Abstract
Our understanding of the genetic basis of myeloproliferative neoplasms began in 2005, when the JAK2 (V617F) mutation was identified in polycythemia vera, essential thrombocythemia, and primary myelofibrosis. JAK2 exon 12 and MPL exon 10 mutations were then detected in subsets of patients, and subclonal driver mutations in other genes were found to be associated with disease progression. Recently, somatic mutations in the gene CALR, encoding calreticulin, have been found in most patients with essential thrombocythemia or primary myelofibrosis with nonmutated JAK2 and MPL. The JAK-STAT pathway appears to be activated in all myeloproliferative neoplasms, regardless of founding driver mutations. These latter, however, have different effects on clinical course and outcomes. Thus, evaluation of JAK2, MPL, and CALR mutation status is important not only for diagnosis but also for prognostication. These genetic data should now also be considered in designing clinical trials.
Introduction
In the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues, Philadelphia-negative myeloproliferative neoplasms include essential thrombocythemia, polycythemia vera, and primary myelofibrosis.1
The identification of JAK2 and MPL as mutant driver genes in myeloproliferative neoplasms
Our knowledge of the genetic basis of these disorders began in 2005, when a unique base substitution in JAK2, the gene encoding Janus kinase 2, was found in patients with polycythemia vera, essential thrombocythemia, and primary myelofibrosis.2-5 The background of our investigations was the previous finding that copy-neutral loss of heterozygosity of chromosome 9p (9pLOH) is the most common chromosomal abnormality in polycythemia vera6 : JAK2 maps on chromosome 9p24, inside the minimal 9pLOH region.3
Using a quantitative polymerase chain reaction–based allelic discrimination assay with a sensitivity of less than 1%, JAK2 (V617F) can be detected in about in 95% of patients with polycythemia vera and in 60% to 65% of those with essential thrombocythemia or primary myelofibrosis (Table 1). The remaining 5% of patients with polycythemia vera carry a somatic mutation of JAK2 exon 12.7,8 Their hematologic phenotype is mainly an isolated erythrocytosis, but their outcomes are similar to those of patients with JAK2 (V617F).9 Therefore, polycythemia vera is a condition almost exclusively associated with gain-of-function mutations of JAK2.
Current WHO classification of Philadelphia-negative myeloproliferative neoplasms, their genetic basis, and clinical relevance of genetic lesions
| Nosologic entities of the WHO classification and founding driver genes and their somatic mutations . | Phenotypic switch and disease progression . | Diagnostic and prognostic implications of genetic lesions . |
|---|---|---|
| Polycythemia vera | ||
| JAK2 (V617F) in about 95% of patients JAK2 exon 12 mutations in about 5% of patients | Patients with polycythemia vera may progress to myelofibrosis, and patients with myelofibrosis evolving from polycythemia vera have a mutant allele burden close to 100%, unambiguously indicating the presence of a dominant clone of cells that are homozygous for the mutation; the occurrence of subclonal driver mutations may lead to leukemic transformation | Polycythemia vera is a condition almost exclusively associated with gain-of-function mutations of JAK2. The combination of erythrocytosis (ie, Hb >17 g/dL in males, and >16 g/dL in females) and JAK2 mutation, in the absence of bone marrow fibrosis, may be considered diagnostic of polycythemia vera |
| Essential thrombocythemia | ||
| JAK2 (V617F) in about 60%-65% of cases MPL exon 10 mutations in about 5% of cases CALR exon 9 indels in about 20%-25% of cases About 5%-10% of patients do not carry any of the above somatic mutations | Transition from heterozygosity to homozygosity for JAK2 (V617F) may be associated with progression from essential thrombocythemia to polycythemia vera; all patients with essential thrombocythemia carrying a somatic mutation in JAK2, MPL, or CARL may progress to secondary myelofibrosis, while the occurrence of subclonal driver mutations may lead to leukemic transformation | The combination of thrombocytosis (>400 × 109/L) and JAK2 (V617F), or an MPL exon 10 mutation, or a CALR exon 9 indel in the absence of bone marrow fibrosis and erythrocytosis may be considered as diagnostic of essential thrombocythemia; patients with a CALR exon 9 mutation have very high platelet counts but a relatively low risk of thrombosis (much lower than that of patients with the JAK2 mutation) |
| Primary myelofibrosis | ||
