Key Points
Distinct clusters exist within polycythemia vera, essential thrombocythemia, and myelofibrosis.
Clusters are not direct surrogates for current prognostic scores.
Abstract
Symptom burden in myeloproliferative neoplasms (MPNs) is heterogeneous even among patients within the same MPN diagnosis. Using cluster analysis from prospectively gathered symptom burden data in 1470 international patients with essential thrombocythemia (ET), polycythemia vera (PV), or myelofibrosis (MF), we assessed for the presence of clusters and relationship to disease features and prognosis. In MF (4 clusters identified), clusters significantly differed by Dynamic International Prognostic Scoring System (DIPSS) risk (P < .001), leukopenia (P = .009), thrombocytopenia (P < .001), and spleen size (P = .02). Although an association existed between clusters and DIPSS risk, high symptom burden was noted in some low and intermediate-1–risk MF patients. In PV (5 clusters identified), total symptom score increased across clusters (P < .001), but clusters did not significantly differ by PV risk or the risk assessment variable of age. Among ET patients (5 clusters identified), clusters differed by gender (P = .04), anemia (P = .01), and prior hemorrhage (P = .047). Total symptom score increased across clusters (P < .001), but clusters did not significantly differ by International Prognostic Score for ET risk including the risk assessment variables. Significant symptom heterogeneity exists within each MPN subtype, sometimes independent of disease features or prognosis.
Introduction
The Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs), including essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF), originate from malignant transformation of oligoclonal and polyclonal myeloid–derived hematopoietic stem cells. Progression to MF (including post-PV MF, post-ET MF, or primary MF) leads to disease burdens that may include cytopenias, splenomegaly, and/or constitutional symptoms, which in aggregate may significantly compromise quality of life.
For physicians, assessing and treating the symptom burden represents one of the most challenging features of managing MPNs because of the paucity of effective treatment regimens and marked heterogeneity between patient presentations. In 2007, our group published the results of a cross-sectional survey of 1179 MPN patients, which helped assess and quantify that these patients do suffer from excessive fatigue compared with age-matched controls and that symptoms compromise social functioning, physical activity, independence in daily tasks, and global quality of life.1 In addition, this survey found that traditional therapies including hydroxyurea, interferon, thalidomide, corticosteroids, androgens, and erythropoietin analogs were suboptimal in reducing symptoms.
To assist with assessment of MPN symptom burden, we first created the Myelofibrosis Symptom Assessment Form (MF-SAF)2 by using the symptoms endorsed by patients in our original online survey1 and then expanded it to include an additional 7 symptoms relevant to ET and PV. This expanded measure, the MPN Symptom Assessment Form (MPN-SAF),3 was validated among an international sample of MPN patients and subsequently translated into 11 languages. To ease survey administration for serial use in assessment of therapeutic interventions, the MPN-SAF Total Symptom Score (MPN-SAF TSS)4 was created as an abbreviated 10-item measure containing only the most representative and pertinent MPN symptoms to be given in conjunction with the Brief Fatigue Inventory (BFI)5 as a convenient assessment of symptom burden.
Subsequent investigations using these patient-reported outcomes (PROs) have identified significant symptomatic heterogeneity between MPN subtypes and their correlations to disease duration and symptomatic burden.6 Our more recent study included an in-depth evaluation of 17 independent symptoms present among an MPN population and found that the prevalence and severity of symptoms varied widely between MPN subtypes (range in prevalence 17%-59% for ET, 18%-68% for PV, and 29%-77% for MF) with much intra- and interpatient symptom variability.3 To date, there have been no investigations into whether the heterogeneity observed within MPN subtypes represents unique symptom clusters. This study represents the first investigation of the existence and inter-relations between MPN symptom clusters via prospectively gathered information on disease symptoms and features. We additionally sought to identify the spectrum and relationship of such clusters in association with both disease features as well as estimated prognosis.
Methods
Survey development and collection
This study has been approved by our institutional review board and designated the reference number 09-008764. This study was conducted in accordance with the Declaration of Helsinki. Patient and physician-reported demographics, MPN disease features, and symptom burden data were collected from an international cohort in a manner described previously.3 Patients were recruited from academic, government, and private medical centers internationally during routine office visits. Physicians were queried on available laboratory and clinical data including hemoglobin level, platelet count, white blood cell count, absolute neutrophil count, percent blasts, and spleen size.
