Key Points
Among women with pure obstetric APS, late pregnancy complications are more frequent in cases of prior fetal loss.
Late pregnancy complications are more frequent among women treated for pure obstetric APS than among nontreated controls.
Abstract
The incidence of pregnancy outcomes for women with the purely obstetric form of antiphospholipid syndrome (APS) treated with prophylactic low-molecular-weight heparin (LMWH) plus low-dose aspirin (LDA) has not been documented. We observed women without a history of thrombosis who had experienced 3 consecutive spontaneous abortions before the 10th week of gestation or 1 fetal loss at or beyond the 10th week. We compared the frequencies of complications during new pregnancies between treated women with APS (n = 513; LMWH + LDA) and women negative for antiphospholipid antibodies as controls (n = 791; no treatment). Among APS women, prior fetal loss was a risk factor for fetal loss, preeclampsia (PE), premature birth, and the occurrence of any placenta-mediated complication. Being positive for anticardiolipin immunoglobulin M antibodies was a risk factor for any placenta-mediated complication. Among women with a history of recurrent abortion, APS women were at a higher risk than other women of PE, placenta-mediated complications, and neonatal mortality. Among women with prior fetal loss, LMWH + LDA–treated APS women had lower pregnancy loss rates but higher PE rates than other women. Improved therapies, in particular better prophylaxis of late pregnancy complications, are urgently needed for obstetric APS and should be evaluated according to the type of pregnancy loss.
Introduction
Purely obstetric antiphospholipid antibody syndrome (APS) is clinically characterized by pregnancy morbidity in women with no history of thrombosis (repeated unexplained abortions before the 10th week, unexplained fetal loss at or after the 10th week, or premature birth before the 34th week because of preeclampsia [PE]).1-4 The defining laboratory criterion for APS is repeated positive test results for the antiphospholipid antibodies (aPLAbs) lupus anticoagulant (LA), anticardiolipin antibody (aCL), and anti-β2 glycoprotein I antibody (aβ2GP1) of immunoglobulin G (IgG) and/or IgM isotype at moderate or high titers.2,4
Obstetric APS without a history of thrombosis is currently managed by administering low-dose aspirin (LDA) and either low-dose unfractionated heparin (twice daily) or low-molecular-weight heparin (LMWH, once daily) pending large, multicenter randomized controlled trials directly comparing the 2 treatments.5,6 Currently, LMWH is preferred for practical and safety reasons. Although the rates of birth of healthy infants to women with obstetric APS are high, placenta-mediated complications of pregnancy are regularly described7,8 but have not been systematically evaluated.
The Nîmes Obstetricians and Hematologists Antiphospholipid Syndrome (NOH-APS) study9 prospectively recorded the frequencies of pregnancy outcomes among women with no history of thrombosis who had experienced pregnancy loss fulfilling the clinical criteria for APS and who were either positive for aPLAbs (APS group) or negative for aPLAbs (control group).
Patients and methods
Patients
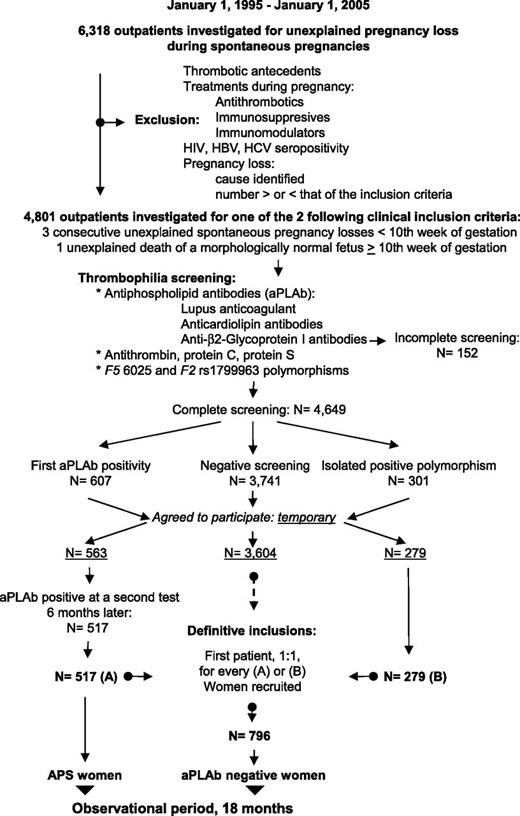
The recruitment is presented in Figure 1 and has been described in detail elsewhere.9 Briefly, 6318 women with pregnancy loss during spontaneous pregnancies were referred, over a 10-year period, for investigation to the Outpatient Department of Hematology, University Hospital of Nîmes (a tertiary referral center). Exclusion criteria were any history of thrombotic events (at least 1 clinical episode of venous, arterial, or small vessel thrombosis, in any tissue or organ except the placenta, with thrombosis being confirmed by objective validated criteria, ie, unambiguous findings by appropriate imaging studies or histopathology) or any treatment given during previous pregnancies that may have modified the natural evolution of their condition, such as antithrombotics or immunosuppressive or immunity-modulating drugs. Women with pregnancy losses explained by infectious, metabolic, anatomic, or hormonal factors were also excluded. Women seropositive for HIV or hepatitis B or C were also excluded. A total of 4801 women fulfilled 1 of the 2 following inclusion criteria: (1) 3 unexplained consecutive spontaneous abortions before the 10th week of gestation (WG) not due to maternal anatomic or hormonal abnormalities or to paternal and maternal chromosomal causes (recurrent embryo loss subgroup); or (2) 1 unexplained death of a morphologically normal fetus (fetal loss) at or after the 10th WG, with the normal fetal morphology having been documented by ultrasound scan or direct examination of the fetus (fetal loss subgroup). Patients were categorized as primary aborters (no previous successful pregnancy) or secondary aborters.
Patient flow diagram. Patient flow diagram for the NOH-APS study on pregnancy and the 2 groups of women with recurrent abortions or 1 fetal death: women with persistent antiphospholipid antibodies (APS women), and women with negative antiphospholipid antibodies and a negative thrombophilia screening (aPLAb negative women). HBV, hepatitis B virus; HCV, hepatitis C virus.
Patient flow diagram. Patient flow diagram for the NOH-APS study on pregnancy and the 2 groups of women with recurrent abortions or 1 fetal death: women with persistent antiphospholipid antibodies (APS women), and women with negative antiphospholipid antibodies and a negative thrombophilia screening (aPLAb negative women). HBV, hepatitis B virus; HCV, hepatitis C virus.
The women underwent standardized thrombophilia screening 3 to 6 months after the last pregnancy loss; screening included a complete whole blood count and tests for fibrinogen, antithrombin, protein C, protein S, polymorphisms F5 rs6025 and F2 rs1799963, the JAK2 V617F mutation, and aPLAbs. The aPLAbs tested were LA, aCL IgM antibodies (aCL-M) and aCL IgG antibodies (aCL-G), aβ2GP1 IgM antibodies (aβ2GP1-M), and aβ2GP1 IgG antibodies (aβ2GP1-G) (cf. infra).9,10 Women with antithrombin, protein C, or protein S deficiency, and women with an abnormal fibrinogen or the JAK2 V617F mutation were not included. Women with completely negative thrombophilia screening results (N = 3741) and who agreed to participate in the observational study (n = 3604) were provisionally assigned to the “negative” group and were numbered according to the order in which they entered the study. Women with an isolated F5 rs6025 polymorphism or isolated F2 rs1799963 polymorphism (N = 301) and who agreed to participate in the observational study (n = 279) constituted the “constitutional thrombophilia” group. Women who were initially positive for aPLAb, with or without the F5 rs6025 or F2 rs1799963 polymorphisms (N = 607), and who agreed to participate in the observational study (n = 563) were provisionally included in the “aPLAb” group and underwent repeat testing 6 months later. Those women who were persistently positive for aPLAbs at the 6-month test (n = 517) were definitively assigned to the “APS” group. As each APS or constitutional thrombophilia woman entered the study, the next woman on the list of the negative group was definitively assigned to the control group and included in the study. In total, 517 APS women and 796 control women were studied (Table 1).
