Key Points
BM donors have a threefold higher risk for life-threatening, serious unexpected, or chronic adverse events vs PBSC donors (0.99% vs 0.31%).
Donors receiving granulocyte colony-stimulating factor for PBSC collection had no evidence of increased risk for cancer, autoimmune illness, and stroke.
Abstract
We compared serious early and late events experienced by 2726 bone marrow (BM) and 6768 peripheral blood stem cell (PBSC) donors who underwent collection of PBSC or BM between 2004 and 2009 as part of a prospective study through the National Marrow Donor Program. Standardized FDA definitions for serious adverse events (SAEs) were used, and all events were reviewed by an independent physician panel. BM donors had an increased risk for SAEs (2.38% for BM vs 0.56% for PBSC; odds ratio [OR], 4.13; P < .001), and women were twice as likely to experience an SAE (OR for men, 0.50; P = .005). Restricting the analysis to life-threatening, unexpected, or chronic/disabling events, BM donors maintained an increased risk for SAEs (0.99% for BM vs 0.31% for PBSC; OR, 3.20; P < .001). Notably, the incidence of cancer, autoimmune illness, and thrombosis after donation was similar in BM vs PBSC donors. In addition, cancer incidence in PBSC donors was less than that reported in the general population (Surveillance, Epidemiology, and End Results Program database). In conclusion, SAEs after donation are rare but more often occurred in BM donors and women. In addition, there was no evidence of increased risk for cancer, autoimmune illness, and stroke in donors receiving granulocyte colony-stimulating factor during this period of observation.
Introduction
The recent celebration of the one millionth hematopoietic cell transplantation not only recognized a now-common procedure potentially beneficial to many individuals with cancer, hematological, or inherited disorders but also highlighted the generosity of thousands of volunteers, as approximately half of these transplant procedures were allogeneic, and thus involved harvesting bone marrow (BM) or peripheral blood stem cells (PBSCs) from related or unrelated donors. Many organizations, including the National Marrow Donor Program (NMDP), the Center for International Blood and Marrow Transplant Research, the World Marrow Donor Association, and a host of international hematopoietic cell transplant registries, are dedicated to ensuring the safety of these donors.1-3 Because the procedures of BM or PBSC collection can, on rare occasions, result in serious and/or chronic conditions that may affect the health of normal volunteers, physicians and donor center personnel have an obligation to understand risks for serious early and significant chronic complications associated with both procedures to inform prospective donors as they decide whether and how to donate.
We recently published a detailed analysis of acute toxicities and time to recovery of nearly 10 000 BM and PBSC donations performed through the NMDP between 2004 and 2009.4 This report covers issues not studied in the earlier effort. First, we compare the incidence of serious adverse events (SAEs) by donation method (marrow vs peripheral blood). Next, given the theoretical concern that short-term recombinant human granulocyte colony-stimulating factor (filgrastim) exposure might increase the rate of hematological malignancies in healthy donors, we analyze the incidence of hematologic malignancies as well as other cancers in BM donors who were not treated with granulocyte colony-stimulating factor (G-CSF) compared with G-CSF-treated PBSC donors. We then compare both of these groups with expected incidences based on Surveillance, Epidemiology, and End Results (SEER) data. Finally, we address the concern raised in the literature that G-CSF use in PBSC donation might be associated with autoimmunity or thrombosis.5-7
Given the large number of donors studied, their close monitoring by the NMDP, and extensive reporting using accurate, verified data tools,1,4,8 and the adjudication of each event by a physician panel, this report provides a definitive assessment of serious and long-term risks associated with BM and PBSC donation. When taken together with our earlier report, donors can now be more reliably counseled on the risks for acute toxicities and rare SAEs associated with donation, as well as the lack of evidence in this large cohort of an increase in the risk for cancer, autoimmune disease, or thrombosis associated with the use of G-CSF.
Methods
Study population
The study population consisted of unrelated donors from the United States whose unstimulated BM or filgrastim recombinant human G-CSF-mobilized PBSC donation was facilitated by the NMDP between January 2004 and July 2009. Only first-time hematopoietic progenitor cell donors for whom data were available from baseline to 2 days postdonation on the NMDP 700-series forms (standardized with overlapping elements for BM and PBSC donors) were included. Donors enrolled on the Bone Marrow Clinical Trials Network (BMT CTN) protocol 0201 (a randomized BM vs PBSC donor trial; n = 273 [reported separately9 ]) and rare donors who donated BM after filgrastim administration were excluded. All donors included in the study provided written informed consent for participation in Center for International Blood and Marrow Transplant Research studies approved by the NMDP institutional research board. The study was conducted in accordance with the Declaration of Helsinki. We identified 9494 individuals eligible for inclusion in this study, including 2726 BM and 6768 PBSC donors. All donors were evaluated for medical suitability, transplantation-transmissible infectious diseases, and contraindications (eg, pregnancy, autoimmune disease, history of thromboembolic disease) for BM collection or PBSC donation.