Symptom burden assessment completed by the patient at a single time point included the BFI and MPN-SAF, which addressed key disease features of fatigue, early satiety, abdominal pain and discomfort, inactivity, headaches, concentration, dizziness, extremity tingling, insomnia, sexual difficulties, mood changes, cough, night sweats, pruritus, bone pain, and fever. Items were scored on a 0 (absent) to 10 (worst imaginable) scale. MPN-SAF TSS items included “worst fatigue” from the BFI plus concentration, early satiety, inactivity, night sweats, itching, bone pain, abdominal discomfort, weight-loss, and fever. For individuals completing at least 6 of the 10 MPN-SAF TSS items, the survey was scored by multiplying the average score across items by 10 to achieve a 0 to 100 scaled score. Survey translations were developed using a PRO translation method7 by teams of 4 international collaborators. Available translations of the survey included Chinese, Danish, Dutch, English (United States and United Kingdom), French, German, Italian, Japanese, Spanish, Swedish, and Portuguese.
Prognostic scoring
Prognostic groups for MF were calculated using the Dynamic International Prognostic Scoring System (DIPSS),8 which uses the factors of age (>65 years), blasts present in peripheral blood (>1%), hemoglobin (<10 g/dL), leukocyte count (>25 × 109/L), and the presence of constitutional symptoms (including weight loss >10%, night sweats, or fevers). This scale can be used at any time during the course of MF to estimate the risk of progression and separates patients into low, intermediate-1, intermediate-2, and high-risk categories. For PV, prognostic scoring used a model developed by Tefferi and colleagues that includes the variables of age (>70 or 60-69 years) and leukocyte count ≥15 × 109/L to indicate low, intermediate-1, intermediate-2, or high risk.9 The International Prognostic Score for ET (IPSET)10 was assessed among ET patients and included the variables of age (>60 years), leukocyte count (≥11 × 109/L), and history of thrombosis to indicate low, medium, or high risk of progression. For the purposes of this study, anemia, thrombocytopenia, and leukopenia were defined as hemoglobin levels <11 g/dL, platelet count <150 × 109/L, and leukocyte count <3.5 × 109/L.
Statistical analysis
Cluster analysis was performed using 19 individual symptom items (the worst fatigue item of the BFI and the 18 individual symptom items of the MPN-SAF) within each disease group independently. The number of clusters was based on consideration of r2 (supplemental Figure A1, available on the Blood Web site) and semipartial r2 while maintaining individual cluster sizes to be at least 5% of the sample in hierarchical clustering using Ward linkage. To be included in the cluster analysis, patients needed to complete all 19 questions in the survey. Thus 1470 of 1885 patients in the database were included. Final cluster assignment within disease was based on the nonhierarchical k-means method using the number of clusters identified via the previous hierarchical clustering procedure. Split-half validation was used to examine the internal validity of the final cluster results.11,12 In split-half validation within each disease group, cluster assignment is performed in a randomly selected pair of split-half subsamples, and the Euclidean distance between each subject’s symptom scores and the mean symptom scores for that patient’s cluster is compared between split-half subsamples using a Kruskal-Wallis test (the size of the difference is described using the Hodges-Lehmann [HL] estimator). A small nonsignificant difference between split-half subsamples would indicate adequate internal validity. In all disease groups, split-half validation indicated adequate internal validity (MF: HL = –0.02, 95% confidence interval [CI] −0.8 to 0.7, P = .95; ET: HL = 0.06, 95% CI −0.5 to 0.7, P = .83; PV: HL = –0.2, 95% CI −0.7 to 0.3, P = .43). Comparisons between symptom clusters were based on analysis of variance (ANOVA) F-tests or Student t tests for continuous variables and χ2 tests for categorical variables. Finally, MPN-SAF TSS was compared across risk groups using ANOVA F-tests.
Results
ET symptom clusters
Subject demographic and disease characteristics.
Analysis included data from 622 prospectively enrolled persons with ET (Chinese 149, French 174, German 20, Italian 65, Dutch 72, English 29, Spanish 67, Swedish 46). Participants were of typical age (median 58 years, range 15-90) for the disease. The group was predominantly female (63.2%; supplemental Table 1).
Cluster overview.
