoform 1a, 1b] and upstream of C12orf12 [hypothetical protein LOC196477]) |
| 15 | 14 | ↑ | ↓ SOCS4 (suppressor of cytokine signaling 4) exon 4 in 3′UT |
| 16 | 14 | ↓ | ↑ GPR132 (G protein-coupled receptor 132) intron 1 |
| 17 | 16 | ↑ | ↑ CREBBP (Creb-binding protein) exon 30 |
| 18 | 16 | ↑ | ↑ KIAA0430 (Limkain b-1 isoform 1, 2, 3) intron 6 |
| 19 | 17 | ↓ | ↓ SUZ12 (suppressor of zeste 12 homolog [Drosophila]) intron 15 |
| No. . | Chromosome . | Direction of transgene . | Gene . |
|---|---|---|---|
| 1 | 1 | ↑ | ↓ DDI2 (DNA damage inducible protein 2) intron 1 |
| 2 | 1 | ↑ | ↑ ZSWIM5 (zinc finger, SWIM-domain-containing 5) intron 1 |
| 3 | 1 | ↑ | ↓ AHS1L (probable histone-lysine N-methyltransferase ASH1L) intron 14 |
| 4 | 3 | ↑ | ↓ CLASP2 (cytoplasmic linker-associated protein 2) intron 12 |
| 5 | 6 | ↑ | Intergenic (located downstream of hypothetical protein LOC100288169 and upstream of DUSP22 [dual-specificity phosphatase 22]) |
| 6 | 7 | ↓ | ↑ DGKB (diacylglyceral kinase β isoform 1, 2) intron 2 |
| 7 | 7 | ↑ | ↑ GTF2IRD2 (general transcription factor II-I repeat domain-containing protein 2A) intron 2 |
| 8 | 7 | ↓ | ↓ PILRB (paired immunoglobulin-like type 2 receptor β precursor) intron 3 |
| 9 | 8 | ↑ | Intergenic (located downstream of UNC5D [netrin receptor UNC5D precursor] and upstream of KCNUI [potassium channel subfamily U member]) |
| 10 | 11 | ↓ | ↓ RBM14-RBM41 (RBM14-RBM4 protein isoform 1, 2) intron 1 |
| 11 | 11 | ↓ | ↑ ARRB1 (β-arrestin-1 isoform A and B) intron 1 |
| 12 | 11 | ↓ | ↑ BSCL2 (Bernadinelli-Seip congenital lipidystrophy 2 [Seipin]) intron 5 |
| 13 | 11 | ↓ | Intergenic (located downstream of LTBP3 [latent transforming growth factor β binding protein 3] and upstream of SSSCA1 [Sjogren syndrome/scleroderma autoantigen 1]) |
| 14 | 12 | ↓ | Intergenic (located downstream of ATP2B1 [plasma membrane calcium-transporting ATPase 1 isoform 1a, 1b] and upstream of C12orf12 [hypothetical protein LOC196477]) |
| 15 | 14 | ↑ | ↓ SOCS4 (suppressor of cytokine signaling 4) exon 4 in 3′UT |
| 16 | 14 | ↓ | ↑ GPR132 (G protein-coupled receptor 132) intron 1 |
| 17 | 16 | ↑ | ↑ CREBBP (Creb-binding protein) exon 30 |
| 18 | 16 | ↑ | ↑ KIAA0430 (Limkain b-1 isoform 1, 2, 3) intron 6 |
| 19 | 17 | ↓ | ↓ SUZ12 (suppressor of zeste 12 homolog [Drosophila]) intron 15 |
Arrows indicate the direction of the transgene or gene relative to the standard chromosomal numbering. The insertion sites listed in this table were from 5 NSG recipients.
Evaluation of clinical efficacy of human platelet–derived FVIII
By using the NSG mouse model, we have examined the feasibility of targeting FVIII expression to human platelets by 2bF8 lentiviral gene transfer to hCB-derived hHSCs. To explore whether 2bF8LV-transduced human platelets can improve the bleeding diathesis in hemophilia A, we generated an immunocompromised hemophilia A mouse model by crossing NSG and FVIIInull mice to produce NSGF8KO mice in which the genetic background is a mixture of NOD, 129/SV, and C57BL/6. 2bF8LV-transduced hCB-derived CD34+ cells were transplanted into NSGF8KO mice preconditioned with busulfan.