BM donation
Before the BM collection, to minimize the likelihood of needing allogeneic transfusion, donor centers were advised to collect 1 to 2 autologous blood units from the donor in relation to the marrow volume they were to donate. Marrow was collected from the donor’s posterior iliac crests in an operating room under either general or regional (spinal or epidural) anesthesia. The NMDP standards require that no more than 20 mL of marrow per kilogram donor weight be removed and recommend that duration of anesthesia should be less than 150 minutes, and duration of the collection procedure itself should be less than 120 minutes.
PBSC donation
All PBSC collections were performed according to the NMDP-sponsored and institutional review board-approved research protocol for manufacturing PBSC products, operated under an investigational new drug application with the US Food and Drug Administration (FDA). Filgrastim-mobilized PBSC collection involved subcutaneous administration of filgrastim for 4 or 5 consecutive days at a daily dose of approximately 10 µg/kg. Administered filgrastim doses were rounded to combinations of 300-µg and 480-µg vials on the basis of the donor’s total body weight, as long as protocol-defined targets of 13.3 µg/kg or less per day were not exceeded. The protocol included provisions for filgrastim dose reductions in the presence of clinically significant symptoms or thrombocytopenia. On day 5 alone, or on days 5 and 6, the donor’s PBSCs were collected by leukapheresis. Donors with preapheresis platelet counts less than 120 × 109/L on the first day of collection or 80 × 109/L on the second day of collection were required to discuss whether collection could be performed safely with an NMDP medical officer, as the goal was to avoid platelet counts less than 50 × 109/L after collection. The volume of whole blood processed was targeted to be between 12 and 24 L per collection, depending on recipient weight and/or immediate preprocedure donor blood CD34+ cell count. The protocol allowed a maximum total blood volume processed to be 24 L, whether collected over 1 or 2 days. If the PBSC product could not be collected using peripheral veins, a central venous catheter (CVC) was used. The insertion of any central catheter took place in a hospital. The subsequent apheresis procedure or procedures were then also performed in a hospital, and donors with CVCs were hospitalized between apheresis procedures.
Data collection
The BM donation processes were facilitated by 81 donor centers and 83 collection centers; the PBSC donation processes were facilitated by 76 donor centers and 98 apheresis centers. Donor centers managed donor medical evaluations, infectious disease marker testing, and filgrastim administration. They were also responsible for coordinating the donation process, monitoring the donor’s recovery, and data reporting. Collection centers performed marrow collections, assisted donor centers with data reporting, and were responsible for submitting Marrow Product Analysis Forms. Apheresis centers performed PBSC collections, coordinated donor filgrastim administration in conjunction with local donor centers as needed, assisted donor centers with data reporting, and were responsible for submitting PBSC Product Analysis Forms.
Data collection began at the time of the donor’s medical evaluation to determine suitability to donate hematopoietic progenitor cells and continued through the filgrastim injections (for PBSC donation), the collection days, and postdonation follow-up times. The development of malignancies, autoimmune diseases, and thrombosis were captured on annual follow-up forms. In addition to this routine data collection, the Stem Cell Donor AE form was designed to capture SAE and unexpected AE information from all donors. An AE is any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medical treatment or procedure, regardless of whether it is considered related to the medical treatment or procedure. Submission of this AE form to the NMDP was required as soon as possible after the report of an AE. A separate form is required for each AE that occurs. A new AE form was required to be completed when there was a change in the donor’s condition, when requested by the NMDP, or when the AE was resolved. All donors in the study were followed-up until July 2011 (BM median follow-up, 36 months, [7 days - 86 months]; PBSC median follow-up, 36 months [6 days - 89 months]).