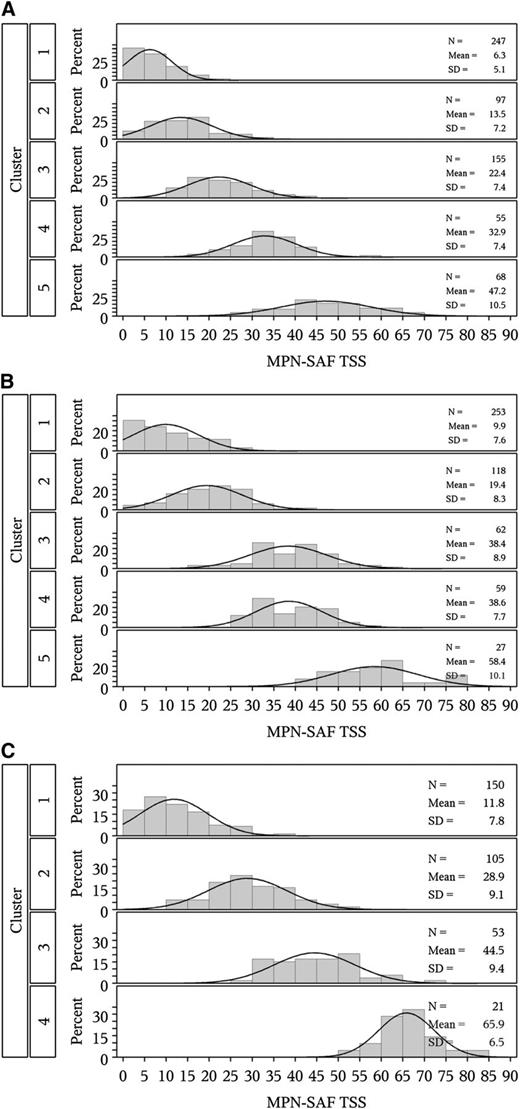
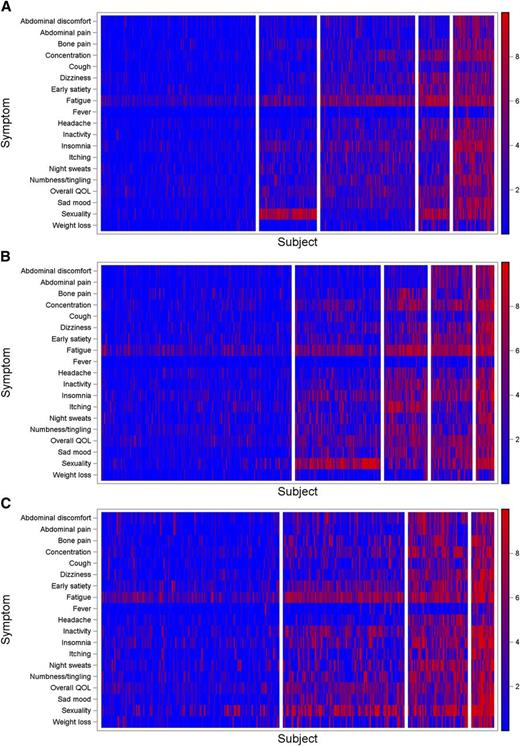
Among all participants, 5 symptom clusters were identified. Distribution of age and gender, along with language, laboratory abnormality (anemia), and prior hemorrhage differed significantly across clusters (P < .05, Table 1 and supplemental Table 1). MPN-SAF TSS also significantly differed across clusters (P < .001, Figure 1A). A heatmap (visual representation of individual symptom levels across patients ordered by cluster) is presented in Figure 2A (also see supplemental Figure 2 for mean symptom scores by cluster). IPSET risk scores did not significantly differ across clusters (P = .43) including the IPSET variable of prior thrombosis (P = .24). No chronological trends were noted among clusters (MPN duration, P = .89).