Because the IL2rg gene is relatively near the F8 locus (the distance between the two genes is 7.5 centimorgans) on the X chromosome, there was some difficulty with crossing to achieve linkage of the defective IL2rg gene with the FVIII knockout gene, but after multiple matings, we successfully identified a crossover between these two genes and obtained NSGF8KO mice for our studies. We found that human platelet chimerism was markedly decreased in recipients that had lost the NOD phenotype during breeding, while leukocyte chimerism was less affected. Three weeks after transplantation, 20.9% ± 23.9% (n = 14) of total platelets in NSGF8KO recipients were of human origin but only 2.1% ± 1.7% (n = 12) at week 5, significantly lower than in NSG recipients (P < .01) (Figure 1C). In contrast, human leukocyte chimerism in NSGF8KO recipients was not significantly different from that in NSG recipients (Figure 1D).
As expected, no FVIII:C was detected in plasma from NSGF8KO recipients (data not shown). Functional platelet-FVIII activity was detected in NSGF8KO recipients at an average level of 0.56 ± 0.42 mU/108 total mouse plus human platelets (Figure 3A), which corresponded to 17.72 ± 8.65 mU/108 human platelets (Figure 3B) when calculated on the basis of human platelet chimerism as determined by flow cytometry. There was no significant difference in the level of platelet FVIII expression between NSG and NSGF8KO groups after xenotransplantation of 2bF8LV-transduced hCB (Figure 3A-B).
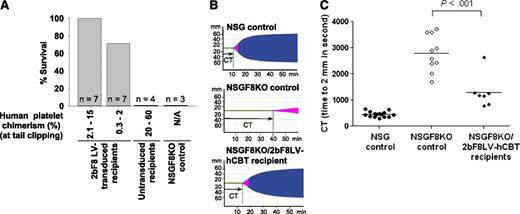
Tail clip survival tests were used to assess phenotypic correction of the FVIIInull coagulation defect in NSGF8KO mice. Four to 6 weeks after transplantation, all recipients survived tail clipping if greater than 2% of platelets were derived from 2bF8LV-transduced hCB CD34+ cells; 5 of 7 mice survived when human platelets were between 0.3% and 2%. In contrast, none of the untransplanted NSGF8KO mice or NSGF8KO mice that received untransduced hCB CD34+ cells survived tail clipping (Figure 5A). To further assess whether 2bF8LV-transduced human platelets improve hemostasis in hemophilia A mice, ROTEM analysis was used to analyze whole blood CT (Figure 5B-C). Six to 8 weeks after transplantation, the whole blood CT was 1296 ± 234 seconds (n = 7) in NSGF8KO/2bF8LV-hCBT recipients, in which human platelet chimerism was 0.4% to 5.5% at the time of the ROTEM analysis. This whole blood CT was significantly shorter than that obtained from NSGF8KO control mice (2792 ± 220 seconds [n = 10]), confirming that hemostasis was improved in NSGF8KO mice that received 2bF8LV-transduced hCB CD34+ cells.
Phenotypic correction assessment. (A) Tail clip survival test assessing the phenotypic correction in 2bF8LV-transduced humanized NSGF8KO mice. The tail clipping test was performed at least 4 weeks after transplantation. Mice surviving beyond 24 hours were considered to have achieved phenotypic correction. Untransduced hCB-transplanted and untransplanted NSGF8KO recipients were tested as controls. Data are summarized from 10 trials of xenotransplants. (B) ROTEM analysis. The ROTEM analysis was performed between 6 and 8 weeks after transplantation when human platelet chimerism was 0.4% to 5.5%. Representative TEMograms (thromboelastometry graphs) from untransplanted NSG, untransplanted NSGF8KO, and 2bF8LV-transduced humanized NSGF8KO (with 2% human platelet chimerism) mice are shown. (C) Whole blood CT determined by ROTEM analysis. Untransplanted NSG and NSGF8KO mice were used as controls. Data are summarized from 5 trials of xenotransplants. N/A, not applicable.
