Key Points
Modified ActRIIB ligand trap promotes terminal erythroid differentiation and mitigates ineffective erythropoiesis in murine β-thalassemia.
This agent reduces anemia, α-globin aggregates, hemolysis, and disease complications such as iron overload, splenomegaly, and bone defects.
Abstract
In β-thalassemia, unequal production of α- and β-globin chains in erythroid precursors causes apoptosis and inhibition of late-stage erythroid differentiation, leading to anemia, ineffective erythropoiesis (IE), and dysregulated iron homeostasis. Here we used a murine model of β-thalassemia intermedia (Hbbth1/th1 mice) to investigate effects of a modified activin receptor type IIB (ActRIIB) ligand trap (RAP-536) that inhibits Smad2/3 signaling. In Hbbth1/th1 mice, treatment with RAP-536 reduced overactivation of Smad2/3 in splenic erythroid precursors. In addition, treatment of Hbbth1/th1 mice with RAP-536 reduced α-globin aggregates in peripheral red cells, decreased the elevated reactive oxygen species present in erythroid precursors and peripheral red cells, and alleviated anemia by promoting differentiation of late-stage erythroid precursors and reducing hemolysis. Notably, RAP-536 treatment mitigated disease complications of IE, including iron overload, splenomegaly, and bone pathology, while reducing erythropoietin levels, improving erythrocyte morphology, and extending erythrocyte life span. These results implicate signaling by the transforming growth factor-β superfamily in late-stage erythropoiesis and reveal potential of a modified ActRIIB ligand trap as a novel therapeutic agent for thalassemia syndrome and other red cell disorders characterized by IE.
Introduction
β-thalassemia, the most common congenital anemia, is caused by mutations that reduce or eliminate production of β-globin.1,2 During late stages of normal erythroid differentiation, hemoglobin synthesis is highly coordinated to minimize accumulation of free globin subunits.3,4 Intracellular accumulation of free α-globin chains and precipitation of α-globin-heme complexes on red cell membranes in β-thalassemia generates proteotoxicity, inhibits late-stage erythroid differentiation, and is also thought to cause hemolysis of erythrocytes.1,2,5,6 Ineffective erythropoiesis (IE) is a hallmark of β-thalassemia and promotes anemia, hypoxia, and elevated erythropoietin (EPO) levels. If prolonged, this condition can lead to erythroid hyperplasia in bone marrow and spleen, dysregulated iron homeostasis, increased levels of reactive oxygen species (ROS) in erythroid cells, and additional complications in both transfusion-dependent and transfusion-independent patients.1,2 Patients with thalassemia intermedia, typically a transfusion-independent form, are afflicted by IE, anemia, and multiple disease complications including endocrinopathies, bone disease, thromboembolism, pulmonary hypertension, cerebrovascular pathology, and liver fibrosis/cirrhosis.2,7-9 Hematopoietic stem cell transplantation is typically curative for patients with severe β-thalassemia in cases where matched donors are available,10 but the mainstay of current treatment, supportive care consisting of regular blood transfusions and iron chelation, fails to address the underlying IE and often exacerbates iron overload.2,11 Therefore, there is a pressing need to identify potential therapeutic targets that promote differentiation of late-stage erythroid precursors for treating IE in patients with β-thalassemia.
Members of the transforming growth factor-β (TGFβ) superfamily have been studied as potential regulators of erythropoiesis. Ligands in this large superfamily, which include TGFβs, activins, growth differentiation factors, and bone morphogenetic proteins (BMPs), signal by triggering formation of activated ternary complexes containing different combinations of type I and type II receptors. Complexes containing activin receptor type IIA (ActRIIA), ActRIIB, or the TGFβ type II receptor regulate gene expression primarily by activating the Smad2/3 subfamily of intracellular effectors, whereas BMP receptors and ligands signal primarily through Smad1/5/8.12 Studies have documented effects of several superfamily ligands on erythroid precursors or cell lines, but the role of this superfamily in regulating erythropoiesis in vivo is not well understood.12-15 Intriguingly, increased Smad2/3 activation is found in hematopoietic progenitors from patients with myelodysplastic syndromes (MDS),16 a heterogeneous group of blood disorders in which IE occurs due to abortive erythroid precursor maturation.17,18 Moreover, pharmacologic inhibition of Smad2/3 signaling has been reported to stimulate effective hematopoiesis and reduce anemia in a murine model of MDS.16
In the present study, we used a receptor fusion protein (RAP-536), consisting of a modified extracellular domain of human ActRIIB linked to the murine IgG2a Fc domain, in a murine model (Hbbth1/th1) of human β-thalassemia intermedia.19 Like its human counterpart (ACE-536), RAP-536 has reduced binding affinity for activins and BMPs compared with wild-type ActRIIB20 and thus functions as a selective ligand trap that modulates Smad2/3 signaling. We find that RAP-536 reduces elevated Smad2/3 phosphorylation, promotes differentiation of late-stage erythroid precursors, alleviates anemia, and improves multiple complications of IE, including iron overload and bone abnormalities in Hbbth1/th1 mice. Our findings demonstrate the potential of a modified ActRIIB ligand trap as a novel therapeutic agent for β-thalassemia.
Materials and methods
Murine model of β-thalassemia
Hbbth1/th1 mice of the B6.D2-Hbbd3th/BrkJ strain and C57BL/6 wild-type mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were bred and maintained in the animal facility at Acceleron Pharma and were age- and gender-matched in all experiments. Genotyping was carried out at Transnetyx (Cordova, TN). Experimental procedures were carried out according to protocols approved by the Acceleron Pharma Institutional Animal Care and Use Committee.
RAP-536
ACE-536 consists of a modified human ActRIIB extracellular domain (residues 24-131 of the native precursor with a L79D substitution) linked to human IgG1-Fc domain and was generated as previously described.20 RAP-536 consists of the same modified extracellular domain linked to the murine IgG2a-Fc domain and was similarly generated. Mice were administered RAP-536 (1 mg/kg, subcutaneously [s.c.]) twice weekly for 2 months beginning at ∼3 months of age. All analyses were carried out at study termination. Additional experiments involved mice treated with RAP-536 at 10 or 30 mg/kg, intraperitoneally, as indicated in the corresponding figure legends.