| JAK2 (V617F) in about 60%-65% of cases MPL exon 10 mutations in about 5% of cases CALR exon 9 indels in about 20%-25% of cases About 5%-10% of patients do not carry any of the above somatic mutations | The occurrence of subclonal driver mutations in genes like ASXL1, DNMT3A, EZH2, IDH1/IDH2, SRSF2, or TP53 is associated with worse clinical course and higher risk of progression to blast phase or leukemic transformation; somatic mutation of ASXL1 appears to have the most detrimental effect | The diagnosis of primary myelofibrosis should now include as a major criterion not only the presence of JAK2 (V617F) or an MPL exon 10 mutation, but also that of a CALR exon 9 indel; patients with myelofibrosis carrying a CALR exon 9 indel have an indolent clinical course, and a far better survival than those carrying JAK2 (V617F) or an MPL mutation, or especially those with nonmutated JAK2, CALR, and MPL; these latter have a very poor prognosis and a particularly high risk of leukemic transformation |
| Nosologic entities of the WHO classification and founding driver genes and their somatic mutations . | Phenotypic switch and disease progression . | Diagnostic and prognostic implications of genetic lesions . |
|---|---|---|
| Polycythemia vera | ||
| JAK2 (V617F) in about 95% of patients JAK2 exon 12 mutations in about 5% of patients | Patients with polycythemia vera may progress to myelofibrosis, and patients with myelofibrosis evolving from polycythemia vera have a mutant allele burden close to 100%, unambiguously indicating the presence of a dominant clone of cells that are homozygous for the mutation; the occurrence of subclonal driver mutations may lead to leukemic transformation | Polycythemia vera is a condition almost exclusively associated with gain-of-function mutations of JAK2. The combination of erythrocytosis (ie, Hb >17 g/dL in males, and >16 g/dL in females) and JAK2 mutation, in the absence of bone marrow fibrosis, may be considered diagnostic of polycythemia vera |
| Essential thrombocythemia | ||
| JAK2 (V617F) in about 60%-65% of cases MPL exon 10 mutations in about 5% of cases CALR exon 9 indels in about 20%-25% of cases About 5%-10% of patients do not carry any of the above somatic mutations | Transition from heterozygosity to homozygosity for JAK2 (V617F) may be associated with progression from essential thrombocythemia to polycythemia vera; all patients with essential thrombocythemia carrying a somatic mutation in JAK2, MPL, or CARL may progress to secondary myelofibrosis, while the occurrence of subclonal driver mutations may lead to leukemic transformation | The combination of thrombocytosis (>400 × 109/L) and JAK2 (V617F), or an MPL exon 10 mutation, or a CALR exon 9 indel in the absence of bone marrow fibrosis and erythrocytosis may be considered as diagnostic of essential thrombocythemia; patients with a CALR exon 9 mutation have very high platelet counts but a relatively low risk of thrombosis (much lower than that of patients with the JAK2 mutation) |
| Primary myelofibrosis | ||
| JAK2 (V617F) in about 60%-65% of cases MPL exon 10 mutations in about 5% of cases CALR exon 9 indels in about 20%-25% of cases About 5%-10% of patients do not carry any of the above somatic mutations | The occurrence of subclonal driver mutations in genes like ASXL1, DNMT3A, EZH2, IDH1/IDH2, SRSF2, or TP53 is associated with worse clinical course and higher risk of progression to blast phase or leukemic transformation; somatic mutation of ASXL1 appears to have the most detrimental effect | The diagnosis of primary myelofibrosis should now include as a major criterion not only the presence of JAK2 (V617F) or an MPL exon 10 mutation, but also that of a CALR exon 9 indel; patients with myelofibrosis carrying a CALR exon 9 indel have an indolent clinical course, and a far better survival than those carrying JAK2 (V617F) or an MPL mutation, or especially those with nonmutated JAK2, CALR, and MPL; these latter have a very poor prognosis and a particularly high risk of leukemic transformation |
The genetic basis of the myeloproliferative neoplasms defined as essential thrombocythemia or primary myelofibrosis is more heterogeneous. Soon after the discovery of JAK2 (V617F), Pikman et al10 identified MPL (W515L) as a novel somatic activating mutation in myelofibrosis. Subsequent studies showed that somatic mutations of MPL exon 10 (mainly involving codon W515) are found in about 5% of patients with essential thrombocythemia or primary myelofibrosis.11-13
Somatic mutations of calreticulin in essential thrombocythemia and primary myelofibrosis
In the last few years, several groups have tried to define the genetic basis of essential thrombocythemia or primary myelofibrosis with nonmutated JAK2 and MPL. We analyzed genomic DNA from granulocytes and T-lymphocytes from 6 patients with primary myelofibrosis, using whole-exome sequencing. Despite problems generated by a repetitive element in the affected genomic region, this approach led us to the identification of somatic mutations of CALR, the gene encoding calreticulin, in all 6 patients.14 At the same time, Nangalia et al15 performed whole-exome sequencing in 151 patients with myeloproliferative neoplasms and found CALR exon 9 mutations in most subjects with nonmutated JAK2.