Associations between cluster and patient disease characteristics by MPN subtype
| . | P value§ . | ||
|---|---|---|---|
| . | ET . | PV . | MF . |
| Age | .01 | .13 | .78 |
| Gender | .04 | .02 | .20 |
| Language | <.001 | <.001 | .24 |
| Risk score* | .43 | .87 | .001 |
| MPN duration, y | .89 | .73 | .15 |
| Laboratory abnormality | .04 | .009 | .79 |
| Prior hemorrhage | .047 | .06 | .22 |
| Prior thrombosis | .24 | .12 | .06 |
| Prior splenectomy | .44 | .43 | .65 |
| Spleen size† | .76 | .002 | .02 |
| Palpable spleen | .75 | .009 | .009 |
| Required red blood cell transfusion | .47 | .63 | .13 |
| Receiving treatment‡ | .21 | .32 | .64 |
| Anemia, hemoglobin <11 g/dL | .01 | .07 | .48 |
| Leukopenia, leukocyte count <3.5 × 109/L | .14 | .01 | .009 |
| Thrombocytopenia, platelet count <150 × 109/L | .28 | .31 | <.001 |
| . | P value§ . | ||
|---|---|---|---|
| . | ET . | PV . | MF . |
| Age | .01 | .13 | .78 |
| Gender | .04 | .02 | .20 |
| Language | <.001 | <.001 | .24 |
| Risk score* | .43 | .87 | .001 |
| MPN duration, y | .89 | .73 | .15 |
| Laboratory abnormality | .04 | .009 | .79 |
| Prior hemorrhage | .047 | .06 | .22 |
| Prior thrombosis | .24 | .12 | .06 |
| Prior splenectomy | .44 | .43 | .65 |
| Spleen size† | .76 | .002 | .02 |
| Palpable spleen | .75 | .009 | .009 |
| Required red blood cell transfusion | .47 | .63 | .13 |
| Receiving treatment‡ | .21 | .32 | .64 |
| Anemia, hemoglobin <11 g/dL | .01 | .07 | .48 |
| Leukopenia, leukocyte count <3.5 × 109/L | .14 | .01 | .009 |
| Thrombocytopenia, platelet count <150 × 109/L | .28 | .31 | <.001 |
Spleen size determined by physical examination, with the mean size being represented by the number of centimeters below the left costal margin.
Treatment vs no treatment/aspirin only.
See supplemental Tables A1-3 for point estimates and measures of variability for each stated P value.
Distribution of MPN-SAF TSS by cluster. (A) Essential thrombocythemia, (B) polycythemia vera, and (C) myelofibrosis.
Distribution of MPN-SAF TSS by cluster. (A) Essential thrombocythemia, (B) polycythemia vera, and (C) myelofibrosis.
Symptom heatmaps. (A) Essential thrombocythemia, (B) polycythemia vera, and (C) myelofibrosis.
Symptom heatmaps. (A) Essential thrombocythemia, (B) polycythemia vera, and (C) myelofibrosis.
Cluster descriptions.
Cluster 1.
Mild ET (n = 247, 40%). The largest cluster, patients had the lowest individual MPN-SAF symptom scores and the lowest MPN-SAF TSS. Mean symptom scores were all ≤1.1 other than fatigue (mean 2.0). The majority of patients (59.4%) had disease duration <3 years and most patients were low (38.2%) or intermediate (45.9%) risk. Anemia was uncommon (21.2%).
Cluster 2.
Moderate-I ET (n = 97, 16%). This cluster was typified by the predominant complaint of sexual dysfunction (mean 7.6) out of proportion to other symptoms (all means ≤2.6 other than fatigue, mean 3.3). These patients had the second-to-lowest overall MPN-SAF TSS. The majority of patients (62.7%) had disease duration <3 years. Subjects in this group were predominantly female (61.7%) and had intermediate risk (54.9%). This cluster had the highest prevalence of anemia (36.3%). Patients also had the lowest rate of prior thrombosis (17.5%).
Cluster 3.
Moderate-II ET (n = 155, 25%). This cluster was characterized by high MPN-SAF symptom scores related to fatigue (mean 5.4) and insomnia (mean 3.5). The majority of patients (55.4%) had disease duration <3 years. Patients were primarily of low (40.8%) or intermediate (45%) risk. This cluster was the least likely to have a laboratory abnormality (23.1%) including anemia (19.8%) and leukopenia (1.7%).
Cluster 4.
High-I ET (n = 55, 9%). Cluster 4 has the highest level of fatigue (mean 7.0), along with a high level of complaints of sexual dysfunction (mean 6.8). This cluster also had the highest percentage of patients in the high IPSET risk score category (24.2%) among ET clusters. The majority of patients (52.6%) had disease duration of 3 years or more. This cluster also had the second highest MPN-SAF TSS. This cluster also had the highest level of anemia (33.3%) and greatest spleen size (mean 1.2 cm below the left costal margin).
Cluster 5.