Clinical characteristics of the women at baseline and the characteristics at follow-up
| . | Control group . | APS group . | P . |
|---|---|---|---|
| N | 796 | 517 | |
| Age, y | 30 (5) [17-44] | 29 (4) [16-41] | .19 |
| >35 y, n (%) | 43 (5.4%) | 15 (2.9%) | .031 |
| BMI, kg/m2 | 25.6 (4.5) [15.3-36.1] | 26.0 (4.6) [15.3-37.0] | .18 |
| >30 kg/m2 | 78 (9.8%) | 60 (11.6%) | .30 |
| <18.5 kg/m2 | 12 (1.5%) | 5 (1%) | .55 |
| Ethnicity | .92 | ||
| White | 753 (94.6%) | 489 (94.6%) | |
| From Europe | 647 (81.3%) | 420 (81.2%) | |
| From North Africa | 106 (13.3%) | 69 (13.4%) | |
| Black Africans | 36 (4.5%) | 22 (4.2%) | |
| Asians | 7 (0.9%) | 6 (1.2%) | |
| PL subtypes | |||
| Embryonic PL <10 WG | 483 (60.7%) | 206 (39.8%) | <.0001 |
| Fetal PL >10 WG | 313 (39.3%) | 311 (60.2%) | |
| Primary PL | 549 (68.9%) | 342 (66.1%) | .29 |
| Secondary PL | 247 (31.1%) | 175 (33.9%) | |
| Inflammatory disease | 7 (0.9%) | 32 (6.2%) | <.0001 |
| Current tobacco users | 83 (10.4%) | 50 (9.7%) | .66 |
| Hypertension | 19 (2.4%) | 17 (3.3%) | .33 |
| Positive history in a first-degree relative | |||
| Venous thromboembolism | 15 (1.9%) | 12 (2.3%) | .59 |
| Atherothrombosis | 96 (12.1%) | 53 (10.3%) | .31 |
| Recurrent abortion | 49 (6.2%) | 14 (2.7%) | .004 |
| Fetal loss | 33 (4.1%) | 6 (1.2%) | .003 |
| Hyperlipidemia | |||
| Hypercholesterolemia | 42 (5.3%) | 31 (6.0%) | .58 |
| Hypertriglyceridemia | 34 (4.3%) | 27 (5.2%) | .42 |
| New pregnancy during the 18-mo observational period | 791 (99.4%) | 513 (99.2%) | .98 |
| Interval between the inclusion and the new pregnancy, days | 156 (29) [95-386] | 143 (33) [91-378] | .32 |
| Length of time between stopping LMWH/LDA treatment and pregnancy related procedures, number of complete hours | NA | 14 (3) [7-29] |
| . | Control group . | APS group . | P . |
|---|---|---|---|
| N | 796 | 517 | |
| Age, y | 30 (5) [17-44] | 29 (4) [16-41] | .19 |
| >35 y, n (%) | 43 (5.4%) | 15 (2.9%) | .031 |
| BMI, kg/m2 | 25.6 (4.5) [15.3-36.1] | 26.0 (4.6) [15.3-37.0] | .18 |
| >30 kg/m2 | 78 (9.8%) | 60 (11.6%) | .30 |
| <18.5 kg/m2 | 12 (1.5%) | 5 (1%) | .55 |
| Ethnicity | .92 | ||
| White | 753 (94.6%) | 489 (94.6%) | |
| From Europe | 647 (81.3%) | 420 (81.2%) | |
| From North Africa | 106 (13.3%) | 69 (13.4%) | |
| Black Africans | 36 (4.5%) | 22 (4.2%) | |
| Asians | 7 (0.9%) | 6 (1.2%) | |
| PL subtypes | |||
| Embryonic PL <10 WG | 483 (60.7%) | 206 (39.8%) | <.0001 |
| Fetal PL >10 WG | 313 (39.3%) | 311 (60.2%) | |
| Primary PL | 549 (68.9%) | 342 (66.1%) | .29 |
| Secondary PL | 247 (31.1%) | 175 (33.9%) | |
| Inflammatory disease | 7 (0.9%) | 32 (6.2%) | <.0001 |
| Current tobacco users | 83 (10.4%) | 50 (9.7%) | .66 |
| Hypertension | 19 (2.4%) | 17 (3.3%) | .33 |
| Positive history in a first-degree relative | |||
| Venous thromboembolism | 15 (1.9%) | 12 (2.3%) | .59 |
| Atherothrombosis | 96 (12.1%) | 53 (10.3%) | .31 |
| Recurrent abortion | 49 (6.2%) | 14 (2.7%) | .004 |
| Fetal loss | 33 (4.1%) | 6 (1.2%) | .003 |
| Hyperlipidemia | |||
| Hypercholesterolemia | 42 (5.3%) | 31 (6.0%) | .58 |
| Hypertriglyceridemia | 34 (4.3%) | 27 (5.2%) | .42 |
| New pregnancy during the 18-mo observational period | 791 (99.4%) | 513 (99.2%) | .98 |
| Interval between the inclusion and the new pregnancy, days | 156 (29) [95-386] | 143 (33) [91-378] | .32 |
| Length of time between stopping LMWH/LDA treatment and pregnancy related procedures, number of complete hours | NA | 14 (3) [7-29] |
The control group comprised women with negative thrombophilia screening results. The APS group comprised women positive for antiphospholipid antibodies. Quantitative data are given as medians (interquartile range) [range] and qualitative data as numbers (percentages).
NA, not applicable; PL, pregnancy loss.
The study was approved by the University Hospital of Nîmes Institutional Review Board and ethics committee and by the local Comité de Protection des Personnes soumises à la Recherche Biomédicale. This clinical investigation was performed in accordance with the Helsinki declaration of 1975 as revised in 1996. All the women gave their informed consent to participate.
Methods
Assays
Plasma samples from 200 healthy women were subjected to the various assays and the results taken to define values for the normal healthy population. The upper threshold of normality was defined as the 99th percentile of the values for these samples.
Screening assays were used to detect LAs (activated partial thromboplastin time: PTT LA, Stago, Asnières, France; dilute Russell's viper venom time: Bioclot LA, Biopool, Umea, Sweden; tissue thromboplastin inhibition test using a 1:500 dilution of thromboplastin: Neoplastin CI Plus, Stago, France). LAs were first identified in samples that were mixed 1:1 with healthy pooled plasma, and the results were then confirmed by neutralization procedures, according to the recommendations of the International Society on Thrombosis and Haemostasis subcommittee. Plasma aCL-G and aCL-M antibody titers were determined by an adaptation10 of the in-house enzyme-linked immunosorbent assay method described by Reber et al.11 Plasma aβ2GP1-G and aβ2GP1-M antibody titers were determined by an enzyme-linked immunosorbent assay developed in-house10 based on β2-glycoprotein I isolated from freshly frozen human citrated plasma.12 All plasmas were assayed for the 4 solid-phase aPLAbs by a method based on calibration curves established using the Sapporo standards HCAL and EY2C9 kindly provided by The Binding Site staff, Saint Egrève, France (normal values: aCL-G <0.85 μg/mL, corresponding to 42.1 GPL units; aCL-M <1.39 μg/mL,corresponding to 42.1 MPL units; aβ2GP1-G <0.89 μg/mL, corresponding to 42.1 GPL units; and aβ2GP1-M <0.99 μg/mL, corresponding to 42.1 MPL units).
Positive results for aPLAbs were categorized as follows according to the recommendations of the International Society on Thrombosis and Haemostasis subcommittee: type I, >1 laboratory criterion present; type IIa, LA present alone; type IIb, aCL-Ab present alone; and type IIc, aβ2GPI-Ab present alone.2 Triple positivity was defined as the association of a positive LA test, a positive aCL-Ab test, and a positive aβ2GP1-Ab test (Table 2).
Prevalence of thrombophilia at baseline
| . | Control group . | APS group . |
|---|---|---|
| Antiphospholipid antibodies aPLAb | ||
| LA | ||
| Positive for LA | 0 | 319 (61.7%) |
| Anticardiolipin IgG | ||
| Positive for aCL-G | 0 | 244 (47.2%) |
| aCL-G titer, µg/mL | 0.39 (0.21) [0.05-0.84] | 0.70 (1.37) [0.05-7.1] |
| Anticardiolipin IgM | ||
| Positive for aCL-M | 0 | 372 (71.9%) |
| aCL-M titer, µg/mL | 0.54 (0.32) [0.08-1.35] | 1.60 (1.55) [0.05-18.1] |
| Anti–β2-glycoprotein I IgG | ||
| Positive for aβ2GP1-G | 0 | 114 (22.1%) |
| aβ2GP1-G titer, µg/mL | 0.23 (0.14) [0.05-0.86] | 0.57 (0.55) [0.03-6.9] |
| Anti–β2-glycoprotein I IgM | ||
| Positive for aβ2GP1-M | 0 | 210 (40.6%) |
| aβ2GP1-M titer, µg/mL | 0.41 (0.24) [0.04-0.98] | 0.72 (0.85) [0.05-19.4] |
| Categories of positive aPLAb* | ||
| I | — | 383 (74.1%) |
| IIa | — | 31 (6%) |
| IIb | — | 103 (19.9%) |
| IIc | — | 0 |
| LA + aCL + aβ2GP1 | — | 149 (28.8%) |
| Constitutional thrombophilias | ||
| F5 rs6025 or F2 rs1799963 | 0 | 17 (3.3%) |
| . | Control group . | APS group . |
|---|---|---|
| Antiphospholipid antibodies aPLAb | ||
| LA | ||
| Positive for LA | 0 | 319 (61.7%) |
| Anticardiolipin IgG | ||
| Positive for aCL-G | 0 | 244 (47.2%) |
| aCL-G titer, µg/mL | 0.39 (0.21) [0.05-0.84] | 0.70 (1.37) [0.05-7.1] |
| Anticardiolipin IgM | ||
| Positive for aCL-M | 0 | 372 (71.9%) |
| aCL-M titer, µg/mL | 0.54 (0.32) [0.08-1.35] | 1.60 (1.55) [0.05-18.1] |
| Anti–β2-glycoprotein I IgG | ||
| Positive for aβ2GP1-G | 0 | 114 (22.1%) |
| aβ2GP1-G titer, µg/mL | 0.23 (0.14) [0.05-0.86] | 0.57 (0.55) [0.03-6.9] |
| Anti–β2-glycoprotein I IgM | ||
| Positive for aβ2GP1-M | 0 | 210 (40.6%) |
| aβ2GP1-M titer, µg/mL | 0.41 (0.24) [0.04-0.98] | 0.72 (0.85) [0.05-19.4] |
| Categories of positive aPLAb* | ||
| I | — | 383 (74.1%) |
| IIa | — | 31 (6%) |
| IIb | — | 103 (19.9%) |
| IIc | — | 0 |
| LA + aCL + aβ2GP1 | — | 149 (28.8%) |
| Constitutional thrombophilias | ||
| F5 rs6025 or F2 rs1799963 | 0 | 17 (3.3%) |
The control group comprised women negative for antiphospholipid antibodies. The APS group comprised women positive for antiphospholipid antibodies. Quantitative data are given as medians (interquartile range) [range], qualitative data as numbers (percentages).