Calreticulin mutations are mutually exclusive with mutations in both JAK2 and MPL and are found in about 20% to 25% of all patients with essential thrombocythemia or primary myelofibrosis,14,15 indicating that CALR is the second most frequently mutated gene in myeloproliferative neoplasms. All CALR mutations are insertions or deletions resulting in a frameshift, and cluster in the last exon (exon 9) of the gene. Thus far, more than 50 different types of mutations in CALR have been detected (M.C., unpublished data, April 18, 2014), but a 52-bp deletion (type 1 mutation) and a 5-bp insertion (type 2) are the most frequent types, overall being found in more than 80% of all patients with mutant CALR.
The C-terminal region of wild-type calreticulin includes a negatively charged calcium-binding domain and the endoplasmic reticulum retention motif (KDEL amino acid sequence) at the end. CALR mutations generate a novel C-terminus of the mutated protein, in which the negatively charged amino acids are replaced by neutral and positively charged amino acids. In addition, the endoplasmic reticulum retention motif is lost in the mutant variants. This suggests that both impaired calcium-binding activity and cellular dislocation may play a role in the abnormal proliferation of cells expressing a mutant calreticulin.14 Our in vitro experiments in interleukin 3–dependent Ba/F3 cells showed that overexpression of type 1 CALR mutation (the 52-bp deletion) led to interleukin 3–independent growth and hypersensitivity to interleukin 3, and that JAK-STAT signaling was involved in these abnormal processes.14
Clinical significance of CALR mutations
Outside essential thrombocythemia and primary myelofibrosis, we found somatic CALR mutations only in patients with the myelodysplastic/myeloproliferative neoplasm defined as refractory anemia with ring sideroblasts associated with marked thrombocytosis,16 and all of them had wild-type JAK2 and MPL.14 This observation is important because it defines a strict relationship between mutant CALR and thrombocytosis phenotype within myeloid neoplasms, indicating that calreticulin mutations primarily affect the biology of megakaryocytes.
To define the clinical effect of the different somatic mutations, we studied the clinical features of essential thrombocythemia according to JAK2 or CALR mutation status and in relation to those of polycythemia vera.17 The risk for thrombosis was similar in JAK2-mutant essential thrombocythemia and in polycythemia vera but was much lower in CALR-mutant essential thrombocythemia. In addition, although the cumulative risk for progression to polycythemia vera was 29% at 15 years in JAK2-mutant essential thrombocythemia, no polycythemic transformation was observed in CALR-mutant essential thrombocythemia. These observations are consistent with the notion that JAK2 (V617F)-mutant essential thrombocythemia and polycythemia vera are different phenotypes in the evolution of a single neoplasm, whereas CALR-mutant essential thrombocythemia is a distinct nosologic entity. More generally, our study17 and a companion one18 indicate that CALR-mutant essential thrombocythemia is a myeloproliferative neoplasm that affects relatively young individuals and is characterized by markedly elevated platelet count but relatively low thrombotic risk.
In our original study, we compared the effect of JAK2, MPL, and CALR mutation on survival of patients with primary myelofibrosis.14 Patients with CALR mutation had better overall survival than those with JAK2 or MPL mutation, even after adjustment for age. This has been recently confirmed by Tefferi et al,19 who, in addition, found that patients with primary myelofibrosis and nonmutated JAK2, MPL, and CALR have a very poor prognosis. Findings of an ongoing collaborative study indeed show that these latter patients have very poor survival, with a particularly high risk for leukemic evolution (M.C., unpublished data, April 18, 2014).
Founding mutations, genetic predisposition, and subclonal evolution in myeloproliferative neoplasms
A driver mutation is a genome abnormality that causes a selective advantage in a cell with capacity for self-renewal, leading to formation of a clone of mutated cells.20 Driver mutations can be subdivided into founding or initiating mutations, which give rise to the initial clone of a malignancy, and subclonal or cooperating mutations, which occur in a cell of an already established clone and generate subclones carrying both the founding and the newly acquired mutation. Subclonal mutations are commonly associated with disease progression.