High-II ET (n = 68, 11%). This profile demonstrated considerable symptomatology in virtually all individual MPN symptoms (all means ≥3.5 except fever [mean 1.2] and weight loss [mean 2.6]). The majority of patients (55.2%) had disease duration <3 years. Patients were represented equally between low (40.7%) and intermediate-1 (40.7%) IPSET risk score categories. This cluster was likely to have a laboratory abnormality (38.2%) including leukopenia (9.1%) and thrombocytopenia (5.5%), along with prior thrombosis (27.7%), prior hemorrhage (11.9%), and the need for red blood cell transfusions (2.9%).
PV symptom clusters
Subject demographic and disease characteristics.
Data from 519 patients were prospectively collected and combined (Chinese 87, French 131, German 25, Italian 49, Dutch 119, English 27, Spanish 42, Swedish 39). Age (median 62 years, range 22-91) and gender (56.9% male) were typical of the disease. Overall, 28% of patients had the presence of at least one laboratory abnormality (supplemental Table 2) including anemia (20.8%), leukopenia (4.3%), and thrombocytopenia (7.5%).
Cluster overview.
Five clusters were identified. Distribution of gender, language, laboratory abnormalities, spleen size, and leukopenia varied significantly across clusters (P < .05; Table 1 and supplemental Table 2). MPN-SAF TSS varied significantly among clusters (P < .001; Figure 1B). A heatmap showing variability of symptoms across clusters is presented in Figure 2B (also see supplemental Figure 2 for mean symptom scores by cluster). No significant association was noted between PV risk group and clusters (P = .87). No association was noted between disease duration and clusters (P = .73).
Cluster descriptions.
Cluster 1.
Mild PV (n = 253, 49%). In this largest cluster, individual symptom scores remained relatively low (all means ≤1.5 except fatigue [mean 2.5]). The majority (56%) of patients had disease duration of 3 years or more. Subjects were mostly male (58.9%). Most patients were in the low (36.5%) or intermediate-1 (34%) risk category. This cluster also had the lowest number of patients with a laboratory abnormality (21.3%) including anemia (15.8%) and leukopenia (1.5%). These patients also had the smallest spleen size (mean 1.7 cm) and the lowest rate of prior hemorrhage (4.3%) of all clusters.
Cluster 2.
Moderate-1 PV (n = 118, 23%). This cluster was typified by the predominant complaint of sexual dysfunction (mean 7.2) followed by fatigue (mean 4.7) and insomnia (mean 3.6), with the second to lowest mean MPN-SAF TSS. The majority of patients (62.5%) had disease duration of 3 years or more. Subjects in this cluster were predominantly male (65.8%) and were equitably dispersed among the PV risk categories of low (32.6%), intermediate-1 (34.9%), and intermediate-2 (30.2%) risk. This cluster had the largest percentage of patients with a laboratory abnormality (41.9%) including anemia (29.1%), thrombocytopenia (11.6%), and leukopenia (10.5%). They also had the highest percentage of patients with a prior splenectomy (1.6%).
Cluster 3.
Moderate-2 PV (n = 62, 12%). This cluster was characterized by high MPN-SAF scores in cytokine-related symptoms including fatigue (mean 6.9) and pruritus (mean 6.9). The majority of patients (68.4%) had disease duration of 3 years or more. Patients in this cluster were of nearly equal gender distribution (48.4% male) and the majority of patients were in the low PV risk category (36.8%). The cluster had the second to lowest percentage of patients with a laboratory abnormality (25.6%) including anemia (18.4%) and leukopenia (2.6%). These patients also had the highest rate of prior hemorrhage (14.8%) among the clusters.
Cluster 4.
High-I PV (n = 59, 11%). Cluster 4 is characterized by a higher proportion of abdominal-related symptoms including abdominal discomfort (mean 4.6), abdominal pain (mean 4.2), and early satiety (mean 5.1) in addition to high fatigue (mean 7.0) and insomnia (mean 5.5). Unlike clusters 1, 2, and 3, the majority of these patients had disease duration <3 years (61.1%). Patients were of equal gender distribution (49.2% male), and the majority of patients were in the low (47.1%) or intermediate-2 (32.4%) PV risk category. A high percentage of patients had anemia (29.4%). This cluster demonstrated the lowest proportion of patients with prior thrombosis (19.3%) and the highest proportion requiring red blood cell transfusions (3.4%).
Cluster 5.