Categories of positive aPLAb: type I, >1 laboratory criterion present; type IIa, LA present alone; type IIb, aCL-Ab present alone; and type IIc, aβ2GPI-Ab present alone.
Follow-up
All patients were informed at the start of the study about the advisability of early blood testing in case of suspicion of a new pregnancy; they were then observed until 18 months after the final recruitments and any new pregnancy noted. The women were instructed, if they had any positive pregnancy test result, to contact their general practitioner for evaluation and, after discussion with a study physician, the possible initiation of prophylaxis.
Patients were clinically reevaluated each year in our outpatient department. Patient loss to follow-up was minimized by directly contacting the general practitioners and the patients themselves. A complete clinical checkup was performed. Any suspicion of developing systemic disease led to further adapted investigations for diagnosis. Symptoms were evaluated and the treatments taken during the year were recorded. There was no systematic aPLAbs assessment if a new pregnancy occurred.
Primary prophylaxis of thrombosis
APS patients were administered a primary prophylaxis treatment of thrombosis when not pregnant; this consisted of 100 mg/day LDA, which although not a standard treatment, is the local routine practice. Compliance was monitored only by self-declaration by the patient-companion couples. Women in the control group did not take any regular prophylaxis for thrombosis.
Antithrombotics during new pregnancies
LMWH (enoxaparin, 40 mg per day [ie, 4000 U/day]) was added to LDA (100 mg/day) for all pregnant APS women from the positive pregnancy test result to delivery; the 2 treatments were administered at the same time. Compliance with the LMWH treatment was monitored only by self-declaration by the patient-companion couples and systematic examination of subcutaneous injection sites at each medical examination. Monitoring and investigation for heparin-induced thrombocytopenia were according to published recommendations.5 Enoxaparin injections and LDA intake were stopped at the onset of uterine contractions indicating imminent delivery.
Patients in the control group did not take any prophylactic drug during pregnancy.
Outcome assessment
Events occurring during the first pregnancy during the observational period were systematically reviewed.
Thrombotic events were objectively confirmed as described elsewhere.9
Women were classified as hypertensive if they were using hypertensive medication or had either a systolic blood pressure of ≥140 mm Hg or a diastolic blood pressure of ≥90 mm Hg, both on 2 readings taken in a supine position 5 minutes apart, and on 2 separate occasions. PE was defined as the diastolic blood pressure increasing to >90 mm Hg, or systolic blood pressure increasing to >140 mm Hg on 2 occasions at least 4 hours apart after 20 WG, accompanied by a significant proteinuria (>0.3 g in a 24-hour urine sample). Women with a preexisting hypertension were scored as having PE if there was a new onset of proteinuria. PE was classified as severe if one or more of the following was observed: severe hypertension (diastolic >110 mm Hg or systolic >160 mm Hg), eclampsia (seizures), pulmonary edema, proteinuria >5 g every 24 hours, renal insufficiency as revealed by abnormal creatininemia levels, abnormally high liver enzyme levels (asparatate aminotransferanse or alanine aminotransferase >70 IU/L) with abdominal pain, or low platelet counts (<100 g/L). HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) was defined according to the criteria established at the University of Tennessee13 : hemolysis on peripheral blood smear (schistocytes) with serum lactate dehydrogenase >600 IU/L, serum aspartate aminotransferase >70 IU/L, and platelet count <100 000/μL. PE with onset before 34 WG was defined as early onset PE.
Biometry-based gestational age was assessed according to charts derived from published data,14,15 and newborn weight percentiles were taken from standard French birth weight charts. Babies with birthweights lower than the corresponding gestational age- and sex-adjusted, customized 10th percentile were scored as small for gestational age (SGA). Severe SGA was defined as weight below the fifth percentile and very severe SGA as below the third percentile.
Placental abruption was defined according to classical clinical prenatal signs and symptoms: vaginal bleeding accompanied by nonreassuring fetal status or uterine hypertonicity, or visualization of abruption by ultrasound scanning, and evidence of retroplacental clots during examination of the delivered placenta. Cases were confirmed by histopathological diagnosis.
Neonatal mortality was defined as death before 28 days of age; death before 8 days of age was recorded as early neonatal mortality and death after 8 days of age as late neonatal mortality.
Major bleeding events were classified according to the definitions of the Control of Anticoagulation Subcommittee of the International Society on Thrombosis and Hemostasis16 : fatal bleeding and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, or pericardial bleeding; intramuscular bleeding with compartment syndrome; and/or bleeding that led to the hemoglobin concentration falling to 20 g/L or lower or that led to transfusion of ≥2 U red blood cells.
All events were adjudicated by a committee of independent experts blind to the thrombophilia screening results.
Statistical analysis
Quantitative data are described by median, interquartile range and range values. Qualitative data are described by values and percentages. Student t test, the Mann-Whitney U test, the Kruskal-Wallis test, the χ2 test, and Fisher’s exact test were used as appropriate for comparisons between baseline characteristics and between risk factors.
Definition of groups and analysis of the associations between biological covariates and pregnancy outcome incidences were based on values obtained at initial inclusion.
All analyses were based on pregnancy outcomes of the first pregnancy after the initial investigations and recruitment and treated as described above.
We compared pregnancy outcomes between groups by estimating a relative risk according to the Mantel-Haenszel method, with a 95% confidence interval, providing a P value from the associated Mantel-Haenszel χ2 test. We then performed a secondary analysis excluding from the APS group the 17 women with constitutional thrombophilia.
Putative predictors of the various outcomes among the clinical predictors (age, body mass index [BMI], family history of thrombosis, family history of pregnancy loss, ethnicity, smoking history, varicose veins, preexisting hypertension, embryonic/fetal pregnancy loss, primary/secondary pregnancy loss, and prior inflammatory disease), the metabolic markers at inclusion (hypercholesterolemia defined as a fasting cholesterol concentration >5.2 mmol/L, and hypertriglyceridemia as a fasting triglyceride concentration >1.7 mmol/L) and the biological predictors at inclusion (positive LA, positive aCL-G, positive aCL-M, positive aβ2GP1-G, positive aβ2GP1-M, triple positivity, and the F5 rs6025 or F2 rs1799963 polymorphisms) were evaluated in women in the APS group, first by univariate analysis then by multivariate analysis. For multivariate models, a stepwise variable selection was performed, starting with all the variables identified by the univariate models as potential predictors at P < .25, with adjustment being finally performed for all variables with P < .25 in the univariate models. The final model included only main effects with P < .10.
All tests were 2 sided and assessed at the 5% significance level. This was an observational study based on our recruitment capacities over a 10-year period, and thus we did not perform sample size calculations prior to the study. The β risk was calculated for each nonsignificant comparison, and all comparisons for which the β value is ≥0.80 are indicated.
Statistical analyses were performed using SAS Windows software (version 9.1).
Results
Baseline patient characteristics
We included 1313 women with unexplained pregnancy loss (Table 1); recurrent unexplained embryonic loss was the most frequent inclusion criterion for the control group and fetal loss the most frequent inclusion criterion for the APS groups. There were more women aged ≥35 years in the control group than the APS group. A history of recurrent abortion or fetal loss was more frequent among the first-degree relatives of women included in the control group. An underlying chronic inflammatory disease was more frequent in the APS group (mainly mild rheumatoid arthritis, Sjogren syndrome, discoid lupus erythematosus, or systemic sclerosis). There was no loss to follow-up during the observation period. The interval between inclusion and the observed new pregnancy did not differ between the 2 groups. Nearly all included women (1304 out of 1313) initiated a new pregnancy, and the fecundity rate was normal.
A total of 46 (8.2%) women of the 563 with a first positive aPLAb screening result were negative in the second test. None of these 46 women had LA, most had an isolated positive aCL-M or aβ2GP1-M result (n = 42: 91.3%), and 4 had an isolated aCL-G result. Women who were not aPLAb positive on repeat testing were not included. Thus, 517 APS women were included, and 42 (8.1%) became pregnant after the systematic once-a-year reevaluation; therefore, after repeat aPLAbs screening, all were persistently positive, with no changes in their aPLAbs pattern.
ACL-M and LA were the most frequently found markers in the APS group (Table 2). Three-quarters of the APS women were positive for >1 marker, 19.9% of the women were positive only for aCL-Ab (8.5% had only aCL-M antibodies). None had only aβ2GP1-Ab. Nearly one-third of the patients exhibited triple positivity,17-19 which was due in part to the high frequency of aCL-M–positive patients. In the APS group, 3.3% of women were positive for the F5 rs6025 or F2 rs1799963 polymorphisms.
Outcomes of new pregnancies in the APS and control groups
Almost all the women had a new pregnancy (Table 3). The rates of preterm birth before 37 or 34 weeks, PE (all PE cases, severe PE, early-onset PE, eclampsia, HELLP syndrome, and PE with an SGA neonate), SGA babies, placenta-mediated complications, and neonatal mortality were all higher for APS women than for control women.