In myeloproliferative neoplasms, somatic mutations of JAK2, MPL, and CALR behave as founding driver mutations responsible for the myeloproliferative phenotype. This does not mean these mutations are necessarily the first somatic event leading to the development of these disorders. In our original paper on JAK2 (V617F),3 we considered the possibility that this mutation occurred as a second event. Indeed, Delhommeau et al21 later found that a somatic mutation of TET2 can precede JAK2 (V617F) in hematopoietic stem cells of patients with myeloproliferative neoplasm, an observation that has been recently confirmed by Lundberg et al.22 Furthermore, Busque et al23 have elegantly shown that TET2 mutations can be found in individuals with clonal hematopoiesis without any hematologic phenotype. The acquisition of JAK2 (V617F) in an individual with mutant TET2-driven clonal hematopoiesis would give rise to a myeloproliferative phenotype.
Two types of genetic predisposition to develop a myeloproliferative neoplasm have been identified so far.24 The first type is population-level genetic predisposition, and the representative example is a haplotype referred to as 46/1 or GGCC of the JAK2 gene itself, which predisposes to the acquisition of JAK2 and MPL mutations.25-27 The second type is familial predisposition, which has been observed in pedigrees with 2 or more individuals affected by myeloproliferative neoplasms.28-30 JAK2 and MPL mutations were shown to be somatically acquired events in these familial cases, as they are in sporadic patients.29,30 More recently, we found that a significant proportion of familial cases of essential thrombocythemia or primary myelofibrosis with nonmutated JAK2 and MPL carry a somatic mutation of CALR.31 The Mendelian genetic basis of this predisposition is largely unknown at present, although we have identified a potential mechanism in some families. In fact, we detected germline mutations in the gene RBBP6, which might predispose to the development of myeloproliferative neoplasms.32
Subclonal evolution is the process by which the founding malignant clone generates subclones through the acquisition of additional driver mutations.20 In myeloproliferative neoplasms, an increased number of chromosomal lesions are significantly associated with disease progression and leukemic transformation.33 Reported subclonal mutations include those in genes such as ASXL1,34 EZH2,35 CBL,36 IDH1/IDH2,37 TP53,38 and SRSF2.39 In primary myelofibrosis, somatic mutations of ASXL1, SRSF2, and EZH2 have been found to be independently associated with poor survival and high risk for leukemic evolution.39 In the recent study by Lundberg et al,22 the presence of at least a subclonal mutation significantly reduced overall survival and increased the risk for leukemic transformation in patients with myeloproliferative neoplasms. Overall, somatic mutation of ASXL1 appears to have the most detrimental effect and represents a reliable predictor of poor outcome in primary myelofibrosis.39
JAK-STAT pathway activation and the pivotal role of the megakaryocyte in the pathophysiology of myeloproliferative neoplasms
Findings of a recent study of gene expression profiling indicate that activated JAK2 signaling is seen in all patients with myeloproliferative neoplasms, regardless of founding driver mutation and clinical diagnosis.40 These findings are consistent with the observation that patients with primary myelofibrosis may respond to JAK inhibitors, regardless of their genotype.41
Various observations suggest that megakaryocytes play a major role in the pathophysiology of myeloproliferative neoplasms, in particular by mediating bone marrow fibrosis.42 In primary myelofibrosis, CD34-positive cells have an increased ability to generate megakaryocytes.43 In addition, driver mutations appear to alter megakaryocyte differentiation, migratory ability, and proplatelet formation, leading to increased platelet production.44,45 In a knock-in mouse model of essential thrombocythemia,45 JAK2 (V617F) was found to lead to intrinsic changes in both megakaryocyte and platelet biology.
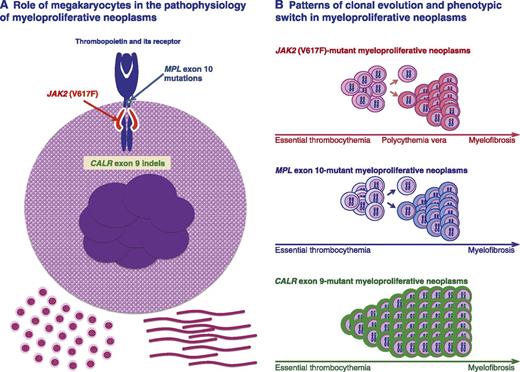
A recent study clearly shows that under normal conditions, megakaryocytes contribute to the bone marrow matrix environment by expressing fibronectin, type IV collagen, and laminin.46 A unifying model of the pathophysiology of myeloproliferative neoplasms implies that the founding driver mutation activates the JAK-STAT pathway in megakaryocytes, resulting in thrombocytosis initially and in bone marrow fibrosis in the long term (Figure 1A).