Adjudication of SAEs
A panel of 5 physicians independent of the collection centers reviewed 451 AE forms describing 328 events that occurred in 296 (10.9%) of 2726 BM donors and 1178 forms describing 972 events that occurred in 854 (12.6%) of 6768 PBSC donors. Each event was reviewed by a minimum of 2 panel members and scored as probable, possible, or not an SAE, using the FDA definition. All events scored as probable or possible on initial screen were adjudicated by the full panel. The FDA definition for SAE was met if any of the following occurred: death, life-threatening event, unplanned inpatient hospitalization or prolongation of existing hospitalization (characterized as planned or unplanned and from anticipated or unanticipated causes), persistent (defined for this study as more than 3 months) or significant (having a major effect on the donor’s ability to function normally) disability, congenital anomaly/birth defect, or other (a significant medical event that could easily have led to one of the other events). We further characterized each event as “expected” (an AE that occurs regularly with collection procedures) or “unexpected.” If the reported event was defined as a persistent disability, we assigned a measure of severity (mild, moderate, or severe). Finally, we categorized the event according to proximal cause: for BM collection, from anesthesia, mechanical related to the operative procedure (ie, nerve/bone/tissue damage), or other/unrelated; and for PBSC collection, from G-CSF administration, the apheresis procedure, or other/unrelated.
Statistical methods
Donor and collection characteristics were quantified by product type. Variables were compared between BM and PBSC, using the Pearson χ-square test for categorical variables and the Kruskal-Wallis test for continuous variables. If a second donation occurred, all outcomes were censored at the time of second donation; for subsequent BM donations, at the time of second collection; and for subsequent peripheral blood donations, at day 1 of filgrastim administration.
Logistic regression was used to compare BM and PBSC donors for the incidence of SAE, after adjusting for donor characteristics and baseline measurements, as listed in Table 1. The effects were estimated via odds ratios (ORs). Two separate analyses were performed when unexpected hospitalizations for expected events were included in the definition of SAE and when they were excluded. Univariate probabilities of the incidence of cancers, autoimmune diseases, and thrombosis were calculated using the Kaplan-Meier estimator; the log-rank test was used for univariate comparisons between BM and PBSC donors, and the χ-square test was used for point-wise comparisons.10 A multivariable model using Poisson regression was used to compare the incidence of cancers and autoimmune diseases between BM and PBSC donors after adjusting for donor characteristics and baseline measurements. The estimated effects of each significant risk factor were given as relative risks. In all multivariate models, donation type was forced into the model, and stepwise model selection was used to determine additional donor characteristics to be included. Finally, the incidence of cancers in donors was compared with the expected rates in the general population, using standardized incidence ratio.
Characteristics of first-time NMDP donors who donated a stem cell product between January 2004 and July 2009
| Characteristic . | BM . | PBSC . | P . |
|---|---|---|---|
| N (%) . | N (%) . | ||
| Number of donors | 2726 | 6768 | |
| Number of donor centers | 81 | 76 | |
| Number of collection centers | 83 | N/A | |
| Number of apheresis centers | N/A | 98 | |
| Donor sex | .168 | ||
| Male | 1638 (60) | 4170 (62) | |
| Female | 1088 (40) | 2598 (38) | |
| Donor race/ethnicity | <.001 | ||
| White | 1935 (71) | 5083 (75) | |
| Hispanic | 294 (11) | 582 (9) | |
| Black/African American | 165 (6) | 320 (5) | |
| Asian/Pacific Islander | 144 (5) | 294 (4) | |
| American Indian/Alaska Native | 42 (2) | 77 (1) | |
| Other/multiple race | 130 (5) | 351 (5) | |
| Decline/unknown | 16 (1) | 61 (1) | |
| Donor age at donation, years | .100 | ||
| 18 to 30 | 973 (36) | 2380 (35) | |
| 31 to 40 | 878 (32) | 2202 (33) | |
| 41 to 50 | 684 (25) | 1616 (24) | |
| 51 to 61 | 191 (7) | 570 (8) | |
| Median (range) | 35 (18-61) | 35 (18-61) | .377 |
| Donor BMI, kg/m2 | .646 | ||