Outcomes of new pregnancies in APS women and in control women
| Group, N . | APS, 517 . | . | Control, 796 . | . | . | . |
|---|---|---|---|---|---|---|
| N | Incidence | N | Incidence | RR, 95%CI | P | |
| New pregnancies | 513 | 0.992 | 791 | 0.994 | ||
| Spontaneous abortions before 10 WG | 91 | 0.177 | 175 | 0.221 | 0.802 (0.639-1.007) | .0572 |
| Ongoing pregnancies at 10 WG | 422 | 0.823 | 621 | 0.785 | 1.048 (0.993-1.106) | .0917 |
| Fetal losses (% of new pregnancies) | 66 | 0.129 | 81 | 0.102 | 1.256 (0.926-1.705) | .143 |
| Fetal losses (% of ongoing pregnancies at 10 WG) | 66 | 0.156 | 81 | 0.130 | 1.199 (0.888-1.620) | .237 |
| Ongoing pregnancy at 20 WG (% of new pregnancies) | 387 | 0.754 | 567 | 0.717 | 1.052 (0.985-1.124) | .129 |
| Live births (% of new pregnancies) | 357 | 0.696 | 540 | 0.683 | 1.019 (0.946-1.098) | .613 |
| Live births (% of ongoing pregnancies at 10 WG) | 357 | 0.846 | 540 | 0.870 | 0.973 (0.925-1.024) | .289 |
| Preterm live births <37 WG (% of live births) | 89 | 0.249 | 74 | 0.137 | 1.819 (1.378-2.402) | <.0001 |
| Preterm live births <34 WG (% of live births) | 44 | 0.123 | 32 | 0.059 | 2.080 (1.346-3.214) | .0010 |
| PE (% of ongoing pregnancies at 20 WG) | 57 | 0.147 | 32 | 0.056 | 2.610 (1.726-4.550) | <.0001 |
| Severe PE | 40 | 0.103 | 21 | 0.037 | 2.791 (1.672-4.657) | .0001 |
| PE <34 WG | 38 | 0.098 | 15 | 0.026 | 3.712 (2.071-6.653) | <.0001 |
| Eclampsia | 12 | 0.031 | 8 | 0.014 | 2.198 (0.907-5.326) | .0813 |
| HELLP syndrome | 16 | 0.045 | 6 | 0.011 | 3.907 (1.543-9.896) | .0041 |
| SGA (% of ongoing pregnancies at 20 WG) | 73 | 0.395 | 80 | 0.141 | 1.337 (1,001-1;786) | .0495 |
| Severe SGA <p5 | 37 | 0.096 | 40 | 0.071 | 1.355 (0.883-2.079) | .164 |
| Very severe SGA <p3 | 26 | 0.067 | 25 | 0.044 | 1.524 (0.894-2.598) | .122 |
| PE + SGA | 39 | 0.100 | 20 | 0.035 | 2.857 (1.693-4.822) | .0001 |
| PA (% of ongoing pregnancies at 20 WG) | 12 | 0.031 | 11 | 0.019 | 1.598 (0.713-3.585) | .255 |
| Placenta-mediated complications: PE and/or SGA and/or PA (% of ongoing pregnancies at 20 WG) | 98 | 0.253 | 99 | 0.175 | 1.450 (1.132-1.858) | .0032 |
| NM (% of live births) | 18 | 0.047 | 8 | 0.014 | 3.529 (1.551-8.031) | .0026 |
| Early NM, day 1-7 | 9 | 0.023 | 5 | 0.009 | 2.723 (0.920-8.058) | .0704 |
| Late NM, day 8-27 | 9 | 0.023 | 3 | 0.005 | 4.538 (1.237-16.65) | .0226 |
| Group, N . | APS, 517 . | . | Control, 796 . | . | . | . |
|---|---|---|---|---|---|---|
| N | Incidence | N | Incidence | RR, 95%CI | P | |
| New pregnancies | 513 | 0.992 | 791 | 0.994 | ||
| Spontaneous abortions before 10 WG | 91 | 0.177 | 175 | 0.221 | 0.802 (0.639-1.007) | .0572 |
| Ongoing pregnancies at 10 WG | 422 | 0.823 | 621 | 0.785 | 1.048 (0.993-1.106) | .0917 |
| Fetal losses (% of new pregnancies) | 66 | 0.129 | 81 | 0.102 | 1.256 (0.926-1.705) | .143 |
| Fetal losses (% of ongoing pregnancies at 10 WG) | 66 | 0.156 | 81 | 0.130 | 1.199 (0.888-1.620) | .237 |
| Ongoing pregnancy at 20 WG (% of new pregnancies) | 387 | 0.754 | 567 | 0.717 | 1.052 (0.985-1.124) | .129 |
| Live births (% of new pregnancies) | 357 | 0.696 | 540 | 0.683 | 1.019 (0.946-1.098) | .613 |
| Live births (% of ongoing pregnancies at 10 WG) | 357 | 0.846 | 540 | 0.870 | 0.973 (0.925-1.024) | .289 |
| Preterm live births <37 WG (% of live births) | 89 | 0.249 | 74 | 0.137 | 1.819 (1.378-2.402) | <.0001 |
| Preterm live births <34 WG (% of live births) | 44 | 0.123 | 32 | 0.059 | 2.080 (1.346-3.214) | .0010 |
| PE (% of ongoing pregnancies at 20 WG) | 57 | 0.147 | 32 | 0.056 | 2.610 (1.726-4.550) | <.0001 |
| Severe PE | 40 | 0.103 | 21 | 0.037 | 2.791 (1.672-4.657) | .0001 |
| PE <34 WG | 38 | 0.098 | 15 | 0.026 | 3.712 (2.071-6.653) | <.0001 |
| Eclampsia | 12 | 0.031 | 8 | 0.014 | 2.198 (0.907-5.326) | .0813 |
| HELLP syndrome | 16 | 0.045 | 6 | 0.011 | 3.907 (1.543-9.896) | .0041 |
| SGA (% of ongoing pregnancies at 20 WG) | 73 | 0.395 | 80 | 0.141 | 1.337 (1,001-1;786) | .0495 |
| Severe SGA <p5 | 37 | 0.096 | 40 | 0.071 | 1.355 (0.883-2.079) | .164 |
| Very severe SGA <p3 | 26 | 0.067 | 25 | 0.044 | 1.524 (0.894-2.598) | .122 |
| PE + SGA | 39 | 0.100 | 20 | 0.035 | 2.857 (1.693-4.822) | .0001 |
| PA (% of ongoing pregnancies at 20 WG) | 12 | 0.031 | 11 | 0.019 | 1.598 (0.713-3.585) | .255 |
| Placenta-mediated complications: PE and/or SGA and/or PA (% of ongoing pregnancies at 20 WG) | 98 | 0.253 | 99 | 0.175 | 1.450 (1.132-1.858) | .0032 |
| NM (% of live births) | 18 | 0.047 | 8 | 0.014 | 3.529 (1.551-8.031) | .0026 |
| Early NM, day 1-7 | 9 | 0.023 | 5 | 0.009 | 2.723 (0.920-8.058) | .0704 |
| Late NM, day 8-27 | 9 | 0.023 | 3 | 0.005 | 4.538 (1.237-16.65) | .0226 |
95% CI, 95% confidence interval; NM, neonatal mortality; PA, placental abruption; RR, relative risk, APS women vs control women.
Outcomes of new pregnancies in women with prior recurrent abortion
The rate of spontaneous abortion recurrence was similar for APS women treated with LMWH and LDA and for control women (Table 4). However, fetal loss rates were higher and consequently live birth rates lower for APS women than for controls. The absolute difference between the relevant clinical incidences (ADI) for the 2 groups shows, in APS women, an excess of fetal loss of 6.4% of the ongoing pregnancies at 10 WG and a deficiency of live birth of 5.9% of the ongoing pregnancies at 10 WG.