High-II PV (n = 27, 5%). In the smallest cluster (n = 27), patients were predominantly female (63%) and comprised the PV risk category Intermediate-1 (45.5%). No patients had thrombocytopenia, but the cluster did demonstrate the highest rate of prior thrombosis (34.8%). The rate of anemia was 27.3%. This profile was characterized by high levels of all individual MPN-SAF symptoms (all means ≥5.4), with the exceptions of fever (mean 2.26) and weight loss (mean 3.1), and is notable for the highest mean MPN-SAF TSS of all PV clusters. The majority of patients (60.0%) had disease duration of 3 years or more.
MF symptom clusters
Subject demographic and disease characteristics.
Data from 329 prospectively enrolled persons with MF were collected (Chinese 102, French 54, German 19, Italian 22, Dutch 45, English 51, Spanish 29, Swedish 7), including 223 PMF, 67 post-ET MF, and 39 post-PV MF patients. Participants were of typical age (median 60 years, range 26-87) and gender for MPN disease (52.6% male).
Cluster overview.
Among all participants, 4 symptom clusters were identified. Among the clusters, disease features including leukopenia, thrombocytopenia, and enlarged spleen varied significantly (P < .05; Table 1 and supplemental Table 3). MPN-SAF TSS also varied significantly among clusters (P < .001; Figure 1C). A heatmap showing variability of symptoms across clusters is presented in Figure 2C (also see supplemental Figure 2 for mean symptom scores by cluster). DIPSS risk was associated with cluster assignment (P = .001) with the proportion of patients with intermediate-2 or high-risk classification increasing from 20.5% in the first cluster to 66.7% in the last cluster (when clusters are arranged by mean MPN-SAF TSS). Longer MF duration trended toward higher cluster as well (P = .06), although association between MPN duration and cluster was not statistically significant (P = .15).
Cluster descriptions.
Cluster 1.
Mild MF (n = 150, 46% [69.3% PMF, 20% post-ET MF, 10.7% post-PV MF]). The largest of all MF clusters, Cluster 1 was characterized by fatigue-dominant complaints (mean 3.1) in the setting of the lowest overall MPN-SAF TSS, the shortest MF disease duration (61.3% <3 years), and the highest proportion of males (58.7%). The majority of patients in this cluster were DIPSS intermediate-1 risk (46.2%), followed by low risk (33.3%). Individuals in this group had the lowest prevalence of a laboratory abnormality (64.6% total; anemia, 55.6%; thrombocytopenia, 19.8%). In addition, these individuals had the lowest proportion of clinical deficiencies including splenomegaly (mean 6.0 cm below the left costal margin), need for red blood cell transfusions (20.4%), prior thrombosis (8.8%), and prior hemorrhage (4.7%). Interestingly, individuals in this group were most likely to have had a prior splenectomy (5.8%).
Cluster 2.
Moderate-I MF (n = 105, 32% [65% PMF, 20% post-ET MF, 15% post-PV MF]). Cluster 2 was the second largest cluster. Patients had the longest MF disease duration of all clusters (mean 6.7 years), with 50% having disease duration <3 years. The majority of patients in this cluster were DIPSS intermediate-1 risk (54.2%), followed by intermediate-2 risk (23.7%). Subjects had a moderate rate of laboratory abnormalities (67.4%; anemia, 56.2%; thrombocytopenia 34.5%) but a high severity of fatigue (mean 6.1), sexual dysfunction (mean 6.0), sad mood (mean 3.6), and inactivity (mean 4.0). Transfusion dependency was present in 21% of the sample, and this cluster also had the largest spleen size (mean 8.7 cm below the left costal margin).
Cluster 3.
Moderate-II MF (n = 53, 16% [64.2% PMF, 24.5% post-ET MF, 11.3% post-PV MF]). The majority of patients (70.6%) had MF disease duration of 3 years or more, with a mean duration of 4.8 years. This cluster had the highest percentage of intermediate-1 risk patients (63.6%) of all clusters. Less than 5% of cluster 3 patients were low or high risk. Cytopenias were intermediate (68.4%; anemia, 65.8%; thrombocytopenia, 27%). Patients in this cluster had many cognitive and nighttime-related complaints including sexual difficulties (mean 6.4), night sweats (mean 6.1), insomnia (mean 5.4), and concentration problems (mean 5.6). In addition, individuals in this cluster had the lowest rate of leukopenia (7.9%), the second smallest spleen size (mean 6.3 cm), and low rates of prior thrombosis (9.6%), prior hemorrhage (7.8%), and requirement for red blood cell transfusions (21.2%). No patients had a history of splenectomy.