| Underweight (<18.5) | 14 (1) | 41 (1) | |
| Normal (18.5-24.9) | 825 (30) | 1970 (29) | |
| Overweight (25-29.9) | 1044 (38) | 2659 (39) | |
| Obese (≥30) | 843 (31) | 2097 (31) | |
| Unknown | 0 | 1 (N/A) | |
| Median (range) | 27.4 (16.1-50.9) | 27.4 (16.2-56.2) | .899 |
| Donor cytomegalovirus | <.001 | ||
| Nonreactive | 1554 (57) | 4124 (61) | |
| Reactive | 1172 (43) | 2642 (39) | |
| Unknown/indeterminate | 0 | 2 (N/A) | |
| Donor white blood cell counts at baseline (×109/L) | |||
| Median (range) | 6.4 (2.3-14.2) | 6.3 (2.3-16.0) | .544 |
| Donor neutrophil counts at baseline (×109/L) | |||
| Median (range) | 4.0 (1.0-12.5) | 4.0 (1.0-12.9) | .276 |
| Donor mononuclear cell counts at baseline (×109/L) | |||
| Median (range) | 2.3 (0.9-7.2) | 2.3 (0.3-7.3) | .633 |
| Donor platelet counts at baseline (×109/L) | |||
| Median (range) | 254 (104-534) | 254 (100-548) | .869 |
| Donor hemoglobin level at baseline (g/dL) | |||
| Male , median (range) | 15.3 (11.1-18.3) | 15.3 (10.9-19.0) | .800 |
| Female, median (range) | 13.5 (8.6-19.0) | 13.4 (9.4-16.5) | .886 |
| Year of donation | <.001 | ||
| 2004 | 491 (18) | 814 (12) | |
| 2005 | 449 (16) | 1102 (16) | |
| 2006 | 479 (18) | 1188 (18) | |
| 2007 | 471 (17) | 1297 (19) | |
| 2008 | 537 (20) | 1497 (22) | |
| 2009 | 299 (11) | 870 (13) |
| Characteristic . | BM . | PBSC . | P . |
|---|---|---|---|
| N (%) . | N (%) . | ||
| Number of donors | 2726 | 6768 | |
| Number of donor centers | 81 | 76 | |
| Number of collection centers | 83 | N/A | |
| Number of apheresis centers | N/A | 98 | |
| Donor sex | .168 | ||
| Male | 1638 (60) | 4170 (62) | |
| Female | 1088 (40) | 2598 (38) | |
| Donor race/ethnicity | <.001 | ||
| White | 1935 (71) | 5083 (75) | |
| Hispanic | 294 (11) | 582 (9) | |
| Black/African American | 165 (6) | 320 (5) | |
| Asian/Pacific Islander | 144 (5) | 294 (4) | |
| American Indian/Alaska Native | 42 (2) | 77 (1) | |
| Other/multiple race | 130 (5) | 351 (5) | |
| Decline/unknown | 16 (1) | 61 (1) | |
| Donor age at donation, years | .100 | ||
| 18 to 30 | 973 (36) | 2380 (35) | |
| 31 to 40 | 878 (32) | 2202 (33) | |
| 41 to 50 | 684 (25) | 1616 (24) | |
| 51 to 61 | 191 (7) | 570 (8) | |
| Median (range) | 35 (18-61) | 35 (18-61) | .377 |
| Donor BMI, kg/m2 | .646 | ||
| Underweight (<18.5) | 14 (1) | 41 (1) | |
| Normal (18.5-24.9) | 825 (30) | 1970 (29) | |
| Overweight (25-29.9) | 1044 (38) | 2659 (39) | |
| Obese (≥30) | 843 (31) | 2097 (31) | |
| Unknown | 0 | 1 (N/A) | |
| Median (range) | 27.4 (16.1-50.9) | 27.4 (16.2-56.2) | .899 |
| Donor cytomegalovirus | <.001 | ||
| Nonreactive | 1554 (57) | 4124 (61) | |
| Reactive | 1172 (43) | 2642 (39) | |
| Unknown/indeterminate | 0 | 2 (N/A) | |
| Donor white blood cell counts at baseline (×109/L) | |||
| Median (range) | 6.4 (2.3-14.2) | 6.3 (2.3-16.0) | .544 |
| Donor neutrophil counts at baseline (×109/L) | |||
| Median (range) | 4.0 (1.0-12.5) | 4.0 (1.0-12.9) | .276 |
| Donor mononuclear cell counts at baseline (×109/L) | |||
| Median (range) | 2.3 (0.9-7.2) | 2.3 (0.3-7.3) | .633 |
| Donor platelet counts at baseline (×109/L) | |||
| Median (range) | 254 (104-534) | 254 (100-548) | .869 |
| Donor hemoglobin level at baseline (g/dL) | |||
| Male , median (range) | 15.3 (11.1-18.3) | 15.3 (10.9-19.0) | .800 |
| Female, median (range) | 13.5 (8.6-19.0) | 13.4 (9.4-16.5) | .886 |
| Year of donation | <.001 | ||
| 2004 | 491 (18) | 814 (12) | |
| 2005 | 449 (16) | 1102 (16) | |
| 2006 | 479 (18) | 1188 (18) | |
| 2007 | 471 (17) | 1297 (19) | |
| 2008 | 537 (20) | 1497 (22) | |
| 2009 | 299 (11) | 870 (13) |
Results
Characteristics of NMDP BM and PBSC donors
Table 1 shows clinical characteristics of BM and PBSC donors enrolled in the study, and supplemental Table 1 (available on the Blood Web site) shows collection characteristics. Donors were more often men (61%), regardless of type of donation. There were no notable differences between BM and PBSC donors with regard to age and body mass index. BM donation was performed under general anesthesia and did not exceed the recommended maximum amount of time or volume of 20 mL/kg the vast majority of time (96% each). The majority of the single-day PB donations were large volume (77% >18 L), whereas 96% (day 5) and 99% (day 6) of donations over the course of 2 days of apheresis were intermediate or small-volume collections (supplemental Table 1).