Role of megakaryocytes in the pathophysiology of myeloproliferative neoplasms, and patterns of clonal evolution and phenotypic switch in these disorders. (A) Megakaryocyte carrying a somatic mutation in JAK2, MPL, or CALR, all representing gain-of-function mutations of thrombopoietin signaling through its receptor. How a mutant calreticulin involves gain of thrombopoietin signaling is currently under investigation, but the available evidence strongly supports this notion. Enhanced thrombopoietin signaling results in excessive platelet production and, likely, also in abnormal megakaryocyte contribution to the bone marrow matrix environment, leading to thrombocytosis initially and to bone marrow fibrosis in the long term. (B) Patterns of clonal evolution and phenotypic switch in different genotypic entities. Clonal evolution of JAK2 (V617F)-mutant myeloproliferative neoplasms is associated with acquired copy-neutral loss of heterozygosity of chromosome 9p, responsible for the transition from heterozygosity to homozygosity for JAK2 (V617F). This may involve a phenotypic switch from essential thrombocythemia to polycythemia vera, and in the long term, from this latter to myelofibrosis. Essential thrombocythemia may also progress directly to myelofibrosis without the intermediate stage of polycythemia vera. Clonal evolution of MPL-mutant myeloproliferative neoplasms is associated with acquired copy-neutral loss of heterozygosity of chromosome 1p, involving transition from heterozygosity to homozygosity for the MPL mutation. This molecular mechanism and/or cooperating mutations may be responsible for a phenotypic switch from essential thrombocythemia to myelofibrosis. Clonal evolution of CALR-mutant myeloproliferative neoplasms appears to be mainly associated with a progressive expansion of a mutant heterozygous clone that eventually becomes fully dominant in the bone marrow. Activation of megakaryocytes by mutant CALR and/or cooperating mutations may be responsible for a phenotypic switch from essential thrombocythemia to myelofibrosis. Within each genotypic entity, thrombocythemia is likely the initial phenotype. Transition from one phenotype to another may or may not occur, depending on several factors, and transition rates may vary considerably. According to the pathophysiologic model depicted here, myelofibrosis is a late stage in the evolution of the different myeloproliferative neoplasms.
Role of megakaryocytes in the pathophysiology of myeloproliferative neoplasms, and patterns of clonal evolution and phenotypic switch in these disorders. (A) Megakaryocyte carrying a somatic mutation in JAK2, MPL, or CALR, all representing gain-of-function mutations of thrombopoietin signaling through its receptor. How a mutant calreticulin involves gain of thrombopoietin signaling is currently under investigation, but the available evidence strongly supports this notion. Enhanced thrombopoietin signaling results in excessive platelet production and, likely, also in abnormal megakaryocyte contribution to the bone marrow matrix environment, leading to thrombocytosis initially and to bone marrow fibrosis in the long term. (B) Patterns of clonal evolution and phenotypic switch in different genotypic entities. Clonal evolution of JAK2 (V617F)-mutant myeloproliferative neoplasms is associated with acquired copy-neutral loss of heterozygosity of chromosome 9p, responsible for the transition from heterozygosity to homozygosity for JAK2 (V617F). This may involve a phenotypic switch from essential thrombocythemia to polycythemia vera, and in the long term, from this latter to myelofibrosis. Essential thrombocythemia may also progress directly to myelofibrosis without the intermediate stage of polycythemia vera. Clonal evolution of MPL-mutant myeloproliferative neoplasms is associated with acquired copy-neutral loss of heterozygosity of chromosome 1p, involving transition from heterozygosity to homozygosity for the MPL mutation. This molecular mechanism and/or cooperating mutations may be responsible for a phenotypic switch from essential thrombocythemia to myelofibrosis. Clonal evolution of CALR-mutant myeloproliferative neoplasms appears to be mainly associated with a progressive expansion of a mutant heterozygous clone that eventually becomes fully dominant in the bone marrow. Activation of megakaryocytes by mutant CALR and/or cooperating mutations may be responsible for a phenotypic switch from essential thrombocythemia to myelofibrosis. Within each genotypic entity, thrombocythemia is likely the initial phenotype. Transition from one phenotype to another may or may not occur, depending on several factors, and transition rates may vary considerably. According to the pathophysiologic model depicted here, myelofibrosis is a late stage in the evolution of the different myeloproliferative neoplasms.