Outcomes of new pregnancies in APS women and in control women with prior recurrent abortions
| . | APS, 206 . | Control, 483 . | . | . | ||
|---|---|---|---|---|---|---|
| Group, N . | N . | Incidence . | N . | Incidence . | RR, 95% CI . | P . |
| New pregnancies | 205 | 0.995 | 480 | 0.994 | ||
| Spontaneous abortions before 10 WG | 38 | 0.185 | 92 | 0.192 | 0.968 (0.689-1.362) | .854 |
| Ongoing pregnancies at 10 WG | 167 | 0.815 | 391 | 0.815 | 1,007 (0,932-1,089) | .853 |
| Fetal losses (% of new pregnancies) | 17 | 0.083 | 9 | 0.019 | 4.429 (2.007-9.772) | .0002 |
| Fetal losses (% of ongoing pregnancies at 10 WG) | 17 | 0.102 | 9 | 0.023 | 4.396 (2.000-9.662) | .0002 |
| Ongoing pregnancy at 20 WG (% of new pregnancies) | 161 | 0.785 | 387 | 0.806 | 0.975 (0.896-1.062) | .565 |
| Live births (% of new pregnancies) | 151 | 0.737 | 382 | 0.796 | 0.959 (0.869-1.057) | .398 |
| Live births (% of ongoing pregnancies at 10 WG) | 151 | 0.904 | 382 | 0.977 | 0.920 (0.873-0.970) | .0020 |
| Preterm live births <37 WG (% of live births) | 21 | 0.139 | 35 | 0.092 | 1.518 (0.914-2.521) | .107 |
| Preterm live births <34 WG (% of live births) | 9 | 0.060 | 13 | 0.034 | 1.751 (0.765-4.012) | .185 |
| PE (% of ongoing pregnancies at 20 WG) | 12 | 0.075 | 11 | 0.028 | 2.622 (1.182-5.820) | .0178 |
| Severe PE | 8 | 0.050 | 6 | 0.016 | 3.205 (1.130-9.090) | .0285 |
| PE <34 WG | 10 | 0.062 | 4 | 0.010 | 6.009 (1.913-18.88) | .0021 |
| Eclampsia | 2 | 0.012 | 3 | 0.008 | 1.603 (0.270-9.500) | .604 |
| HELLP syndrome | 3 | 0.018 | 2 | 0.005 | 3.606 (0.608-21.34) | .158 |
| SGA (% of ongoing pregnancies at 20 WG) | 27 | 0.168 | 43 | 0.111 | 1.509 (0.967-2.355) | .070 |
| Severe SGA <p5 | 13 | 0.081 | 21 | 0.054 | 1.488 (0.764-2.899) | .243 |
| Very severe SGA <p3 | 9 | 0.056 | 13 | 0.034 | 1.664 (0.726-3.816) | .229 |
| PE + SGA | 9 | 0.056 | 7 | 0.018 | 3.091 (1.171-8.157) | .0227 |
| PA (% of ongoing pregnancies at 20 WG) | 4 | 0.025 | 5 | 0.013 | 1.923 (0.523-7.069) | .325 |
| Placenta-mediated complications: PE and/or SGA and/or PA (% of ongoing pregnancies at 20 WG) | 33 | 0.205 | 49 | 0.127 | 1.619 (1.084-2.418) | .0186 |
| NM (% of live births) | 6 | 0.040 | 4 | 0.010 | 3.795 (1.086-13.259) | .0367 |
| Early NM, day 1-7 | 4 | 0.027 | 2 | 0.005 | 5.060 (0.937-27.336) | .0596 |
| Late NM, day 8-27 | 2 | 0.013 | 2 | 0.005 | 2.530 (0.360-17.798) | .351 |
| . | APS, 206 . | Control, 483 . | . | . | ||
|---|---|---|---|---|---|---|
| Group, N . | N . | Incidence . | N . | Incidence . | RR, 95% CI . | P . |
| New pregnancies | 205 | 0.995 | 480 | 0.994 | ||
| Spontaneous abortions before 10 WG | 38 | 0.185 | 92 | 0.192 | 0.968 (0.689-1.362) | .854 |
| Ongoing pregnancies at 10 WG | 167 | 0.815 | 391 | 0.815 | 1,007 (0,932-1,089) | .853 |
| Fetal losses (% of new pregnancies) | 17 | 0.083 | 9 | 0.019 | 4.429 (2.007-9.772) | .0002 |
| Fetal losses (% of ongoing pregnancies at 10 WG) | 17 | 0.102 | 9 | 0.023 | 4.396 (2.000-9.662) | .0002 |
| Ongoing pregnancy at 20 WG (% of new pregnancies) | 161 | 0.785 | 387 | 0.806 | 0.975 (0.896-1.062) | .565 |
| Live births (% of new pregnancies) | 151 | 0.737 | 382 | 0.796 | 0.959 (0.869-1.057) | .398 |
| Live births (% of ongoing pregnancies at 10 WG) | 151 | 0.904 | 382 | 0.977 | 0.920 (0.873-0.970) | .0020 |
| Preterm live births <37 WG (% of live births) | 21 | 0.139 | 35 | 0.092 | 1.518 (0.914-2.521) | .107 |
| Preterm live births <34 WG (% of live births) | 9 | 0.060 | 13 | 0.034 | 1.751 (0.765-4.012) | .185 |
| PE (% of ongoing pregnancies at 20 WG) | 12 | 0.075 | 11 | 0.028 | 2.622 (1.182-5.820) | .0178 |
| Severe PE | 8 | 0.050 | 6 | 0.016 | 3.205 (1.130-9.090) | .0285 |
| PE <34 WG | 10 | 0.062 | 4 | 0.010 | 6.009 (1.913-18.88) | .0021 |
| Eclampsia | 2 | 0.012 | 3 | 0.008 | 1.603 (0.270-9.500) | .604 |
| HELLP syndrome | 3 | 0.018 | 2 | 0.005 | 3.606 (0.608-21.34) | .158 |
| SGA (% of ongoing pregnancies at 20 WG) | 27 | 0.168 | 43 | 0.111 | 1.509 (0.967-2.355) | .070 |
| Severe SGA <p5 | 13 | 0.081 | 21 | 0.054 | 1.488 (0.764-2.899) | .243 |
| Very severe SGA <p3 | 9 | 0.056 | 13 | 0.034 | 1.664 (0.726-3.816) | .229 |
| PE + SGA | 9 | 0.056 | 7 | 0.018 | 3.091 (1.171-8.157) | .0227 |
| PA (% of ongoing pregnancies at 20 WG) | 4 | 0.025 | 5 | 0.013 | 1.923 (0.523-7.069) | .325 |
| Placenta-mediated complications: PE and/or SGA and/or PA (% of ongoing pregnancies at 20 WG) | 33 | 0.205 | 49 | 0.127 | 1.619 (1.084-2.418) | .0186 |
| NM (% of live births) | 6 | 0.040 | 4 | 0.010 | 3.795 (1.086-13.259) | .0367 |
| Early NM, day 1-7 | 4 | 0.027 | 2 | 0.005 | 5.060 (0.937-27.336) | .0596 |
| Late NM, day 8-27 | 2 | 0.013 | 2 | 0.005 | 2.530 (0.360-17.798) | .351 |
95% CI, 95% confidence interval; NM, neonatal mortality; PA, placental abruption; RR, relative risk, APS women vs control women.
The APS group also had higher rates of PE (excess of total PE cases, ADI calculated for ongoing pregnancies at 20 WG: +4.7%; of severe PE: +3.4%; of early-onset PE: +5.2%; of PE with birth of a SGA baby: +3.8%), placenta-mediated complications as a whole (+7.8%), and neonatal mortality (+3%) (Table 3).
The β value for all the nonsignificant comparisons was <0.80. The exclusion of the 7 APS women bearing a F5 rs6025 or F2 rs179963 polymorphism did not significantly affect these results.
Outcomes of new pregnancies in women with prior fetal loss
The rates of spontaneous abortion (ADI: −9.5%) and of fetal loss recurrence (ADI on ongoing pregnancies at 10 WG: −12.1%) were lower in the treated APS subgroup than in the control group, such that there was a higher rate of live births (+16.1%) (Table 5). However, this subgroup was also at a higher risk of developing PE during the new pregnancy, both as a whole (ADI: +8.2%) and as an early-onset PE (+4.5%).
Outcomes of new pregnancies in APS women and in control women with prior fetal loss
| . | APS, 311 . | Control, 313 . | . | . | ||
|---|---|---|---|---|---|---|
| Group, N . | N . | Incidence . | N . | Incidence . | RR, 95% CI . | P . |
| New pregnancies | 308 | 0.990 | 311 | 0.994 | ||
| Spontaneous abortions before 10 WG | 53 | 0.172 | 83 | 0.267 | 0.679 (0.503-0.917) | .0116 |
| Ongoing pregnancies at 10 WG | 255 | 0.828 | 230 | 0.740 | 1.116 (1.025-1.214) | .0110 |
| Fetal losses (% of new pregnancies) | 49 | 0.159 | 72 | 0.232 | 0.685 (0.494-0.950) | .0234 |
| Fetal losses (% of ongoing pregnancies at 10 WG) | 49 | 0.192 | 72 | 0.313 | 0.614 (0.447-0.842) | .0025 |
| Ongoing pregnancy at 20 WG (% of new pregnancies) | 226 | 0.734 | 180 | 0.579 | 1.264 (1.124-1.421) | .0001 |
| Live births (% of new pregnancies) | 206 | 0.669 | 158 | 0.508 | 1.312 (1.146-1.503) | .0001 |
| Live births (% of ongoing pregnancies at 10 WG) | 206 | 0.808 | 158 | 0.687 | 1.176 (1.058-1.307) | .0027 |
| Preterm live births <37 WG (% of live births) | 68 | 0.330 | 39 | 0.247 | 1.337 (0.957-1.869) | .0887 |
| Preterm live births <34 WG (% of live births) | 35 | 0.170 | 19 | 0.120 | 1.413 (0.841-2.373) | .192 |
| PE (% of ongoing pregnancies at 20 WG) | 45 | 0.199 | 21 | 0.117 | 1.707 (1.057-2.757) | .0289 |
| Severe PE | 32 | 0.142 | 15 | 0.083 | 1.614 (0.904-2.884) | .106 |
| PE <34 WG | 28 | 0.124 | 11 | 0.061 | 2.028 (1.038-3.960) | .0385 |
| Eclampsia | 10 | 0.044 | 5 | 0.028 | 1.593 (0.554-4.577) | .387 |