Blood chemistry
Complete blood counts were determined using a Hematrue blood analyzer (Heska). Bilirubin content in the blood samples was determined using the VetScan VS2 System (Abaxis). Reticulocytes in peripheral blood were measured by flow cytometry after staining with thiazole orange (Sigma-Aldrich). Blood smears were stained with Sure stain (Sigma-Aldrich) to visualize morphology. To reveal Heinz bodies, blood cells were stained supravitally with new methylene blue (Sigma-Aldrich) and counterstained with Wright-Giemsa stain.
Flow cytometry
Erythroid differentiation was monitored by flow cytometry with fluorescently labeled mouse-specific antibodies against CD71 and Ter119 as previously described.21,22 Propidium iodide-positive dead cells were excluded from the analysis.
Labeling with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Invitrogen) and flow cytometry were used to determine ROS levels in erythroblasts and peripheral red cells as previously described.22 ROS levels were also determined in red cells after treatment with hydrogen peroxide (50 μM) for 30 minutes at 37°C.
Red blood cell (RBC) life span was determined as previously described.23
mRNA quantitation
RNA isolation and quantitative real-time polymerase chain reaction were performed as described24 with a Bio-Rad CFX Connect analyzer (Bio-Rad). Data were analyzed with Bio-Rad CFX Manager-3.0 software according to the manufacturer’s instructions by the ΔΔCt method normalized first to 18S RNA levels and then to wild-type levels for the gene of interest. Fold difference was calculated from ΔΔCt values using the following formula: fold difference = 2(−ΔΔCt). Taqman primers were purchased from Life Technologies (Hamp: Mm00519025_m1, Bmp6: Mm01332882_m1, Gdf15: Mm00442228_m1, and Twsg1: Mm00452254_m1).
Bone mineral density
Dual-energy X-ray absorptiometry was performed with a Lunar PIXImus II densitometer (GE Medical Systems) to determine bone mineral density in the right femur of anesthetized mice at 0, 2, 4, 6, and 8 weeks of treatment. Data were analyzed using PIXImus software (v2.10).
Bone architecture
Trabecular structure of the tibia was evaluated after study termination using a vivaCT-75 small-angle-cone-beam microcomputed tomography imaging system (Scanco Medical AG, Bassersdorf, Switzerland) with a resolution of 20.5- to 156-μm nominal pixel size as previously described.25 Detailed methodology is included in the supplemental Methods provided on the Blood Web site.
Globin chain analysis
Blood samples were normalized by hematocrit level. Membrane-bound α-globin aggregates were extracted as previously described26 and reconstituted in 20% acetonitrile. Samples were passed through a 0.2-μm syringe filter (Corning) and injected (100 μL) onto a reverse phase Vydac C4 column (214TPC4, 250 × 4.6 mm; Grace Vydac) at 1 mL/min for analysis with an Agilent 1100 series high-performance liquid chromatograph (Agilent Technologies). Elution was performed with an optimized acetonitrile gradient (35-45%) for 60 minutes. Elution signal was collected at 210 nm via an in-line diode-array detector. Analyses were performed based on peak areas for the globin chains. Peak areas were integrated by Agilent Chem Station software, averaged, and compared between the treatment groups as previously described.23
Phospho-Smad2/3 detection
Immunohistochemistry was performed on formalin-fixed and paraffin-embedded spleen sections (5 µm) at the Lombardi Comprehensive Cancer Center (Washington, DC) using an antibody against phospho-Smad2/3 (ab52903; Abcam). Western blot analysis was performed using an antibody against phospho-Smad2/3 (Santa Cruz Biotechnology sc-11769, 1:200 dilution) as previously described.22 Antibodies against Smad2/3 (Cell Signaling) and glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology) were used for detecting total Smad2 and glyceraldehyde-3-phosphate dehydrogenase proteins as loading controls in the gel.
Analysis of iron status
Serum levels of iron, ferritin, and transferrin saturation were measured at Molecular Diagnostic Services (San Diego, CA). Dry-tissue iron content in liver, kidney, and spleen was measured at the Diagnostic Center for Population and Animal Health at Michigan State University. Staining with Perls’ Prussian blue was performed on formalin-fixed and paraffin-embedded tissue sections by IDEXX Laboratories (West Sacramento, CA).
Photomicroscopy
All photomicrographs are brightfield images obtained with an Eclipse 80i microscope (Nikon Instruments, Melville, NY) fitted with a 4× objective, 20× objective, or 100× oil immersion objective (all Nikon; numerical aperature, 0.13) and attached to a Nikon camera system operating with NIS-Elements v3.10 imaging software (Nikon Instruments).
Statistical analysis
Data are reported as means ± standard error of the mean (SEM) unless indicated otherwise. Non-normally distributed variables were log-transformed so that data were normally distributed prior to statistical analysis. Otherwise, data were analyzed by a nonpaired Student t test for between-group comparisons.
Results
RAP-536 alleviates anemia in Hbbth1/th1 mice
We investigated effects of RAP-536 in the Hbbth1/th1 mouse model of β-thalassemia intermedia, which is characterized by abortive maturation of erythroid precursors, ineffective erythropoiesis, and a hypochromic, microcytic anemia.19 As expected, vehicle-treated Hbbth1/th1 mice were severely anemic and exhibited significantly altered RBC parameters compared with age-matched wild-type mice (Table 1). Compared with vehicle, RAP-536 treatment significantly increased RBC number by 29% (P < .001), hemoglobin concentration by 16% (P < .001), and hematocrit by 19% (P < .001). RAP-536 treatment led to modest decreases of 8% in mean cell volume, 10% in mean cell hemoglobin, and 2% in mean cell hemoglobin concentration compared with vehicle. As expected, vehicle-treated Hbbth1/th1 mice exhibited severe reticulocytosis and an increase in red cell distribution width area (RDWa, indicative of immature erythrocytes) compared with wild-type mice. The correction of anemia in Hbbth1/th1 mice treated with RAP-536 was accompanied by a 33% reduction in reticulocytes (P < .01) and a 19% reduction in RDWa (P < .01), both compared with vehicle. In wild-type mice, treatment with RAP-536 for 2 months similarly produced significant increases in RBC, hemoglobin, and hematocrit, as well as modest decreases in mean cell volume and mean cell hemoglobin compared with vehicle (Table 1). Together these data demonstrate that RAP-536 promotes erythropoiesis in both wild-type mice and β-thalassemic mice, thereby reducing anemia and reticulocytosis in the latter.