Genotypic and phenotypic entities
Table 1 reports the current WHO classification of myeloproliferative neoplasms and summarizes the clinical relevance of founding driver mutations. There is no question that the diagnostic approach to myeloproliferative neoplasms must now include evaluation of the mutation status of JAK2, MPL, and CALR. Moreover, this information is also extremely useful for prognostication and individual risk assessment.
The definition of the genomic landscape of myeloproliferative neoplasms has more general implications. In our opinion, the genotype/phenotype relationships established so far justify a genetic classification of these disorders, as schematically represented in Figure 1B.
It is well established that JAK2 (V617F)-mutant essential thrombocythemia may transform into polycythemia vera and that this latter may progress to secondary myelofibrosis (Figure 1B). Campbell et al47 elegantly showed that JAK2 (V617F)-mutant essential thrombocythemia and polycythemia vera form a biological continuum, with the degree of erythrocytosis determined by physiological or genetic modifiers. The JAK2 (V617F) mutant allele burden represents a major factor in causing the different phenotypes, a notion that is supported by several observations. Acquired copy-neutral loss of heterozygosity of chromosome 9p is responsible for the transition from heterozygosity to homozygosity for the JAK2 (V617F) mutation, and in turn, for higher mutant allele burden.3 The JAK2 (V617F) mutant allele burden is higher in polycythemia vera than in essential thrombocythemia and is very high in myelofibrosis evolving from polycythemia vera.17 In a knock-in mouse model, Li et al48 have recently demonstrated that homozygosity for human JAK2 (V617F) results in a phenotypic switch from an essential thrombocythemia-like to a polycythemia vera–like phenotype. Similar observations had been previously made by Tiedt et al.49
MPL exon 10 mutations are found in essential thrombocythemia and primary myelofibrosis, and most of them involve a W515 substitution.13 Defour et al50 have recently shown that this tryptophan at the transmembrane–cytosolic junction plays a crucial role in modulating thrombopoietin receptor dimerization and activation. We previously reported that acquired copy-neutral loss of heterozygosity of chromosome 1p, involving transition from heterozygosity to homozygosity for the MPL mutation, is a molecular mechanism associated with myelofibrotic transformation in these patients.13 This mechanism may therefore play a role in the transition from MPL exon 10–mutant essential thrombocythemia to myelofibrosis (Figure 1B).
In addition, CALR exon 9 mutations are found in essential thrombocythemia and primary myelofibrosis, but acquired copy-neutral loss of heterozygosity of chromosome 19p, involving transition from heterozygosity to homozygosity for the CALR mutation, appears to be a relatively uncommon event.17 In CALR-mutated patients, disease evolution is mainly characterized by the expansion of a heterozygous clone that becomes fully dominant in the bone marrow and activates megakaryocytes. In our original work,14 we found that the 52-bp deletion (type 1 mutation) was significantly more frequent in primary myelofibrosis than in essential thrombocythemia, suggesting it may activate megakaryocytes more strongly than other CALR indels.
Conclusions
A revision of the WHO classification of tumors of hematopoietic and lymphoid tissues is ongoing. The classification of myeloproliferative neoplasms will hopefully take into account the recent genetic advances in the field. At present, the clinical phenotype (essential thrombocythemia, polycythemia vera, or myelofibrosis) continues to be important in clinical decision-making. However, because of their clinical relevance, genetic data should be now properly considered both in clinical decision-making and in designing clinical trials.
Acknowledgments
The studies on myeloproliferative neoplasms conducted at the Department of Hematology Oncology, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, and Department of Molecular Medicine, University of Pavia, Pavia, Italy, are supported by a grant from the Associazione Italiana per la Ricerca sul Cancro (Special Program Molecular Clinical Oncology 5 per Mille, project 1005 to M.C.). Those conducted at the Center for Molecular Medicine, Austrian Academy of Sciences, Vienna, Austria, are supported by a grant from the Austrian Science Fund (FWF4702-B20 to R.K.).
Authorship
Contribution: M.C. and R.K. wrote the paper.
Conflict-of-interest disclosure: R.K. has pending patent applications on the use of calreticulin gene mutations for the diagnosis and therapeutic targeting of myeloproliferative neoplasms. The remaining author declares no competing financial interests.
Correspondence: Mario Cazzola, Department of Hematology Oncology, Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.