| HELLP syndrome | 13 | 0.058 | 4 | 0.022 | 2.589 (0.859-7.803) | .0911 |
| SGA (% of ongoing pregnancies at 20 WG) | 46 | 0.204 | 37 | 0.206 | 0.990 (0.673-1.457) | .960 |
| Severe SGA <p5 | 24 | 0.106 | 19 | 0.106 | 1.006 (0.569-1.777) | .983 |
| Very severe SGA <p3 | 17 | 0.075 | 12 | 0.067 | 1.128 (0.553-2.301) | .740 |
| PE + SGA | 30 | 0.133 | 13 | 0.072 | 1.838 (0.988-3.419) | .0546 |
| PA (% of ongoing pregnancies at 20 WG) | 8 | 0.035 | 6 | 0.033 | 1.062 (0.375-3.005) | .910 |
| Placenta-mediated complications: PE and/or SGA and/or PA (% of ongoing pregnancies at 20 WG) | 65 | 0.288 | 50 | 0.278 | 1.035 (0.758-1.415) | .827 |
| NM (% of live births) | 12 | 0.058 | 4 | 0.025 | 2.301 (0.756-6.999) | .142 |
| Early NM, day 1-7 | 5 | 0.024 | 3 | 0.019 | 1.278 (0.310-5.269) | .734 |
| Late NM, day 8-27 | 7 | 0.034 | 1 | 0.006 | 5.369 (0.667-43.19) | .114 |
| . | APS, 311 . | Control, 313 . | . | . | ||
|---|---|---|---|---|---|---|
| Group, N . | N . | Incidence . | N . | Incidence . | RR, 95% CI . | P . |
| New pregnancies | 308 | 0.990 | 311 | 0.994 | ||
| Spontaneous abortions before 10 WG | 53 | 0.172 | 83 | 0.267 | 0.679 (0.503-0.917) | .0116 |
| Ongoing pregnancies at 10 WG | 255 | 0.828 | 230 | 0.740 | 1.116 (1.025-1.214) | .0110 |
| Fetal losses (% of new pregnancies) | 49 | 0.159 | 72 | 0.232 | 0.685 (0.494-0.950) | .0234 |
| Fetal losses (% of ongoing pregnancies at 10 WG) | 49 | 0.192 | 72 | 0.313 | 0.614 (0.447-0.842) | .0025 |
| Ongoing pregnancy at 20 WG (% of new pregnancies) | 226 | 0.734 | 180 | 0.579 | 1.264 (1.124-1.421) | .0001 |
| Live births (% of new pregnancies) | 206 | 0.669 | 158 | 0.508 | 1.312 (1.146-1.503) | .0001 |
| Live births (% of ongoing pregnancies at 10 WG) | 206 | 0.808 | 158 | 0.687 | 1.176 (1.058-1.307) | .0027 |
| Preterm live births <37 WG (% of live births) | 68 | 0.330 | 39 | 0.247 | 1.337 (0.957-1.869) | .0887 |
| Preterm live births <34 WG (% of live births) | 35 | 0.170 | 19 | 0.120 | 1.413 (0.841-2.373) | .192 |
| PE (% of ongoing pregnancies at 20 WG) | 45 | 0.199 | 21 | 0.117 | 1.707 (1.057-2.757) | .0289 |
| Severe PE | 32 | 0.142 | 15 | 0.083 | 1.614 (0.904-2.884) | .106 |
| PE <34 WG | 28 | 0.124 | 11 | 0.061 | 2.028 (1.038-3.960) | .0385 |
| Eclampsia | 10 | 0.044 | 5 | 0.028 | 1.593 (0.554-4.577) | .387 |
| HELLP syndrome | 13 | 0.058 | 4 | 0.022 | 2.589 (0.859-7.803) | .0911 |
| SGA (% of ongoing pregnancies at 20 WG) | 46 | 0.204 | 37 | 0.206 | 0.990 (0.673-1.457) | .960 |
| Severe SGA <p5 | 24 | 0.106 | 19 | 0.106 | 1.006 (0.569-1.777) | .983 |
| Very severe SGA <p3 | 17 | 0.075 | 12 | 0.067 | 1.128 (0.553-2.301) | .740 |
| PE + SGA | 30 | 0.133 | 13 | 0.072 | 1.838 (0.988-3.419) | .0546 |
| PA (% of ongoing pregnancies at 20 WG) | 8 | 0.035 | 6 | 0.033 | 1.062 (0.375-3.005) | .910 |
| Placenta-mediated complications: PE and/or SGA and/or PA (% of ongoing pregnancies at 20 WG) | 65 | 0.288 | 50 | 0.278 | 1.035 (0.758-1.415) | .827 |
| NM (% of live births) | 12 | 0.058 | 4 | 0.025 | 2.301 (0.756-6.999) | .142 |
| Early NM, day 1-7 | 5 | 0.024 | 3 | 0.019 | 1.278 (0.310-5.269) | .734 |
| Late NM, day 8-27 | 7 | 0.034 | 1 | 0.006 | 5.369 (0.667-43.19) | .114 |
95% CI, 95% confidence interval; NM, neonatal mortality; PA, placental abruption; RR, relative risk, APS women vs control women.
The β value for all comparisons not showing a significant difference was <0.80. The exclusion of the 10 APS women bearing a F5 rs6025 or F2 rs179963 polymorphism did not significantly affect the findings.
Safety outcomes for pregnant women taking antithrombotic treatment
Antithrombotics were given to 513 pregnant women. No treatment had to be stopped for safety reasons; we observed no major bleeding events.16 There were no cases of heparin-induced thrombocytopenia, symptomatic osteoporosis, or thrombosis. Patients who received epidural or spinal analgesia or anesthesia developed no complications, with no case of epidural hematoma or of hemorrhagic or neurologic complications.
Six patients showed allergic skin reactions after enoxaparin injections (1.2%) and these symptoms disappeared after switching for another LMWH. Easy bruising, at least at injection sites, was frequent (n = 387: 75.4%). Primary postpartum hemorrhage occurred in 36 of the 513 treated patients (7.0%) and in 51 of the 796 control women (6.4%, P = .72); there was severe primary postpartum hemorrhage (estimated blood loss >1000 mL) in 6 APS women (1.2%) and in 10 controls (1.3%, P = .85).
Predictors in the APS group (Table 6)
In APS patients, prior fetal loss was identified by the final multivariate models as a risk factor for the following adverse late pregnancy outcomes: fetal loss recurrence, PE, premature birth before 37 WG or before 34 WG, and placenta-mediated complications. Varicose veins was unexpectedly identified as an independent protective factor against the occurrence of placenta-mediated complications. High BMI values increased the PE risk.
Predictors of pregnancy outcomes in APS women
| Outcome, models . | Variables . | OR (95%CI) . | P . |
|---|---|---|---|
| Abortion (embryonic loss) | |||
| Univariate | aβ2GP1-M | 0.52 (0.32-0.86) | .013 |
| Hypertriglyceridemia | 0.37 (0.08-1.59) | .18 | |
| Varicose veins | 0.69 (0.39-1.24) | .21 | |
| Multivariate* | aβ2GP1-M | 0.55 (0.30-0.89) | .021 |
| Fetal loss | |||
| Univariate | Prior fetal loss | 2.10 (1.16-3.79) | .015 |
| Familial atherothrombosis | 1.75 (0.82-3.75) | .15 | |
| Familial venous thromboembolism | 2.78 (0.68-11.4) | .17 | |
| Primary loss | 0.72 (0.42-1.23) | .22 | |
| Multivariate* | Prior fetal loss | 2.08 (1.13-3.83) | .019 |
| PE | |||
| Univariate | Prior fetal loss | 2.73 (1.40-5.34) | .003 |
| BMI >30 kg/m2 | 2.22 (1.08-4.54) | .030 | |
| Varicose veins | 0.48 (0.22-1.05) | .07 | |
| Tobacco smoking | 1.94 (0.87-4.31) | .10 | |
| aCL-M | 1.70 (0.85-3.41) | .13 | |
| LA | 1.55 (0.85-2.83) | .16 | |
| Multivariate* | Prior fetal loss | 2.68 (1.35-5.32) | .005 |
| BMI >30 kg/m2 | 2.23 (1.05-4.73) | .037 | |
| Varicose veins | 0.50 (0.20-1.10) | .09 | |
| Early-onset PE | |||
| Univariate | BMI >30 kg/m2 | 2.10 (0.91-4.88) | .08 |
| Prior fetal loss | 1.91 (0.90-4.05) | .09 | |
| Varicose veins | 0.46 (0.17-1.21) | .11 | |
| aCL-M | 1.77 (0.76-4.14) | .19 | |
| Tobacco smoking | 1.86 (0.73-4.75) | .20 | |
| Multivariate* | Prior fetal loss | 1.99 (0.93-4.26) | .08 |
| Varicose veins | 0.42 (0.16-1.13) | .09 | |
| Premature birth before 37 WG | |||
| Univariate | Prior fetal loss | 3.03 (1.76-5.22) | <.0001 |
| aCL-M | 1.63 (0.91-2.91) | .10 | |
| Hypertriglyceridemia | 2.10 (0.83-5.31) | .12 | |
| aCL-G | 1.41 (0.87-2.29) | .16 | |
| Familial atherothrombosis | 0.51 (0.19-1.36) | .18 | |
| Age >35 y | 0.26 (0.03-2.08) | .21 | |
| Multivariate* | Prior fetal loss | 2.95 (1.69-5.15) | .0001 |
| aCL-G | 1.58 (0.95-2.64) | .08 | |
| Hypertriglyceridemia | 2.39 (0.88-6.50) | .09 | |
| Premature birth before 34 WG | |||
| Univariate | Prior fetal loss | 3.21 (1.49-6.90) | .003 |
| Varicose veins | 0.56 (0.24-1.30) | .18 | |
| Tobacco smoking | 1.85 (0.76-4.51) | .18 | |
| aβ2GP1-G | 1.59 (0.79-3.22) | .19 | |
| Hypertension | 2.21 (0.58-8.36) | .24 | |
| Multivariate* | Prior fetal loss | 3.24 (1.47-7.13) | .004 |
| Tobacco smoking | 2.18 (0.86-5.52) | .10 | |