Incidence and nature of SAEs in BM vs PBSC donors
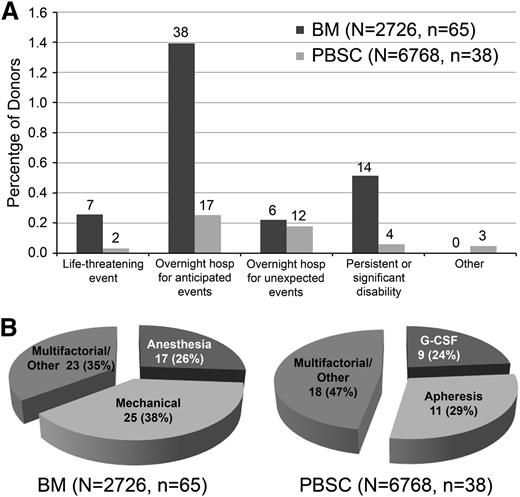
Table 2 provides a detailed description of all SAEs and time to resolution. Of note, no deaths occurred in this population. The most common events for either type of donation were unplanned hospitalizations resulting from expected events, such as pain, nausea, or fainting. When donors had more than 1 reason for hospitalization (eg, both pain and nausea), classification was according to the primary reason recorded for their hospitalization. Because some donor registries routinely hospitalize donors for a period of time after their collection procedures, the number of patients with SAEs in the category “unplanned hospitalization for expected events” will vary between registries, as an unplanned hospitalization cannot occur when the patient is already hospitalized. Given that some registries would not consider events in this category an SAE, we analyzed the data both including and excluding this category. Figure 1A-B compares SAEs experienced by BM and PBSC donors according to FDA-defined categories, nature, and duration of symptoms.
Specific SAEs in BM vs PBSC donors
| Event . | BM . | PBSC . | Time to resolution . |
|---|---|---|---|
| Life-threatening events | 7 | 2 | |
| Major hypotension with electrocardiogram change/hypokalemia | 1 | <1 d | |
| Abdominal thrombosis, Escherichia coli septicemia | 1 | >3 mo | |
| Severe laryngospasm after extubation requiring extensive resuscitation | 1 | <1 d | |
| Postoperative hypotension, pulmonary edema | 1 | <1 d | |
| Laryngospasm, noncardiogenic pulmonary edema | 1 | <1 d | |
| Asystole ×30 s, arrhythmias, desaturation | 1 | <1 d | |
| Severe pain and anemia (hematocrit 15%) | 1 | <2 d | |
| Intracranial hemorrhage not requiring surgery | 1 | >3 mo | |
| After apheresis, fainted, pulseless requiring resuscitation, pericarditis | 1 | >3 mo | |
| Unplanned hospitalization for expected events | 38 | 17 | |
| Nausea/emesis | 8 | 6 | <1-2 d |
| Pain | 8 | 5 | <1-2 d |
| Syncope, light-headedness | 7 | 2 | <1-2 d (8 donors), <2 wk (1 donor) |
| Anesthesia issues | |||
| Hypotension | 9 | <1-2 d | |
| Urinary retention | 2 | <1 d | |
| Bradycardia | 2 | <1 d | |
| Reactive airway issues | 1 | <1 d | |
| Fever | 1 | <1 d | |
| Symptomatic hypokalemia | 3 | <1 d | |
| Panic attack in a patient with known panic disorder | 1 | <1 d | |
| Unplanned hospitalization for unexpected events | 6 | 12 | |
| Infection | |||
| Pneumonia | 1 | 1 | <2 wk |
| Sacral cellulites | 1 | <2 wk | |
| Osteomyelitis | 1 | <1 wk | |
| Possible infection: high fever | 1 | <1 wk | |
| Pulmonary edema and chest pain | 1 | <1 d | |
| Unusual pain, inflammation | |||
| Pleuritic chest pain | 1 | <1 d | |
| Chest pain/shortness of breath | 1 | <1 d | |
| Flare of old pancreatitis | 1 | <1 mo | |
| Pericarditis | 2 | <2 wk, <2 mo | |
| Inflammatory issues | |||
| Temporary granulomatous adenopathy/inflammation | 1 | >3 mo | |
| Joint inflammation | 1 | <2 wk | |
| Worrisome pain | |||
| Radiating chest pain | 1 | <1 wk | |
| Severe headache/weakness | 1 | <2 d | |
| Unusual CVC issues | |||
| Severe emesis causing CVC loss | 1 | <1 mo | |
| Tachyarrhythmia requiring monitoring with CVC placement | 1 | <1 d | |
| Uncontrolled bleeding after CVC removal | 1 | <1 d | |
| Chronic (>3 mo) or disabling events | 14 | 4 | |
| Prolonged hip, back, or joint pain | 6 | 1 | >3 mo |