Cluster 4.
High MF (n = 21; 6% [80.1% PMF, 14.2% post-ET MF, 4.8% post-PV MF]). Cluster 4 was the most symptomatic cohort with the highest proportion of subjects with PMF. Mean MF disease duration was higher than most clusters (6.5 years) but less than that observed in cluster 2. This cluster had the highest percentage of DIPSS high-risk patients (33.3%) and no patients were low risk. This cluster had lower levels of end-organ complaints including abdominal pain (mean 4.8), cough (mean 5.7), and headaches (mean 4.1) compared with other symptoms. Symptoms including fatigue (mean 8.0), sexual difficulties (mean 8.9), sad mood (mean 7.8), and insomnia (mean 8.0) were predominant. Subjects also had the highest prevalence of prior thrombosis (28.6%), prior hemorrhage (14.3%), and transfusions (42.9%). In addition, this cohort had the largest prevalence of laboratory abnormalities (76.5%), with frequent anemia (70.6%), thrombocytopenia (70.6%), and leukopenia (41.2%). No subjects had a history of prior splenectomy.
Discussion
MPNs including MF, PV, and ET can be grouped into specific profiles with similar symptomology and physical and laboratory findings. Our previous studies have indicated that symptom burden among MPN patients is severe and heterogeneous within disease subtypes despite active treatment with standard therapies.3 However, the question of whether the heterogeneity observed in each MPN subtype was attributable to the existence of unique symptom clusters remained unknown. This study represents the first examination of these differences in symptom burden by cluster analysis.
As previously described, significant heterogeneity was observed both between and within MPN subtypes and likely originates from variances in biological activity of disease, genetic mutations, and cultural influences present within the MPN population. In addition to JAK2V617F, which occurs at a frequency between 55% and 90% in MPNs, mutations including MPL,13 EZH2,14 ASXL1,15 IDH1/2,16 TET2,17 CBL,18 IKZF1,19 and p5320 have been identified as contributing to cellular deregulation. Such mutations have been found to affect hematopoietic response and cytokine signaling, which likely results in many of the variances observed in laboratory and physical examination findings. In myelofibrosis, association existed between clusters and DIPSS risk groups. However, significant symptom burden was also detected within low and intermediate-1 risk disease, suggesting that the phenotypic heterogeneity observed within MPNs is not solely a surrogate for disease prognosis, as currently assessed by MPN prognostic scores, and that stages within each MPN subtype may exist as a combination of assessed symptomatic burden and prognosis. The absence of linear chronological progression between symptoms and risk scores within ET and PV further support the existence of biological subsets within MPN subtypes and suggest that current risk scores cannot be applied as surrogates of disease severity.
Analysis of laboratory abnormalities in MF revealed a direct association with symptom severity, suggesting that the roles anemia, leukopenia, and thrombocytopenia play in MPN symptom development should not be disregarded. Anemia may result in increased fatigue, and thrombocytopenia may reflect platelet sequestration within the spleen, which may secondarily result in abdominal pain/discomfort, early satiety, and weight loss. Leukopenia may cause an increased risk of infection and subsequent fatigue and fevers. The associations between symptoms and laboratory abnormalities in PV and ET were less robust. This can be explained by the decreased prevalence and severity of laboratory abnormalities compared with MF. In addition, this study included post-PV and post-ET MF within the MF analysis, which represent later and more symptomatic stages of ET and PV. The correlation between decreased splenomegaly and minimal cytopenias in MF and PV cluster 1 is consistent with previous findings that splenic sequestration can lead to anemia and thrombocytopenia.
Cluster 2 in both ET and PV was dominated by significant complaints of sexual dysfunction. Uniquely, these 2 clusters were expressed in both Eastern and Western language groups and differed between males and females (PV = Dutch, M > F; ET = Chinese, F > M). These differences may originate from the prevalence of each gender surveyed within the MPN subgroup (M > F in PV, F > M in ET). The pathophysiology behind sexual complaints is likely related to both microvascular and macrovascular pathology, along with elevated cytokines that inhibit autoregulatory function inherent to reproductive processes. Intriguingly, cluster 2 in ET had the lowest history of prior thrombosis, suggesting that thrombocytosis and thrombosis may play a lesser role in the development of sexual dysfunction than has been historically speculated. Differences in the prevalence of specific languages between clusters were also observed (insignificant for MF, P < .001 for ET/PV clusters). It remains to be determined whether the increased expression of certain symptoms within language groups reflects cultural values or phenotypic variations of the same disease process as a result of geographical segregation.