| Varicose veins | 0.48 (0.20-1.16) | .10 | |
| SGA neonate | |||
| Univariate | aCL-M | 2.02 (1.06-3.85) | .033 |
| Varicose veins | 0.55 (0.28-1.08) | .08 | |
| LA | 1.60 (0.92-2.76) | .10 | |
| aβ2GP1-M | 1.43 (0.86-2.39) | .17 | |
| Primary loss | 1.47 (0.83-2.58) | .19 | |
| Multivariate* | LA | 1.67 (0.97-2.89) | .07 |
| Any placenta-mediated complications | |||
| Univariate | Prior fetal loss | 1.77 (1.07-2.90) | .025 |
| aCL-M | 1.82 (1.03-3.23) | .040 | |
| Varicose veins | 0.53 (0.29-0.98) | .041 | |
| BMI >30 kg/m2 | 1.56 (0.79-3.08) | .21 | |
| LA | 1.33 (0.81-2.18) | .25 | |
| Multivariate* | aCL-M | 2.02 (1.09.3.73) | .025 |
| Varicose veins | 0.50 (0.27-0.93) | .028 | |
| Prior fetal loss | 1.67 (1.01-2.80) | .049 | |
| LA | 1.54 (0.91-2.61) | .11 |
| Outcome, models . | Variables . | OR (95%CI) . | P . |
|---|---|---|---|
| Abortion (embryonic loss) | |||
| Univariate | aβ2GP1-M | 0.52 (0.32-0.86) | .013 |
| Hypertriglyceridemia | 0.37 (0.08-1.59) | .18 | |
| Varicose veins | 0.69 (0.39-1.24) | .21 | |
| Multivariate* | aβ2GP1-M | 0.55 (0.30-0.89) | .021 |
| Fetal loss | |||
| Univariate | Prior fetal loss | 2.10 (1.16-3.79) | .015 |
| Familial atherothrombosis | 1.75 (0.82-3.75) | .15 | |
| Familial venous thromboembolism | 2.78 (0.68-11.4) | .17 | |
| Primary loss | 0.72 (0.42-1.23) | .22 | |
| Multivariate* | Prior fetal loss | 2.08 (1.13-3.83) | .019 |
| PE | |||
| Univariate | Prior fetal loss | 2.73 (1.40-5.34) | .003 |
| BMI >30 kg/m2 | 2.22 (1.08-4.54) | .030 | |
| Varicose veins | 0.48 (0.22-1.05) | .07 | |
| Tobacco smoking | 1.94 (0.87-4.31) | .10 | |
| aCL-M | 1.70 (0.85-3.41) | .13 | |
| LA | 1.55 (0.85-2.83) | .16 | |
| Multivariate* | Prior fetal loss | 2.68 (1.35-5.32) | .005 |
| BMI >30 kg/m2 | 2.23 (1.05-4.73) | .037 | |
| Varicose veins | 0.50 (0.20-1.10) | .09 | |
| Early-onset PE | |||
| Univariate | BMI >30 kg/m2 | 2.10 (0.91-4.88) | .08 |
| Prior fetal loss | 1.91 (0.90-4.05) | .09 | |
| Varicose veins | 0.46 (0.17-1.21) | .11 | |
| aCL-M | 1.77 (0.76-4.14) | .19 | |
| Tobacco smoking | 1.86 (0.73-4.75) | .20 | |
| Multivariate* | Prior fetal loss | 1.99 (0.93-4.26) | .08 |
| Varicose veins | 0.42 (0.16-1.13) | .09 | |
| Premature birth before 37 WG | |||
| Univariate | Prior fetal loss | 3.03 (1.76-5.22) | <.0001 |
| aCL-M | 1.63 (0.91-2.91) | .10 | |
| Hypertriglyceridemia | 2.10 (0.83-5.31) | .12 | |
| aCL-G | 1.41 (0.87-2.29) | .16 | |
| Familial atherothrombosis | 0.51 (0.19-1.36) | .18 | |
| Age >35 y | 0.26 (0.03-2.08) | .21 | |
| Multivariate* | Prior fetal loss | 2.95 (1.69-5.15) | .0001 |
| aCL-G | 1.58 (0.95-2.64) | .08 | |
| Hypertriglyceridemia | 2.39 (0.88-6.50) | .09 | |
| Premature birth before 34 WG | |||
| Univariate | Prior fetal loss | 3.21 (1.49-6.90) | .003 |
| Varicose veins | 0.56 (0.24-1.30) | .18 | |
| Tobacco smoking | 1.85 (0.76-4.51) | .18 | |
| aβ2GP1-G | 1.59 (0.79-3.22) | .19 | |
| Hypertension | 2.21 (0.58-8.36) | .24 | |
| Multivariate* | Prior fetal loss | 3.24 (1.47-7.13) | .004 |
| Tobacco smoking | 2.18 (0.86-5.52) | .10 | |
| Varicose veins | 0.48 (0.20-1.16) | .10 | |
| SGA neonate | |||
| Univariate | aCL-M | 2.02 (1.06-3.85) | .033 |
| Varicose veins | 0.55 (0.28-1.08) | .08 | |
| LA | 1.60 (0.92-2.76) | .10 | |
| aβ2GP1-M | 1.43 (0.86-2.39) | .17 | |
| Primary loss | 1.47 (0.83-2.58) | .19 | |
| Multivariate* | LA | 1.67 (0.97-2.89) | .07 |
| Any placenta-mediated complications | |||
| Univariate | Prior fetal loss | 1.77 (1.07-2.90) | .025 |
| aCL-M | 1.82 (1.03-3.23) | .040 | |
| Varicose veins | 0.53 (0.29-0.98) | .041 | |
| BMI >30 kg/m2 | 1.56 (0.79-3.08) | .21 | |
| LA | 1.33 (0.81-2.18) | .25 | |
| Multivariate* | aCL-M | 2.02 (1.09.3.73) | .025 |
| Varicose veins | 0.50 (0.27-0.93) | .028 | |
| Prior fetal loss | 1.67 (1.01-2.80) | .049 | |
| LA | 1.54 (0.91-2.61) | .11 |
Results are restricted to putative predictors with P value < .25 in the univariate analysis and to those with a P value < .10 in the multivariate analysis, adjusted for predictors with P < .25 in the univariate analysis.
aβ2GP1-G, positive for anti-β2-glycoprotein I IgG; aβ2GP1-M, positive anti-β2-glycoprotein I IgM; aCL-G, positive for anticardiolipin IgG; aCL-M, positive for anticardiolipin IgM; LA, positive for lupus anticoagulant.
Adjusted for the individual variables identified as significant by univariate analysis.
Being positive for aCL-M was an independent risk predictor for placenta-mediated complications. Positive aβ2GP1-M results were also paradoxically associated with lower abortion rates. Positive results for LA, aβ2GP1-G, or aCL-G in treated APS women did not indicate significant risks and the triple positivity pattern had no predictive value (Table 7).
Antiphospholipid antibodies and prediction of pregnancy outcomes in treated APS women
| Outcome, models . | aPLAbs . | aOR (95%CI) . | P . |
|---|---|---|---|
| Abortion (embryonic loss) | |||
| LA | 0.98 (0.62-1.57) | .95 | |
| aCL-G | 1.08 (0.64-1.84) | .75 | |
| aβ2GP1-G | 0.72 (0.35-1.43) | .33 | |
| Triple positivity | 0.83 (0.49-1.39) | .48 | |
| Fetal loss | |||
| LA | 1.28 (0.73-2.24) | .38 | |
| aCL-G | 1.16 (0.74-1.82) | .53 | |
| aβ2GP1-G | 1.29 (0.76-2.18) | .34 | |
| Triple positivity | 1.13 (0.64-1.99) | .67 | |
| PE | |||
| LA | 1.67 (0.87-3.18) | .14 | |
| aCL-G | 0.81 (0.46-1.42) | .46 | |
| aβ2GP1-G | 1.14 (0.58-2.22) | .71 | |
| Triple positivity | 1.61 (0.90-2.87) | .14 | |
| Premature birth | |||
| LA | 0.87 (0.53-1.42) | .57 | |
| aCL-G | 1.41 (0.87-2.29) | .16 | |
| aβ2GP1-G | 1.24 (0.70-2.18) | .46 | |
| Triple positivity | 1.64 (0.92-2.93) | .13 | |
| SGA neonate | |||
| LA | 1.67 (0.97-2.89) | .07 | |
| aCL-G | 0.97 (0.58-1.63) | .92 | |
| aβ2GP1-G | 0.98 (0.54-1.84) | .97 | |
| Triple positivity | 1.49 (0.88-2.49) | .15 | |
| Any placenta-mediated complications | |||
| LA | 1.54 (0.91-2.61) | .11 | |
| aCL-G | 0.93 (0.58-1.48) | .75 | |
| aβ2GP1-G | 1.07 (0.61-1.87) | .82 | |
| Triple positivity | 1.57 (0.86-2.52) | .16 |
| Outcome, models . | aPLAbs . | aOR (95%CI) . | P . |
|---|---|---|---|
| Abortion (embryonic loss) | |||
| LA | 0.98 (0.62-1.57) | .95 | |
| aCL-G | 1.08 (0.64-1.84) | .75 | |
| aβ2GP1-G | 0.72 (0.35-1.43) | .33 | |
| Triple positivity | 0.83 (0.49-1.39) | .48 | |
| Fetal loss | |||
| LA | 1.28 (0.73-2.24) | .38 | |
| aCL-G | 1.16 (0.74-1.82) | .53 | |
| aβ2GP1-G | 1.29 (0.76-2.18) | .34 | |
| Triple positivity | 1.13 (0.64-1.99) | .67 | |
| PE | |||
| LA | 1.67 (0.87-3.18) | .14 | |
| aCL-G | 0.81 (0.46-1.42) | .46 | |
| aβ2GP1-G | 1.14 (0.58-2.22) | .71 | |
| Triple positivity | 1.61 (0.90-2.87) | .14 | |
| Premature birth | |||
| LA | 0.87 (0.53-1.42) | .57 | |
| aCL-G | 1.41 (0.87-2.29) | .16 | |
| aβ2GP1-G | 1.24 (0.70-2.18) | .46 | |
| Triple positivity | 1.64 (0.92-2.93) | .13 | |
| SGA neonate | |||
| LA | 1.67 (0.97-2.89) | .07 | |
| aCL-G | 0.97 (0.58-1.63) | .92 | |
| aβ2GP1-G | 0.98 (0.54-1.84) | .97 | |
| Triple positivity | 1.49 (0.88-2.49) | .15 | |
| Any placenta-mediated complications | |||
| LA | 1.54 (0.91-2.61) | .11 | |
| aCL-G | 0.93 (0.58-1.48) | .75 | |
| aβ2GP1-G | 1.07 (0.61-1.87) | .82 | |
| Triple positivity | 1.57 (0.86-2.52) | .16 |
All results are adjusted for predictors with P < .25 in the univariate analysis (adjusted odds ration [aOR]) (see Table 6).