| Chronic numbness, burning, or neuropathic pain | 5 | 1 | >3 mo |
| Unable to walk after harvest, eventually able to walk with a cane (did not become chronic) | 3 | <3 mo (1 donor), >3 mo (2 donors) | |
| Prolonged muscle pain, rash | 1 | >3 mo | |
| Incapacitating chronic dizziness | 1 | >3 mo | |
| Other Events | 0 | 3 | |
| Significant hematuria during granulocyte colony stimulating factor therapy. No specific renal diagnosis made, all resolved | 3 | <1 d (1 donor), >3 mo (2 donors) | |
| Total | 65 | 38 |
| Event . | BM . | PBSC . | Time to resolution . |
|---|---|---|---|
| Life-threatening events | 7 | 2 | |
| Major hypotension with electrocardiogram change/hypokalemia | 1 | <1 d | |
| Abdominal thrombosis, Escherichia coli septicemia | 1 | >3 mo | |
| Severe laryngospasm after extubation requiring extensive resuscitation | 1 | <1 d | |
| Postoperative hypotension, pulmonary edema | 1 | <1 d | |
| Laryngospasm, noncardiogenic pulmonary edema | 1 | <1 d | |
| Asystole ×30 s, arrhythmias, desaturation | 1 | <1 d | |
| Severe pain and anemia (hematocrit 15%) | 1 | <2 d | |
| Intracranial hemorrhage not requiring surgery | 1 | >3 mo | |
| After apheresis, fainted, pulseless requiring resuscitation, pericarditis | 1 | >3 mo | |
| Unplanned hospitalization for expected events | 38 | 17 | |
| Nausea/emesis | 8 | 6 | <1-2 d |
| Pain | 8 | 5 | <1-2 d |
| Syncope, light-headedness | 7 | 2 | <1-2 d (8 donors), <2 wk (1 donor) |
| Anesthesia issues | |||
| Hypotension | 9 | <1-2 d | |
| Urinary retention | 2 | <1 d | |
| Bradycardia | 2 | <1 d | |
| Reactive airway issues | 1 | <1 d | |
| Fever | 1 | <1 d | |
| Symptomatic hypokalemia | 3 | <1 d | |
| Panic attack in a patient with known panic disorder | 1 | <1 d | |
| Unplanned hospitalization for unexpected events | 6 | 12 | |
| Infection | |||
| Pneumonia | 1 | 1 | <2 wk |
| Sacral cellulites | 1 | <2 wk | |
| Osteomyelitis | 1 | <1 wk | |
| Possible infection: high fever | 1 | <1 wk | |
| Pulmonary edema and chest pain | 1 | <1 d | |
| Unusual pain, inflammation | |||
| Pleuritic chest pain | 1 | <1 d | |
| Chest pain/shortness of breath | 1 | <1 d | |
| Flare of old pancreatitis | 1 | <1 mo | |
| Pericarditis | 2 | <2 wk, <2 mo | |
| Inflammatory issues | |||
| Temporary granulomatous adenopathy/inflammation | 1 | >3 mo | |
| Joint inflammation | 1 | <2 wk | |
| Worrisome pain | |||
| Radiating chest pain | 1 | <1 wk | |
| Severe headache/weakness | 1 | <2 d | |
| Unusual CVC issues | |||
| Severe emesis causing CVC loss | 1 | <1 mo | |
| Tachyarrhythmia requiring monitoring with CVC placement | 1 | <1 d | |
| Uncontrolled bleeding after CVC removal | 1 | <1 d | |
| Chronic (>3 mo) or disabling events | 14 | 4 | |
| Prolonged hip, back, or joint pain | 6 | 1 | >3 mo |
| Chronic numbness, burning, or neuropathic pain | 5 | 1 | >3 mo |
| Unable to walk after harvest, eventually able to walk with a cane (did not become chronic) | 3 | <3 mo (1 donor), >3 mo (2 donors) | |
| Prolonged muscle pain, rash | 1 | >3 mo | |
| Incapacitating chronic dizziness | 1 | >3 mo | |
| Other Events | 0 | 3 | |
| Significant hematuria during granulocyte colony stimulating factor therapy. No specific renal diagnosis made, all resolved | 3 | <1 d (1 donor), >3 mo (2 donors) | |
| Total | 65 | 38 |
Classification of SAEs experienced by BM and PBSC donors. (A) SAEs by category. The number above the bar indicates the number of SAEs in that category. (B) SAEs by proximal cause.