Limitations to this study include the possibility of inappropriate categorization of early MF patients into the ET and PV cohorts. As noted in supplemental Tables 2 and 3, a subset of PV and ET patients demonstrated hemoglobin levels <11 g/dL and/or transfusion dependency. The anemia in these populations may stem from a variety of sources including other medical comorbidities and do not necessarily infer miscategorization. Given the large number of patients and international nature of the study, the primary authors did not personally review bone marrow histology, and individual contributing institutions were required to follow standard-of-care diagnostic techniques in determining formal diagnosis. As previously described, the majority of ET and PV patients were receiving concurrent therapy for their MPN disorders, likely contributing to the development of anemia. No statistical differences were noted between ET and PV clusters when treatment history was analyzed. In addition, the number of ET and PV patients with anemia far exceeds the number expected to transform to MF from the time of bone marrow biopsy to study initiation. Patient characteristics (for all combined clusters) within each MPN category including patient demographics, examination findings, and MPN history are consistent with the known literature on these topics, further supporting the limited impact of miscategorization. This study is also notable for having a limited number of patients within MF Cluster 4 and PV Cluster 5. As we identified, the MF symptom clusters correlated with DIPSS risk scores, and thus the low number of patients in MF cluster 4 may be a reflection of disease severity and higher mortality rate, hence limiting population size. The low number of patients in the highly symptomatic PV cluster 5 is congruent with current literature suggesting a limited number of PV patients within the community who describe a high symptom burden.
Within the past decade, investigations of pharmacotherapies capable of ameliorating MPN symptoms have uncovered a variety of compounds demonstrating efficacy in improving symptoms, quality of life, and overall prognosis. JAK2 inhibitors are among those therapies that have been approved for the treatment of MPN-related symptoms. Ruxolitinib was the JAK1/JAK2 inhibitor to first gain Food and Drug Administration approval in November 2011 after its efficacy was demonstrated in 2 randomized controlled studies, The Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment Trials (COMFORT-I and COMFORT-II). In both investigations, ruxolitinib was compared against placebo (COMFORT-I)21 or best available therapy (COMFORT-II)22 in intermediate and high-risk MF and demonstrated efficacy in reducing splenic size, improving symptoms, and imparting survival advantage. Similarly, evaluation of ruxolitinib in PV has shown significant reduction in splenic length, leukocytosis, and JAK2 burden.23 In ET, improvements in thrombocytosis and splenomegaly were noted.23 Other JAK2 inhibitors including CYT387,24 pactritinib (SB1518),25 SAR302503 (TG101348),26 and CEP701 (Lestaurtinib)27 have documented similar results. Ruxolitinib is currently approved only for MF patients with intermediate and high-risk disease. However, the results of this study demonstrate that even low-risk MF populations may have significant symptom burden and that these symptoms do not necessarily follow linear chronological progression across DIPSS risk categories. Results were similarly seen within ET and PV populations. Given this information, broader therapeutic application of these and other novel agents to lower-risk MPN populations is a subject deserving of ongoing investigation.
In conclusion, our cluster analysis suggests that heterogeneic phenotypes exist within MPN subgroups and that these clusters are not direct surrogates for prognostic scores. Significant symptomatic burden may be observed in patients with low and intermediate-risk scores. Recognition of such subsets may affect the choice, timing, and goals of therapy, as well as underscore the role for serial assessment of symptom burden in a clinical setting.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
Presented at the 2013 European Hematology Association meeting (Stockholm, Sweden).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This article was produced through collaborative efforts by the MPN International Quality of Life Study Group. Study group participants are listed in Appendix 1 in the supplemental material online. Additional contributors to the authorship of this article are listed in Appendix 2. The authors thank their French collaborators for their contributions to this project (listed in Appendix 3).
Authorship
Contribution: R.A.M. designed and supervised the study; H.L.G., R.M.S., A.C.D., and R.A.M. interpreted results and drafted the paper; A.C.D. performed statistical analysis; and all remaining authors were instrumental in data acquisition and article review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruben A. Mesa, Mayo Clinic, 13400 E Shea Blvd, Scottsdale, AZ 85259; e-mail: mesa.ruben@mayo.edu.
References
Author notes
H.L.G. and R.M.S. contributed equally to this study.