Phenotypic correction assessment. (A) Tail clip survival test assessing the phenotypic correction in 2bF8LV-transduced humanized NSGF8KO mice. The tail clipping test was performed at least 4 weeks after transplantation. Mice surviving beyond 24 hours were considered to have achieved phenotypic correction. Untransduced hCB-transplanted and untransplanted NSGF8KO recipients were tested as controls. Data are summarized from 10 trials of xenotransplants. (B) ROTEM analysis. The ROTEM analysis was performed between 6 and 8 weeks after transplantation when human platelet chimerism was 0.4% to 5.5%. Representative TEMograms (thromboelastometry graphs) from untransplanted NSG, untransplanted NSGF8KO, and 2bF8LV-transduced humanized NSGF8KO (with 2% human platelet chimerism) mice are shown. (C) Whole blood CT determined by ROTEM analysis. Untransplanted NSG and NSGF8KO mice were used as controls. Data are summarized from 5 trials of xenotransplants. N/A, not applicable.
Taken together, our results show that 2bF8 lentiviral gene delivery to hHSCs can efficiently introduce FVIII expression in human platelets and that 2bF8LV-transduced human platelets can improve hemostasis in a hemophilia A mouse model.
Discussion
Previous studies by our group10,11,16,17 and others20-23 using mouse models have demonstrated that platelet-targeted expression of FVIII may be a promising approach for gene therapy of hemophilia A and hemophilia A with inhibitors. In this study, we established a novel immunocompromised hemophilia A mouse model and used humanized mouse models to explore the feasibility and efficacy of targeting FVIII expression to human platelets for hemophilia A gene therapy. FVIII expression was introduced by ex vivo transduction of hCB-derived hHSCs followed by xenotransplantation into immunocompromised hemophilia A mice. We conclude that 2bF8 lentiviral gene delivery to hHSCs can efficiently introduce functional FVIII expression in human platelets and that human platelet–derived FVIII can ameliorate the bleeding diathesis in hemophilia A mice.
Human thrombocytopoiesis in the 2bF8LV genetically modified humanized NSG model
Our study demonstrated that 2bF8LV can efficiently transduce hCB CD34+ cells, resulting in successfully genetically manipulated hHSCs. In preliminary experiments, we transplanted the transduced hCB CD34+ cells into NSG mice preconditioned with a sublethal irradiation dose of 200 or 300 cGy total-body irradiation, but the mortality was extremely high. It has been reported that busulfan chemotherapy is an efficient preconditioning regimen to achieve successful engraftments in humanized models for white blood cell–related studies.24-27 Thus, for the first time, we used busulfan chemotherapy as a preconditioning regimen for xenotransplants to study the feasibility of 2bF8 gene delivery to hHSCs and the clinical efficacy of human platelet–derived FVIII in gene therapy of hemophilia A. We found that the transduced repopulating hHSCs were able to successfully engraft into immunocompromised animals preconditioned with busulfan and give rise to human blood cells, including platelets that contain functional FVIII activity.
Since the final target for FVIII expression in our gene therapy approach is platelets, it is important to investigate whether 2bF8LV-transdcued hHSCs can differentiate into human platelets. Human platelet chimerism peaked at 2 to 3 weeks after xenotransplantation when up to 93% of platelets were of human origin, but by week 5, they had dropped to 3% to 18%, although leukocyte chimerism was still maintained at greater than 47%. The rapid reduction of human platelet chimerism in NSG recipients also occurs in nontransduced transplants (data not shown), so it is not due to 2bF8 genetic modification. This is a common problem in studies of human thrombocytopoiesis in immunocompromised mouse models, and the cause is still unknown.28-31 There was a small population that was positive for both human and mouse platelet markers, which could be due to low level platelet activation during sample processing resulting in human and mouse platelet association.
We had monitored some animals for up to 7 months after transplantation and found that leukocyte chimerism was sustained, but the reduction of human platelet chimerism between weeks 3 and 7 was remarkable, decreasing by 70% to 95%. 2bF8 proviral DNA was consistently detected in peripheral blood cell–derived DNA at all time points during the study course, indicating that long-term repopulating hHSCs were successfully engrafted and were genetically modified by 2bF8LV. Although the temporal window for studying human platelets derived from 2bF8LV-transduced hCBT in the NSG mouse model was narrow, there were up to 133 × 103 human platelets per microliter circulating in the peripheral blood in xenotransplanted recipients, with the highest chimerism between weeks 2 and 3, nearly reaching the range of normal platelet number in humans (150 to 450 × 103 platelets per microliter).