RAP-536 alleviates anemia in Hbbth1/th1 mice and increases RBC numbers in wild-type mice
| Group . | RBC (106 cells/µL) . | Hb (g/dL) . | Hct (%) . | MCV (fL) . | MCH (pg) . | MCHC (g/dL) . | Retic (%) . | RDWa (fL) . |
|---|---|---|---|---|---|---|---|---|
| Wild type + vehicle | 9.2 ± 0.1 | 13.7 ± 0.1 | 40.1 ± 0.5 | 43.5 ± 0.4 | 14.9 ± 0.1 | 34.4 ± 0.3 | 3.1 ± 0.1 | 28.8 ± 0.3 |
| Wild type + RAP-536 | 10.5 ± 0.1* | 15.0 ± 0.2* | 43.4 ± 0.7* | 41.2 ± 0.4† | 14.2 ± 0.1* | 34.6 ± 0.3 | 3.1 ± 0.1 | 28.6 ± 0.2 |
| Hbbth1/th1 + vehicle | 6.3 ± 0.2* | 9.0 ± 0.1* | 25.3 ± 0.6* | 40.2 ± 1.0† | 14.4 ± 0.6 | 35.7 ± 0.7 | 47.0 ± 5.8* | 41.8 ± 1.7* |
| Hbbth1/th1 + RAP-536 | 8.1 ± 0.2‡ | 10.4 ± 0.2‡ | 30.0 ± 0.7‡ | 37.0 ± 0.6§ | 12.9 ± 0.4 | 34.9 ± 0.9 | 31.3 ± 2.2§ | 33.7 ± 1.3¶ |
| Group . | RBC (106 cells/µL) . | Hb (g/dL) . | Hct (%) . | MCV (fL) . | MCH (pg) . | MCHC (g/dL) . | Retic (%) . | RDWa (fL) . |
|---|---|---|---|---|---|---|---|---|
| Wild type + vehicle | 9.2 ± 0.1 | 13.7 ± 0.1 | 40.1 ± 0.5 | 43.5 ± 0.4 | 14.9 ± 0.1 | 34.4 ± 0.3 | 3.1 ± 0.1 | 28.8 ± 0.3 |
| Wild type + RAP-536 | 10.5 ± 0.1* | 15.0 ± 0.2* | 43.4 ± 0.7* | 41.2 ± 0.4† | 14.2 ± 0.1* | 34.6 ± 0.3 | 3.1 ± 0.1 | 28.6 ± 0.2 |
| Hbbth1/th1 + vehicle | 6.3 ± 0.2* | 9.0 ± 0.1* | 25.3 ± 0.6* | 40.2 ± 1.0† | 14.4 ± 0.6 | 35.7 ± 0.7 | 47.0 ± 5.8* | 41.8 ± 1.7* |
| Hbbth1/th1 + RAP-536 | 8.1 ± 0.2‡ | 10.4 ± 0.2‡ | 30.0 ± 0.7‡ | 37.0 ± 0.6§ | 12.9 ± 0.4 | 34.9 ± 0.9 | 31.3 ± 2.2§ | 33.7 ± 1.3¶ |
RBC parameters in β-thalassemic (Hbbth1/th1) mice and age-matched C57BL/6 wild-type mice treated with RAP-536 (1 mg/kg, s.c., twice weekly) or vehicle for 2 months starting at 3 months of age. Results are expressed as mean ± SEM (n = 12-17 mice per group). Hb, hemoglobin; Hct, hematocrit; MCV, mean cell volume; MCH, mean cell hemoglobin; MCHC, mean corpuscular hemoglobin concentration; Retic, reticulocytes.
P < .001 vs wild type + vehicle.
P < .01 vs wild type + vehicle.
P < .001 vs Hbbth1/th1 + vehicle.
P < .05 vs Hbbth1/th1 + vehicle.
P < .01 vs Hbbth1/th1 + vehicle.
RAP-536 mitigates ineffective erythropoiesis in Hbbth1/th1 mice
Because ACE-536 inhibits ligands known to signal through Smad2/3,20 we examined the effect of RAP-536 on Smad2/3 activation in erythropoietic tissue from β-thalassemic mice. Immunostaining for phosphorylated Smad2/3 (phospho-Smad2/3) was elevated in erythroid precursors from spleens of Hbbth1/th1 mice compared with wild-type mice, and treatment of Hbbth1/th1 mice with a single dose of RAP-536 reduced this staining within 12 h (Figure 1; supplemental Figure 1A). A similar effect of RAP-536 on phospho-Smad2 levels in spleen extracts was detected by western blotting (supplemental Figure 1B). These results indicate that elevated Smad2/3 activation occurs during ineffective erythropoiesis in erythroid precursors of β-thalassemic mice compared with wild-type mice, and RAP-536 treatment can inhibit this activation.
RAP-536 inhibits Smad2/3 activation in Hbbth1/th1 mice. (Upper) Representative phospho-Smad2/3 immunostaining (brown) in hematoxylin-counterstained spleen sections from a wild-type mouse (Wt), a vehicle-treated Hbbth1/th1 mouse (th1/th1), and an Hbbth1/th1 mouse treated for 12 hours with a single dose of RAP-536 (30 mg/kg, intraperitoneally, n = 3-4 mice per group). (Insets) Higher magnification. (Lower) Representative Ter119 immunostaining (brown) denoting red pulp in adjacent hematoxylin-counterstained spleen sections. *White pulp. Images were obtained with a 20× objective or 100× oil immersion objective. Bar = 100 or 15 µm (insets).