aβ2GP1-G, positive for anti-β2-glycoprotein I IgG; aCL-G, positive for anticardiolipin IgG; aPLAbs, antiphospholipid antibodies; LA, positive for lupus anticoagulant; triple positivity, association of a positive LA test, a positive aCL-Ab test, and a positive aβ2GP1-Ab test.
Discussion
Our findings in this observational study of patients with pregnancy loss show that the prognosis of new pregnancies in women treated for APS depends largely on the initial presentation. APS patients with a prior fetal loss were at higher risk of late pregnancy complications than APS patients with recurrent spontaneous abortions. This relationship with the initial clinical subtype may have distorted assessments of antithrombotic treatment efficacy in APS patients.
Antithrombotic treatment of APS women failed to reduce the incidence of placenta-mediated adverse pregnancy outcomes to normal control values. This suggests that available treatments are of limited efficacy. However, we do not conclude that antithrombotics had no effects on pregnancy outcomes, but only that they were insufficiently effective. We therefore recommend the continued use of antithrombotic therapy in these patients with obstetric APS with no prior history of thrombosis, at least until more effective treatments become available. APS patients with pregnancy loss probably share nonantithrombotic responsive, but currently unknown risk factors for fetal loss. Abortions were less frequent among APS women with prior fetal loss than among controls, but this was not the case for APS women with prior abortions: some aPLAbs may have a limited precipitating effect on spontaneous abortions, as previously suggested.8,20 In addition to the possibility that the response to antithrombotics is only partial, the treatment administered may have been inadequate: higher doses of LMWH and/or aspirin might be more effective, and indeed, the optimal doses have not been established.
We found live birth rates of 80% for control women with prior recurrent abortions and 50% for control women with prior fetal loss, values consistent with available reported results. A study of 95 women with a second trimester pregnancy loss described a rate of stillbirths of 11% for the following pregnancy.21 Another study of 230 women with fetal loss defined as in our study described 839 subsequent pregnancies: 44% resulted in spontaneous abortion and 25% in fetal loss; only 24% led to live births.22 Patients with a prior second-trimester loss were 10.8 times more likely to have a recurrence (27%) or a spontaneous preterm birth in their next pregnancy.23 Fetal loss thus defines a population of women with a poor prognosis for the subsequent pregnancy. We recommend systematic dichotomization of pregnancy loss according to the 10 WG threshold, as in the clinical criteria of APS.4
Our APS women developed more late pregnancy complications (mainly PE) than controls. Adverse pregnancy outcomes, including PE and SGA, were observed for 19.4% of 144 treated APS patients, but only the minority of these women had prior pregnancy loss, such that this factor could not predict adverse pregnancy outcomes.8 Other recent retrospective work on pregnant APS patients, including also thrombotic APS, did not focus on placenta-mediated complications.17,18 A study considering high antibody titers found an association with some adverse pregnancy outcomes, but included thrombotic and obstetric patients, without analyzing the effect of subcategorization of pregnancy losses.24
The presence of aCL-M was a risk factor for placenta-mediated complications and LA for SGA neonates. There is substantial debate about the relevance of solid-phase aPLAb of the IgM isotype, as assessed from data relative to thrombotic APS. Our findings for aCL-M are concordant with the suggestions that IgM isotype should not be dropped for the diagnosis of obstetric APS.10,25
Prior studies report an association between LA positivity and pregnancy loss despite antithrombotic treatments.17,18 These studies included APS patients with a history of thrombosis, a history of both thrombosis and pregnancy morbidity being a risk factor for pregnancy failure.18 Past thrombosis may thus act as a confounding factor on the risk of pregnancy loss in LA-positive patients. LAs are a heterogeneous group of antibodies, and their activity can be β2GP1 dependent or independent; β2GP1-dependent LA correlates with thrombosis.26 LAs in pure obstetric APS may thus be less thrombogenic, and antithrombotics may inhibit their abortive properties. Previous studies also showed that triple positivity was associated with worse prognosis.18,19 However, these studies were not restricted to pure obstetric APS18 or only included thrombotic APS with no pregnancy-related morbidity.19 Because we suspect that LAs in cases of pure obstetric APS are not strongly thrombogenic, their β2GP1 dependence being questionable, the only limited impact of triple positivities is not surprising.
Varicose veins in APS women were identified as a protective factor against the occurrence of any placenta-mediated complications. Primary varicose veins have been associated with an altered transcription of vascular endothelial growth factor and its receptors, including soluble flt-127 ; PE is strongly related to the antiangiogenic state.28
Our study has various limitations. It was not a randomized controlled trial and the patients included were heterogeneous. There was no rigorous assessment of compliance with LMWH treatment. We were unable to control for cointerventions. Outcome assessment was not performed blind to the APS or control status of the women. Genome-wide scanning of affected sibling pairs with miscarriages suggests genetic linkage,29 and mutations in complement regulatory proteins in APS patients predispose to PE30 ; this genetic background may act as a confounder. We only included pure obstetric APS with pregnancy loss, fulfilling 2 of the 3 pregnancy morbidity criteria of the APS classification.2 This study does not apply to obstetric APS women with a premature birth due to placental insufficiency. This was not an academic multicenter study, although several of the primary and secondary centers in our administrative area participated in the initial screening of the patients. Some patients may have been treated independently from our network. Embryonic karyotyping is not obligatory according to the APS criteria,2 and indeed, there was no karyotype analysis of most of the lost embryos. The study does not allow analysis of the consequences of aPLAbs on preclinical, very early losses: it was not the first pregnancy during follow-up that was studied in every case. We did not check aPLAbs when women obtained a new pregnancy. However, the time lag before a new pregnancy was not very long, and no consistent variations were observed among women reevaluated before becoming pregnant.
Our study also has several strengths. It received substantial support from the Nîmes Obstetricians and Haematologists (NOHA) administrative region-hospital medical network,31 which was able to recruit a substantial number of patients.9 New pregnancies were observed after inclusion, and there was no loss to follow-up, in part because of the relatively short lengths of time between screening/inclusion and the new pregnancies. Pregnancy outcomes were objectively diagnosed using guidelines and international criteria/definitions. We therefore believe that very few pregnancy-related events were not recorded in the database.
In summary, among obstetric APS patients with prior pregnancy loss who are treated during a new pregnancy, the subgroup of women with prior fetal loss were at increased risk of placenta-mediated late pregnancy complications, mainly PE. Obstetric APS patients developed more late pregnancy complications than nontreated controls. New therapeutic developments are urgently needed and should be evaluated according to prior pregnancy loss types.
There is an Inside Blood commentary on this article in this issue.
Presented in part as a poster communication at the XIV Congress of the International Society on Thrombosis and Haemostasis, Amsterdam, The Netherlands, June 29 to July 4, 2013.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the study participants (the patients who agreed to join us in this long-term adventure); H. Bres for technical assistance; the NOHA network of gynecologists, obstetricians, and general practitioners who actively contributed to the study; the adjudication committee (S. Ripart-Neveu and E. Arnaud); M. L. Tailland, D. Dupaigne, C. Ferrer, V. le Touzey, M. Hoffet, A. Cornille, B. Fatton, E. Mousty, F. Masia, L. Boileau, F. Grojean, R. de Tayrac, and A. Sotto for their efficient help in the management of the patients; the research staff of the “Direction de la Recherche Clinique et de l’Innovation” of the University Hospital of Nîmes (S. Clément, C. Meyzonnier, N. Best, S. Granier, B. Lafont, H. Obert, H. Léal, O. Albert, C. Suehs, P. Rataboul, and M. P. Francheschi); the staff of the “Departement de Biostatistiquee, Epidémiologie, Santé Publique et Information Médicale BESPIM” of the University Hospital of Nîmes P. Landais, J. P. Daurès, and S. Leroy); and Prof Philippe de Moerloose, Geneva, Switzerland, for thoughtful discussions and reviewing of the manuscript.
Authorship
Contribution: J.-C.G., J.-P.B., and P.M. designed the research and wrote the paper; G.L.-L. and J.-C.G. performed the statistical analyses; S.B., T.M., J.-C.G., J.-P.B., and P.M. performed the research and contributed to the analysis of the data; and S.B., E.C.-N., E.M., and G.L.-L. contributed analytical tools and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Christophe Gris, Laboratory of Hematology, University Hospital Caremeau, Place du Pr. Robert Debré, 30029 Nîmes Cedex 9, France; e-mail: jean.christophe.gris@chu-nimes.fr.