Because high levels of white blood cell chimerism were sustained in 2bF8LV-transduced recipients, we suspect the quick reduction of human platelets in xenotransplant recipients may be due to incompatibility of murine molecular signals involving thrombocytopoiesis or maintenance of the proper hematopoietic microenvironment, for human platelet production. Although we did infuse recombinant human thrombopoietin (rhTPO) into 3 of the NSG mice that received 2bF8LV-transduced hCB CD34+ cells, human platelet chimerism did not significantly increase (data not shown). Recent studies done by Hu and Yang32 have demonstrated that poor human platelet reconstitution in their xenotransplantation models is mainly caused by macrophage-mediated rejection. The macrophage rejection mechanism cannot explain the phenotype of blood cells in our xenotransplanted recipients, in which human platelets peak in the early phase (2 weeks) when there were 12% murine endogenous leukocytes including macrophages and decrease dramatically at 5 weeks when murine endogenous leukocytes are barely detectable (0.9%). These results indicate that there are other mechanisms that are still unknown playing roles in human thrombocytopoiesis in immunocompromised mouse models.
Human thrombocytopoiesis in the 2bF8LV genetically modified humanized NSGF8KO model
Although we obtained high levels of human platelet chimerism in NSG mice that received 2bF8LV-transduced hCB CD34+ cells, the human platelet chimerism in NSGF8KO recipients was dramatically lower. This might be owing to loss of the NOD background during crossbreeding of NSG mice to the FVIIInull background as well as scid “leakiness.”26 It is known that the NOD background contributes to the absence of hemolytic complement, defective macrophages, and enhanced human HSC engraftment.33-35 For genotyping of NSGF8KO mice, we used PCR to detect the FVIIInull allele, the scid mutation, and the IL-2Rγnull gene. However, there is currently no genotyping approach available to track the NOD background. Thus, the genetic background of NOD was likely diluted during crossbreeding of NSG into FVIIInull background, so macrophages from resulting NSGF8KO mice might function normally and phagocytize human platelets in xenotransplanted recipients.
The scid mutation prevents maturation of T and B cells,36 but scid leakiness can result in the production of some functional B and T cells as the mice age,37 causing a rejection of xenografts. It has been reported that such leakiness is higher in mice housed under nonspecific pathogen-free conditions, which is the type of facility in which we house our mice, and leakiness is generally higher on the C57BL/6 genetic background.38-40 The FVIIInull mice crossed with NSG mice were on a mixed genetic background of C57BL/6 and 129/SV, so NSGF8KO mice inherited a mixed genetic background of C57BL/6, 129/SV, and NOD. Backcrossing NSGF8KO mice to a pure NOD background will need to be pursued in future studies.
FVIII expressed in human platelets has functional activity
Although human platelet reconstitution was not sustained in 2bF8LV-transduced hCBT recipients, platelets produced by 2bF8LV-transduced hCB CD34+ cells contained functional FVIII activity. When the levels of platelet FVIII expression were converted to average per human platelet values according to chimerism levels determined by flow cytometry, the amount of FVIII in 2bF8LV-transduced human platelets (17.77 mU/108 platelets) is significantly greater than those obtained from transduced or transgenic mouse platelets (1.56 mU/108 platelets or 1.41 mU/108 platelets, respectively) in our previous studies.11,16 This phenomenon was consistent for both NSG and NSGF8KO mice that received 2bF8LV-transduced hCB CD34+ cells.
It is still unclear why the average amount of FVIII in human platelets derived from 2bF8 genetically engineered hHSCs is greater than in mouse HSCs, but there are three potential explanations. First, human platelets are larger than mouse platelets. Human platelets are approximately 2.0 to 4.0 μm in diameter with a mean volume of 7.8 fL, whereas mouse platelets are 1.0 to 2.0 μm in diameter with a mean volume of 5.5 fL.41,42 Thus, each 2bF8LV-transduced human platelet has a higher capacity to carry cargo including transgene FVIII protein than mouse platelets. Second, 2bF8LV may transduce hCB-derived HSCs more efficiently than mouse bone marrow-derived HSCs. The vector we used to deliver 2bF8 transgene to HSCs was a second-generation HIV type 1–based vector, which has been reported to transduce human cells more efficiently than its mouse counterparts.43-45 Indeed, the average copy number of 2bF8 proviral DNA per human cell was significantly higher than that obtained from the transduced mouse cells in our previous reports.10,11 A third reason for potentially higher expression (not infection) is that human platelets contain human VWF, which works better with human FVIII.