RAP-536 inhibits Smad2/3 activation in Hbbth1/th1 mice. (Upper) Representative phospho-Smad2/3 immunostaining (brown) in hematoxylin-counterstained spleen sections from a wild-type mouse (Wt), a vehicle-treated Hbbth1/th1 mouse (th1/th1), and an Hbbth1/th1 mouse treated for 12 hours with a single dose of RAP-536 (30 mg/kg, intraperitoneally, n = 3-4 mice per group). (Insets) Higher magnification. (Lower) Representative Ter119 immunostaining (brown) denoting red pulp in adjacent hematoxylin-counterstained spleen sections. *White pulp. Images were obtained with a 20× objective or 100× oil immersion objective. Bar = 100 or 15 µm (insets).
In untreated Hbbth1/th1 mice, tissue hypoxia initiates intra- and extramedullary erythropoiesis, leading eventually to erythroid hyperplasia.19 We therefore investigated whether RAP-536 alters erythroid differentiation in these mice. As expected, vehicle-treated Hbbth1/th1 mice displayed significantly increased numbers of basophilic erythroblasts (R1, CD71highTer119highFSChigh) and orthochromatic erythroblasts and reticulocytes (R3, CD71lowTer119highFSClow) in bone marrow and spleen compared with wild type (supplemental Figure 1C-D). Vehicle-treated Hbbth1/th1 mice also exhibited a significant increase in late basophilic and polychromatic erythroblasts (R2, CD71highTer119highFSClow) in the spleen. Compared with vehicle, RAP-536 treatment in Hbbth1/th1 mice caused an overall increase in the proportion of late-stage erythroid precursors. Specifically, in bone marrow, there was a significant decrease in the R1 population combined with a significant increase in the R3 population, whereas in the spleen, there were significant decreases in both the R1 and R2 populations together with a significant increase in the R3 population (Figure 2A-B). Administration of RAP-536 to EPO-pretreated wild-type mice promoted terminal erythroid differentiation in the bone marrow and spleen and increased peripheral red cells compared with vehicle (supplemental Figure 2). Together, these data indicate that inhibition of Smad2/3 signaling by RAP-536 can promote differentiation of terminal erythroid precursors in both Hbbth1/th1 mice and wild-type mice.
RAP-536 promotes erythroid differentiation in Hbbth1/th1 mice. (A) Representative flow cytometric density plots for Ter119+ erythroid precursors from bone marrow (BM) and spleen separated into basophilic erythroblasts (R1), late basophilic and polychromatic erythroblasts (R2), and orthochromatic reticulocytes and reticulocytes (R3) on the basis of CD71 expression and cell size (Fsc, forward scatter) as previously described.21 Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 (1 mg/kg, s.c.) or vehicle twice weekly for 2 months. (B) Quantitative flow cytometric data of erythroid precursors from bone marrow and spleen indicate that RAP-536 reduced the proportion of cells in R1 and R2 and concurrently increased the proportion in R3 compared with vehicle. n = 5 to 7 mice per group. (C) Serum EPO concentrations (n = 6-11 mice per group). (D) (Left) Spleen weight (n = 13-17 mice per group). (Right) Spleen size shown in representative images obtained in a study separate from that shown at left but using the same dosing schedule in 10- to 12-month-old mice. Data in B to D are means ± SEM. *P < .05 and **P < .01, RAP-536 vs vehicle; ###P < .001 vs wild type.
RAP-536 promotes erythroid differentiation in Hbbth1/th1 mice. (A) Representative flow cytometric density plots for Ter119+ erythroid precursors from bone marrow (BM) and spleen separated into basophilic erythroblasts (R1), late basophilic and polychromatic erythroblasts (R2), and orthochromatic reticulocytes and reticulocytes (R3) on the basis of CD71 expression and cell size (Fsc, forward scatter) as previously described.21 Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 (1 mg/kg, s.c.) or vehicle twice weekly for 2 months. (B) Quantitative flow cytometric data of erythroid precursors from bone marrow and spleen indicate that RAP-536 reduced the proportion of cells in R1 and R2 and concurrently increased the proportion in R3 compared with vehicle. n = 5 to 7 mice per group. (C) Serum EPO concentrations (n = 6-11 mice per group). (D) (Left) Spleen weight (n = 13-17 mice per group). (Right) Spleen size shown in representative images obtained in a study separate from that shown at left but using the same dosing schedule in 10- to 12-month-old mice. Data in B to D are means ± SEM. *P < .05 and **P < .01, RAP-536 vs vehicle; ###P < .001 vs wild type.
Tissue hypoxia arising from anemia elevates expression of EPO as the principal feedback mechanism to increase RBC production. In vehicle-treated Hbbth1/th1 mice, serum EPO levels were nearly 25-fold higher than those in wild-type mice (70 ± 14 vs 1770 ± 517 pg/mL, P < .001; Figure 2C). Treatment with RAP-536 reduced serum EPO levels in Hbbth1/th1 mice by ∼60% compared with vehicle (640 ± 111 vs 1770 ± 517 pg/mL, P < .05; Figure 2C). This reduction in EPO levels is likely due to alleviation of hypoxia by increased numbers of functional RBCs following RAP-536 treatment (Table 1). In β-thalassemia, increased EPO levels trigger both intra- and extramedullary erythropoiesis. Due to the latter, spleen weight was nearly eightfold higher in vehicle-treated Hbbth1/th1 mice compared with wild-type mice (P < .001) (Figure 2D). Consistent with anemia correction and decreased EPO levels, RAP-536 treatment reduced splenomegaly by 36% in Hbbth1/th1 mice compared with vehicle (385 ± 28 vs 604 ± 45 mg, P < .05; Figure 2D), indicating mitigation of ineffective extramedullary erythropoiesis.