Classification of SAEs experienced by BM and PBSC donors. (A) SAEs by category. The number above the bar indicates the number of SAEs in that category. (B) SAEs by proximal cause.
In virtually every category, a higher percentage of BM donors experienced AEs compared with PBSC donors. The one exception was hematuria during G-CSF therapy (categorized as “other”), which occurred in 3 PBSC donors. When unplanned hospitalizations for expected events are included, the rate of SAEs is more than fourfold higher in donors giving marrow vs PBSCs (2.38% vs 0.56%; P < .001). With exclusion of these hospitalizations, BM donors still had a threefold increase in events (0.99% vs 0.31%; P < .001).
The large majority of SAEs for either type of donation were acute in nature (Figure 1A) and resolved within a few days (Table 2). Expected events that occurred in both groups were nausea, pain, and fainting. Life-threatening events were very rare for both types of donation (0.26% for BM and 0.03% for PBSC donors). Life-threatening and unexpected events differed between the 2 groups (Table 2). BM donors experienced complications of anesthesia (laryngospasm, arrhythmia, and hypotension) and local infections, whereas PBSC donors experienced complications associated with CVC placement and inflammatory issues. As anticipated, the proximal causes of many SAEs in BM donors were anesthesia and mechanical damage to local tissue, and PBSC donor SAEs were usually associated with G-CSF and apheresis. The proximal causes of a large percentage of events, however, were multifactorial and could not be ascribed to any one portion of the collection procedure (Figure 1B).
Multivariate risk analysis for SAEs
We performed multivariate analyses to determine whether any donor characteristics correlated with the occurrence of SAEs, first including (Table 3) and then excluding unplanned hospitalizations for expected events (Table 3). Of note, BM donation was significantly more likely to result in an SAE in both analyses (ORs, 4.13 and 3.20, respectively; P < .001 for both). Female donors were twice as likely to experience an SAE compared with men (OR, 0.50; P = .005), but this was driven primarily by a higher rate of unplanned hospitalization for expected events such as pain or fainting. Interestingly, donors in the more recent era (2007-2009; OR, 0.56; P < .001) were less likely to experience an SAE when unplanned hospitalizations for expected events were included in the analysis. There was no significant effect of year of donation when unplanned hospitalizations for expected events were excluded from the analysis, a finding that suggests that collection centers and apheresis centers became more skilled at avoiding unplanned hospitalizations over time.
Logistic regression model for SAEs after donation
| Variable . | n . | Number of events . | Odds ratio . | 95% confidence interval . | P . |
|---|---|---|---|---|---|
| Including unplanned hospitalizations for expected events | |||||
| Product | |||||
| PBSC | 6768 | 38 | 1.00 | ||