Our previous studies have demonstrated that hFVIII expressed in mouse platelets can rescue the bleeding diathesis in hemophilia A mice.10,11,16,46 In this study, which used a humanized NSGF8KO model, we demonstrated that 2bF8LV can efficiently introduce FVIII expression in human platelets and that human platelet–derived FVIII can restore hemostasis in a hemophilia A mouse model as long as human platelets persist. The bleeding diathesis was significantly improved, even with a small proportion of platelets (0.3% to 2%) derived from 2bF8LV-transduced hCB CD34+ cells. Our data suggest that sufficiently high transduction efficiencies can be practically achieved to ensure adequate transgene delivery to hHSCs to produce therapeutic levels of platelet-FVIII using 2bF8 lentiviral gene transfer.
In conclusion, we have demonstrated for the first time that 2bF8 lentiviral gene delivery to hHSCs can efficiently introduce therapeutic levels of FVIII expression in human platelets and that this can ameliorate the hemophilic phenotype in an immunocompromised hemophilia A mouse model. Our data strongly suggest that 2bF8 lentiviral gene delivery to hHSCs can be a promising approach for gene therapy of hemophilia A. In the scenario of a hemophilia A patient, autologous cells from cord blood or mobilized HSCs will be transduced with 2bF8LV ex vivo followed by auto-transplantation of the transduced cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. Kazazian at the University of Pennsylvania School of Medicine for FVIIInull mice.
This work was supported by National Institutes of Health, Heart, Lung and Blood Institute grants HL-102035 (Q.S.) and HL-44612 (R.R.M.), Hemophilia Association of New York grant (Q.S.), and generous gifts from the Children’s Hospital Foundation (Q.S.) and Midwest Athletes Against Childhood Cancer Fund (Q.S.).
Authorship
Contribution: Q.S. designed and performed research, analyzed data, and wrote the manuscript; E.L.K. and Y.C. performed research and analyzed data; J.A.S., and S.A.F. performed research, analyzed data, and made comments to the manuscript; and R.R.M. helped design the project and made comments to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qizhen Shi, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: qizhen.shi@bcw.edu.

![Figure 3. Analysis of platelet FVIII expression in 2bF8LV-transduced xenotransplanted recipients. Platelets were lysed in 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). The levels of platelet-derived FVIII expression were determined by chromogenic FVIII activity (FVIII:C) assay of platelet lysates from recipients at least 3 weeks after transplantation. Bars represent mean ± SD. The average FVIII level was calculated for individual mice analyzed more than once over the course of study. Platelets from NSG mice, C57BL/6 mice, and normal human individuals were used as controls. (A) Quantitative evaluation of FVIII activity levels in platelet lysates from 2bF8LV-transduced hCBT NSG or NSGF8KO recipients. (B) Quantitative evaluation of FVIII activity levels in hCB-derived human platelets based on human platelet numbers as determined by flow cytometry. Data are summarized from 10 trials of xenotransplants. NA, not applicable.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/3/10.1182_blood-2013-08-520478/4/m_395f3.jpeg?Expires=1767727993&Signature=gkHzg2YzveCRa6vGkbYNpLcUcmu2m4rsGQ-VzaYniQ88odA~-QCPPaVdGgw82Y1-8Lx4SzK4XO1OF2prwGcQgw6H1M8hdC9WA8gWVh7koC9FWj692YonASW6doSi9jGUtYUrrZq0rZWES9yBph2ODsP72OpFque4SsKSvzmrWzYLgd~B22gYic8Hv0pE92QWaMc7TxMtSnao5DRK5~oXUJZpEoiqm~lWWZ4BR14DLkUDy~mxatBA05W9OOw16XHdqxQApRgC-VrRSYqHl45ccLgtd9RHzX5Ny3wrjoPYw-gCgQNMasLQ4bJYANhx3jsXvY8UQy4814VnvaxANV7KzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)