RAP-536 restores iron homeostasis in Hbbth1/th1 mice
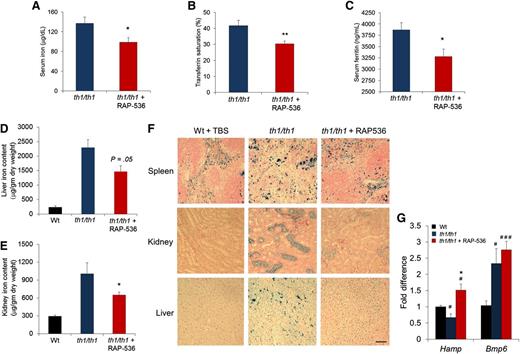
Given the importance of iron dysregulation in β-thalassemia, we investigated effects of RAP-536 on iron homeostasis in the murine model. As expected, Hbbth1/th1 mice displayed evidence of substantial iron overload, including elevated serum concentrations of the iron-storage protein ferritin (3870 ± 159 vs 887 ± 79 ng/mL, P < .0001), as well as high iron levels in liver (2296 ± 277 vs 236 ± 45 μg/mg dry weight, P < .0001) and kidney (1005 ± 184 vs 29 ± 19 μg/mg dry weight, P < .01) compared with wild-type littermates (Figure 3C-F). RAP-536 treatment reduced serum ferritin concentrations in Hbbth1/th1 mice by 15% compared with vehicle (3279 ± 167 vs 3871 ± 159 ng/mL, P < .05; Figure 3C). RAP-536 treatment in Hbbth1/th1 mice also significantly reduced serum iron concentrations (by 28%, 99 ± 9 vs 137 ± 13 μg/dL, P < .05; Figure 3A) and transferrin saturation (by 29%, 30 ± 2 vs 42 ± 3%, P < .01; Figure 3B), even though these iron parameters were not significantly elevated in the Hbbth1/th1 mice compared with wild type (data not shown). Importantly, RAP-536 treatment in Hbbth1/th1 mice reduced iron levels in liver (1464 ± 199 vs 2296 ± 277 μg/mg dry weight, P = .053) and kidney (648 ± 50 vs 1005 ± 184 μg/mg dry weight, P < .05) compared with vehicle (Figure 3D-E). These data are further supported by results of histochemical staining with Perls’ Prussian blue in renal cortex and hepatic Kuppfer cells, where RAP-536 treatment in Hbbth1/th1 mice normalized patterns of tissue iron staining (Figure 3F). Although treatment of Hbbth1/th1 mice with RAP-536 did not statistically reduce splenic iron levels expressed as dry weight (data not shown), RAP-536 altered the distribution of splenic iron and restored splenic architecture, as determined in representative spleen sections (Figure 3F; supplemental Figure 3), indicating an overall reduction in red pulp area (extramedullary erythropoiesis) and reduced splenomegaly (Figure 2D). RAP-536 did not affect iron parameters in wild-type mice after either 2 months of treatment (supplemental Figure 4) or 1 week of treatment (data not shown), thereby providing evidence that RAP-536 affects iron homeostasis indirectly in Hbbth1/th1 mice.
Effect of RAP-536 on iron overloading and Hamp expression in Hbbth1/th1 mice.Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 or vehicle as in Figure 2, and age-matched wild-type mice treated with vehicle were used as additional controls. (A-C) Serum iron concentration, transferrin saturation, and serum ferritin concentration (n = 6-8 mice per group). (D-E) Tissue iron levels in liver and kidney (n = 5-7 mice per group). (F) Representative images of formalin-fixed sections from spleen, kidney, and liver stained with Perls’ Prussian blue reveal reduced iron content following RAP-536 treatment. Images were obtained with a 20× objective; bar = 100 µm. (G) Expression of Hamp and Bmp6 in liver determined by quantitative real-time polymerase chain reaction (n = 5-7 mice per group). Data are means ± SEM. #P < .05 and ###P < .001 vs wild type; *P < .05 and **P < .01, RAP-536 vs vehicle.
Effect of RAP-536 on iron overloading and Hamp expression in Hbbth1/th1 mice.Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 or vehicle as in Figure 2, and age-matched wild-type mice treated with vehicle were used as additional controls. (A-C) Serum iron concentration, transferrin saturation, and serum ferritin concentration (n = 6-8 mice per group). (D-E) Tissue iron levels in liver and kidney (n = 5-7 mice per group). (F) Representative images of formalin-fixed sections from spleen, kidney, and liver stained with Perls’ Prussian blue reveal reduced iron content following RAP-536 treatment. Images were obtained with a 20× objective; bar = 100 µm. (G) Expression of Hamp and Bmp6 in liver determined by quantitative real-time polymerase chain reaction (n = 5-7 mice per group). Data are means ± SEM. #P < .05 and ###P < .001 vs wild type; *P < .05 and **P < .01, RAP-536 vs vehicle.
We then investigated hepatic expression of Hamp (hepcidin) and Bmp6, critical genes in the regulation of iron homeostasis.27 Elevated concentrations of EPO that occur in β-thalassemia inhibit hepcidin expression indirectly by stimulating erythropoiesis. Consistent with the severe anemia and elevated EPO concentrations (Figure 2C) present in this murine model, levels of Hamp mRNA in Hbbth1/th1 mice were lower than those in wild-type mice (P < .05) (Figure 3G; supplemental Figure 5A). RAP-536 treatment of 2 months in Hbbth1/th1 mice led to increased Hamp mRNA levels approximately 2 times those in vehicle-treated controls (P < .05) but notably produced little change in Hamp expression in wild-type mice (supplemental Figure 5A-B). As expected, levels of Bmp6 mRNA in Hbbth1/th1 mice were elevated compared with those in wild-type mice (Figure 3G; supplemental Figure 5B); however, treatment of Hbbth1/th1 mice with RAP-536 did not reduce Bmp6 expression (Figure 3G) despite the significant effect of RAP-536 on liver iron content in these mice (Figure 3D). A similar pattern has been observed with matriptase-2 haploinsufficiency (but not complete deletion) in th3/+ thalassemic mice,28 and it seems likely that elevated Bmp6 expression serves to strongly promote Hamp expression until liver iron content approaches closer to normal levels. Finally, treatment of Hbbth1/th1 mice with RAP-536 did not significantly alter splenic expression of Gdf15 or twisted gastrulation (Twsg1) (supplemental Figure 5C-D), identified previously as potential erythroid regulators.29,30 Together, these results demonstrate that RAP-536 treatment improves iron homeostasis in Hbbth1/th1 mice by increasing Hamp expression and reducing iron overload.
RAP-536 reduces hemolysis, α-globin aggregates, and ROS in Hbbth1/th1 mice
Erythrocytic damage and hemolysis are features of β-thalassemia caused by aggregation of unpaired α-globin chains and oxidative stress. Compared with wild-type mice, vehicle-treated Hbbth1/th1 mice had elevated concentrations of total serum bilirubin (Figure 4A) and a reduction of mean erythrocyte life span by approximately half (42 vs 20 days, respectively) (supplemental Figure 6A). Treatment of Hbbth1/th1 mice with RAP-536 reduced mean concentrations of total bilirubin by nearly 40% (Figure 4A) and increased mean erythrocyte life span by a similar percentage compared with vehicle-treated Hbbth1/th1 mice (28 vs 20 days, respectively) (supplemental Figure 6A). Consistent with these results, RAP-536 reduced the occurrence of abnormal erythrocyte morphology and hemolytic debris compared with vehicle (Figure 4B) Together, these results indicate that RAP-536 reduces hemolysis in Hbbth1/th1 mice.
Effects of RAP-536 on hemolysis, RBC morphology, Heinz bodies, and α-globin chain distribution in Hbbth1/th1 mice.Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 or vehicle as in Figure 2, and age-matched wild-type mice treated with vehicle were used as additional controls. (A) Total bilirubin concentration (n = 10-13 mice per group). (B) Blood smears treated with Wright-Giemsa stain to reveal effects of RAP-536 on RBC morphology, reticulocyte number, cellular debris, and degree of poikilocytosis. (C) Representative high-performance liquid chromatogram showing reduction of α-globin aggregates in erythrocyte membranes from Hbbth1/th1 mice treated with RAP-536. Mean levels of α-globin aggregates were reduced 38% by RAP-536 treatment. n = 3 to 5 mice per group. (D) Blood cells stained supravitally with new methylene blue and counterstained with Wright-Giemsa to reveal Heinz bodies. Images in B and D were obtained with a 100× oil immersion objective; bar = 10 µm. Data are means ± SEM. ###P < .001 vs wild type; **P < .01, RAP-536 vs vehicle.
Effects of RAP-536 on hemolysis, RBC morphology, Heinz bodies, and α-globin chain distribution in Hbbth1/th1 mice.Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 or vehicle as in Figure 2, and age-matched wild-type mice treated with vehicle were used as additional controls. (A) Total bilirubin concentration (n = 10-13 mice per group). (B) Blood smears treated with Wright-Giemsa stain to reveal effects of RAP-536 on RBC morphology, reticulocyte number, cellular debris, and degree of poikilocytosis. (C) Representative high-performance liquid chromatogram showing reduction of α-globin aggregates in erythrocyte membranes from Hbbth1/th1 mice treated with RAP-536. Mean levels of α-globin aggregates were reduced 38% by RAP-536 treatment. n = 3 to 5 mice per group. (D) Blood cells stained supravitally with new methylene blue and counterstained with Wright-Giemsa to reveal Heinz bodies. Images in B and D were obtained with a 100× oil immersion objective; bar = 10 µm. Data are means ± SEM. ###P < .001 vs wild type; **P < .01, RAP-536 vs vehicle.
We then investigated whether this effect of RAP-536 on red cell quality in β-thalassemic mice could be caused by reduced levels of α-globin aggregation. Heinz bodies (denatured α-globin aggregates) in peripheral erythrocytes were markedly reduced following treatment with RAP-536 (Figure 4D). We next used reverse-phase high-performance liquid chromatography to quantitate α-globin aggregates in isolated membranes of Hbbth1/th1 erythrocytes as a function of RAP-536 treatment (supplemental Figure 6B). Compared with vehicle-treated Hbbth1/th1 mice, those treated with RAP-536 exhibited a mean reduction of 38% (P < .05) in membrane-associated α-globin aggregates (Figure 4C, blue vs red peak). α-Globin was undetectable in wild-type membrane fractions (Figure 4C, black line), and β-globin was undetectable in membrane fractions from all groups as expected. These results indicate that RAP-536 reduces hemolysis in Hbbth1/th1 mice at least partly by reducing levels of α-globin aggregates in erythrocytes.
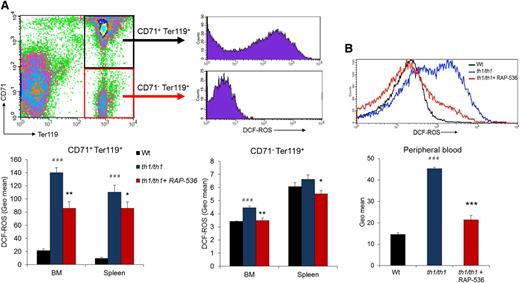
Because oxidative stress is heavily implicated in hemolysis and the etiology of β-thalassemia generally, we also investigated the effect of RAP-536 on ROS in Hbbth1/th1 mice. Flow cytometric analyses were carried out using the cell-permeable ROS indicator CM-H2DCFDA in immature erythroblasts (CD71highTer119high) and mature erythroblasts (CD71lowTer119high) from bone and spleen, as well as in peripheral red cells. As expected, ROS levels were elevated in all cell types investigated from vehicle-treated Hbbth1/th1 mice compared with wild-type mice (Figure 5). Treatment of Hbbth1/th1 mice with RAP-536 reduced ROS levels significantly in immature (CD71+Ter119+) and mature (CD71−Ter119+) erythroblasts (Figure 5A), as well as in peripheral red cells (Figure 5B; P < .0001), where ROS levels were nearly normalized. In addition to basal ROS levels, we also determined ROS levels in erythrocytes after exogenous challenge with H2O2. We observed that peroxide-stimulated ROS levels were reduced in RAP-536-treated Hbbth1/th1 mice compared with vehicle (supplemental Figure 7). These results demonstrate that RAP-536 reduces oxidative stress from Hbbth1/th1 mice under basal conditions but also confers protection against oxidative stress associated with an episodic challenge.
Effect of RAP-536 on ROS in Hbbth1/th1 mice.Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 or vehicle as in Figure 2, and age-matched wild-type mice treated with vehicle were used as additional controls. (A) Representative flow cytometric gating and quantitative data for ROS obtained by measuring H2DCF-DA fluorescence in immature (CD71+Ter119+) and mature (CD71–Ter119+) erythroblasts from bone marrow and spleen. (B) Representative histogram overlay and quantitative data for ROS in peripheral erythrocytes under basal conditions (without exogenous peroxide) (n = 4-6 mice per group). Data are means ± SEM. ###P < .001 vs wild type; *P < .05, **P < .01, and ***P < .001, RAP-536 vs vehicle.
Effect of RAP-536 on ROS in Hbbth1/th1 mice.Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 or vehicle as in Figure 2, and age-matched wild-type mice treated with vehicle were used as additional controls. (A) Representative flow cytometric gating and quantitative data for ROS obtained by measuring H2DCF-DA fluorescence in immature (CD71+Ter119+) and mature (CD71–Ter119+) erythroblasts from bone marrow and spleen. (B) Representative histogram overlay and quantitative data for ROS in peripheral erythrocytes under basal conditions (without exogenous peroxide) (n = 4-6 mice per group). Data are means ± SEM. ###P < .001 vs wild type; *P < .05, **P < .01, and ***P < .001, RAP-536 vs vehicle.
Together, the above findings support a mechanistic model in which RAP-536 treatment in β-thalassemic mice can reduce membrane aggregates of α-globin and intracellular levels of ROS during erythroid differentiation, leading to peripheral red cells with reduced levels of basal oxidative stress, improved resistance to stress, improved morphology, reduced hemolysis, and increased cell survival.
RAP-536 corrects bone abnormalities in Hbbth1/th1 mice
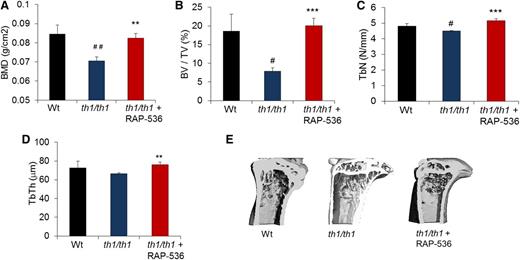
Patients with severe β-thalassemia often develop increased bone turnover, decreased bone mineral density, bone fragility, and bone deformities due in part to intramedullary erythroid hyperplasia.31 We therefore investigated whether RAP-536 treatment can alleviate bone complications occurring in Hbbth1/th1 mice. As determined by dual-energy X-ray absorptiometry and microcomputed tomography, tibiae from vehicle-treated Hbbth1/th1 mice exhibited lower bone mineral density (0.070 ± 0.002 vs 0.084 ± 0.004 g/cm2, P < .01), trabecular bone volume (7.8 ± 0.9 vs 18.6 ± 4.5%, P < .05), and trabecular number (4.5 ± 0.01 vs 4.8 ± 0.15 N/mm, P < .05) compared with wild type (Figure 6A-C), likely in part as a consequence of increased erythropoietic activity and bone marrow expansion.32 Treatment of Hbbth1/th1 mice with RAP-536 significantly increased bone mineral density (by 17%, 0.082 ± 0.002 vs 0.070 ± 0.002 g/cm2, P < .001), trabecular bone volume (by 100%, 20.01 ± 2.03 vs7.8 ± 0.9%, P < .001), trabecular number (by 14%, 5.15 ± 0.01 vs 4.5 ± 0.01 N/mm, P < .001), and trabecular thickness (by 14%, 75.91 ± 2.74 vs 66.31 ± 1.17 N/mm, P < .01), completely restoring these parameters to wild-type levels (Figure 6A-D). Representative 3-dimensional images confirm that structural deterioration in the tibia of Hbbth1/th1 mice, particularly a marked decrease in cortical thickness and an increase in marrow space compared with wild type, was reversed on treatment with RAP-536 (Figure 6E). Importantly, RAP-536 treatment in wild-type mice did not alter the majority of bone parameters evaluated (supplemental Figure 8), thereby providing evidence that RAP-536 affects bone indirectly in Hbbth1/th1 mice.
Effect of RAP-536 on bone in Hbbth1/th1 mice.Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 or vehicle as in Figure 2, and age-matched wild-type mice treated with vehicle were used as additional controls. (A) BMD (n = 8-13 mice per group). (B-D) BV/TV, TbN, and TbTh (n = 4-6 mice per group). (E) Representative 3-dimensional images of proximal tibiae generated from microcomputed tomography analysis. Data are means ± SEM. #P < .05 and ##P < .01 vs wild type; *P < .05, **P < .01, and ***P < .001, RAP-536 vs vehicle. BMD, bone mineral density; BV/TV, bone volume fraction; TbN, trabecular number; TbTh, trabecular thickness.
Effect of RAP-536 on bone in Hbbth1/th1 mice.Hbbth1/th1 mice (3-4 months old) were treated with RAP-536 or vehicle as in Figure 2, and age-matched wild-type mice treated with vehicle were used as additional controls. (A) BMD (n = 8-13 mice per group). (B-D) BV/TV, TbN, and TbTh (n = 4-6 mice per group). (E) Representative 3-dimensional images of proximal tibiae generated from microcomputed tomography analysis. Data are means ± SEM. #P < .05 and ##P < .01 vs wild type; *P < .05, **P < .01, and ***P < .001, RAP-536 vs vehicle. BMD, bone mineral density; BV/TV, bone volume fraction; TbN, trabecular number; TbTh, trabecular thickness.
Together, these findings provide compelling evidence that RAP-536 mitigates ineffective erythropoiesis in Hbbth1/th1 mice, thereby alleviating anemia, extramedullary erythropoiesis, erythroid hyperplasia, and bone abnormalities, at least in part by promoting terminal erythroid differentiation and maturation.
Discussion
In β-thalassemia, anemia is caused primarily by IE arising from inhibition of late-stage erythroid differentiation and exacerbated by hemolysis. Here we show that a modified ActRIIB ligand trap can alleviate anemia in Hbbth1/th1 mice in part by promoting differentiation of late-stage erythroid precursors and reducing hemolysis, effects likely due to reduced α-globin aggregates in erythrocytes and decreased ROS in erythroid precursors and peripheral erythrocytes. In addition to improving these fundamental features of the disease, RAP-536 treatment in Hbbth1/th1 mice produced beneficial effects on several interrelated disease complications. First, RAP-536 reduced the characteristically elevated concentrations of circulating EPO, an effect that confirms functional RBC production because reduced EPO levels generally result from diminished hypoxic stimulus. Second, RAP-536 reduced erythroid hyperplasia as demonstrated by a marked reduction in splenomegaly. Finally, RAP-536 corrected bone pathology and reduced abnormal iron homeostasis, two major complications with serious consequences in β-thalassemia. In wild-type mice, treatment with RAP-536 promoted late-stage erythroid differentiation and increased RBCs but did not alter other end points such as iron or bone parameters, thereby providing evidence that RAP-536-induced changes in Hbbth1/th1 mice are indirect effects likely mediated by reduced anemia.
Growing evidence implicates Smad2/3 signaling in the regulation of erythroid differentiation. Our findings of elevated Smad2/3 activation, erythroid hyperplasia, and inhibition of erythroid differentiation in Hbbth1/th1 mice are consistent with reports of constitutive overactivation of Smad2/3 in erythroid tissue from patients with MDS,16 another disease characterized by erythroid hyperplasia and abortive maturation.33-35 We hypothesize that one or more TGFβ superfamily ligands that activate the Smad2/3 pathway negatively regulate terminal erythroid differentiation, perhaps by promoting erythroid expansion during ineffective erythropoiesis, and that RAP-536 exerts beneficial effects in β-thalassemic mice by blunting elevated activity in this inhibitory pathway.
In contrast to Smad2/3 signaling, the Smad1/5/8 pathway is heavily implicated in hepatic regulation of iron homeostasis,36 as well as in stress erythropoiesis.37 In several murine models of β-thalassemia, experimental interventions intended to alter systemic iron availability have been found to improve not only iron overloading but also erythroid differentiation.26,38-40 Significantly, these studies did not find evidence of a direct effect of their respective interventions on terminal erythroid differentiation in wild-type mice. Although RAP-536 does not inhibit Smad1/5/8 signaling in vitro20 or alter iron homeostasis in wild-type mice, we show here that RAP-536 improves iron homeostasis in β-thalassemic mice. By directly promoting erythroid differentiation and reducing anemia in these mice, RAP-536 may indirectly alleviate suppression of hepcidin and trigger a beneficial cycle that reduces iron absorption and further mitigates ineffective erythropoiesis. Recently, pharmacologic depletion of macrophages was found to reduce erythroid precursor proliferation and promote differentiation in β-thalassemic mice through inhibition of a direct iron-independent pathway termed stress erythropoiesis macrophage-supporting activity.41 As noted in that study, broad therapeutic effects may be obtained in β-thalassemic mice, and perhaps in patients, by direct intervention in the erythroid differentiation pathway.
The presence of free α-globin chains is strongly associated with pathophysiology in β-thalassemia. Such chains are unstable and form cytotoxic α-globin aggregates that precipitate in erythrocyte membranes, thereby causing membrane damage due to oxidative stress and ultimately leading to a shortened erythrocyte life span.3 In the present study, we found that inhibition of Smad2/3 signaling reduces oxidative stress in erythrocytes, reduces accumulation of α-globin aggregates in erythrocyte membranes, improves erythrocyte morphology, and improves RBC life span. Although the mechanism responsible for this reduction in α-globin accumulation is unknown, potential explanations include an absolute decrease in α-globin chain production, enhancement of the known ability of erythroid cells to remove misfolded α-globin chains via proteosomes,4 or hepcidin-mediated reduction in iron availability for synthesis of heme and globin.22,42,43
Taken together, our results show that RAP-536, a modified ActRIIB ligand trap with Smad2/3 inhibitory activity, promotes terminal erythroid differentiation and mitigates IE in a mouse model of β-thalassemia. Studies have recently demonstrated a role for Smad2/3 signaling in the pathophysiology of IE in both β-thalassemia and MDS.44,45 Inhibitors of Smad2/3 signaling might therefore be useful for ameliorating anemia in these conditions. ACE-536 has completed a phase 1 clinical study in healthy volunteers,46 and phase 2 studies are ongoing in patients with β-thalassemia and in those with MDS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr John Knopf and Dr John Quisel for critical review, Abigail Pullen for assistance with mouse husbandry and genotyping, and the Cell Biology, Protein Purification, and Preclinical Pharmacology groups at Acceleron Pharma for valuable contributions in support of this work. The authors also thank Dr Deborah Berry, Lombardi Comprehensive Cancer Center, Georgetown University, for expert immunohistochemical services.
This study was supported by Acceleron Pharma.
Authorship
Contribution: R.N.V.S.S., S.M.C., R.L., R.S.P., S.R., A.V.G., and R.K. planned and designed the experiments; R.N.V.S.S., S.M.C., R.L., S.W., and S.G. conducted the experiments; R.N.V.S.S., S.M.C., R.L., S.W., and A.W.M. collected and interpreted data; and R.N.V.S.S., M.J.A., and R.K. drafted and revised the manuscript.
Conflict-of-interest disclosure: R.N.V.S.S., R.L., S.W., M.J.A., A.W.M., A.V.G., R.S.P., and R.K. are employees of Acceleron Pharma with ownership interest in the company, and S.M.C. is an employee of Acceleron Pharma. S.R. is a consultant for Novartis, Biomarin, Alexion, Bayer, Exigo, and Isis Pharmaceuticals and holds equity/ownership interest in Merganser Biotech, Inc. The remaining authors declare no competing financial interests.
Correspondence: Ravindra Kumar, Acceleron Pharma, 128 Sidney St, Cambridge, MA 02139; e-mail: rkumar@acceleronpharma.com.