| BM | 2726 | 65 | 4.13 | 2.76-6.19 | <.001 |
| Sex | |||||
| Female | 3686 | 58 | 1.00 | ||
| Male | 5808 | 45 | 0.50 | 0.34-0.75 | .005 |
| Year of donation | |||||
| 2004-2006 | 4523 | 66 | 1.00 | ||
| 2007-2009 | 4971 | 37 | 0.56 | 0.37-0.84 | <.001 |
| Excluding unplanned hospitalizations for expected events | |||||
| Product | |||||
| PBSC | 6768 | 21 | 1.00 | ||
| BM | 2726 | 27 | 3.20 | 1.81-5.68 | <.001 |
| White blood cell counts at baseline (×109/L) | |||||
| <7.6 | 7106 | 27 | 1.00 | ||
| ≥7.6 | 2388 | 21 | 2.32 | 1.31-4.11 | .004 |
| Variable . | n . | Number of events . | Odds ratio . | 95% confidence interval . | P . |
|---|---|---|---|---|---|
| Including unplanned hospitalizations for expected events | |||||
| Product | |||||
| PBSC | 6768 | 38 | 1.00 | ||
| BM | 2726 | 65 | 4.13 | 2.76-6.19 | <.001 |
| Sex | |||||
| Female | 3686 | 58 | 1.00 | ||
| Male | 5808 | 45 | 0.50 | 0.34-0.75 | .005 |
| Year of donation | |||||
| 2004-2006 | 4523 | 66 | 1.00 | ||
| 2007-2009 | 4971 | 37 | 0.56 | 0.37-0.84 | <.001 |
| Excluding unplanned hospitalizations for expected events | |||||
| Product | |||||
| PBSC | 6768 | 21 | 1.00 | ||
| BM | 2726 | 27 | 3.20 | 1.81-5.68 | <.001 |
| White blood cell counts at baseline (×109/L) | |||||
| <7.6 | 7106 | 27 | 1.00 | ||
| ≥7.6 | 2388 | 21 | 2.32 | 1.31-4.11 | .004 |
Incidence of reported cancer, thrombosis, and autoimmune illness after donation
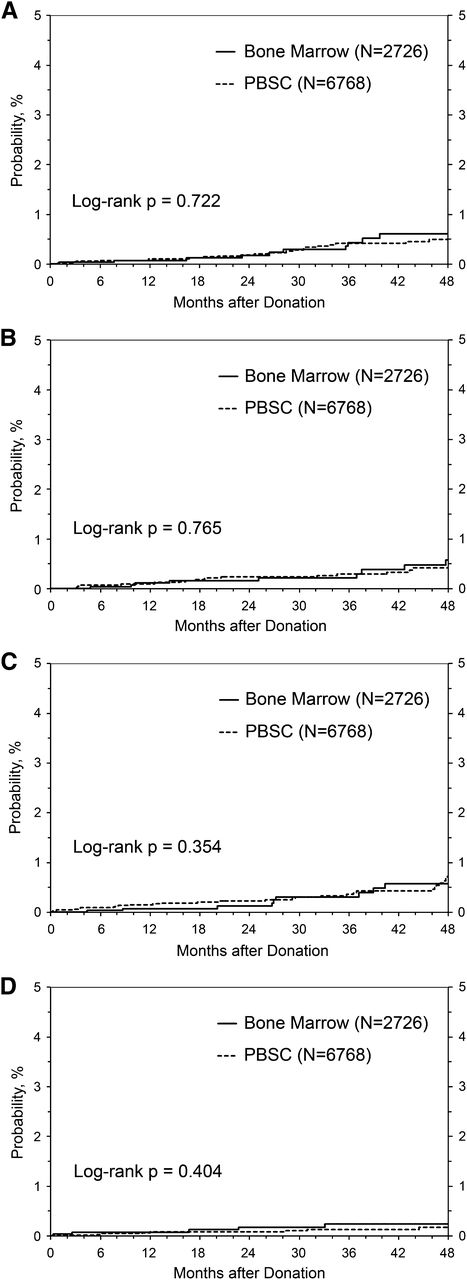
The extensive follow-up in our data set allowed us to compare the incidence of developing cancer, thrombosis (including stroke and deep vein thrombosis), and autoimmune illness after short-term exposure to G-CSF between donors of PBSC (all of whom received filgrastim, G-CSF) and donors of BM who did not receive filgrastim. Follow-up for BM donors totaled 8642 donor-years (median follow-up, 36 months; interquartile range, 23-59 months; range, 1 week-86 months), whereas follow-up for PBSC donors totaled 20 570 donor-years (median, 36 months; interquartile range, 23-49 months; range, 1 week-89 months). Nonmelanoma skin cancer is common in adults and could potentially obscure an otherwise small increased risk for cancer from BM or PBSC donation. Therefore, we analyzed the risk of being diagnosed with nonmelanoma skin cancer separately from all other cancers (Figure 2). The incidence of cancer in either category by 4 years after donation was not different between donors who received and did not receive filgrastim (Figure 2A-B). Likewise, the incidence of autoimmune disease (Figure 2C) or thrombosis (Figure 2D) by 4 years postdonation did not differ according to filgrastim exposure.
Risk of cancer, autoimmunity, and thrombosis in G-CSF–treated PBSC donors vs BM donors. (A) Incidence of reported cancer after donation (excluding nonnelanoma skin cancer). (B) Incidence of reported nonmelanoma skin cancer after donation. (C) Incidence of reported autoimmunity after donation. (D) Incidence of reported thrombosis after donation.
Risk of cancer, autoimmunity, and thrombosis in G-CSF–treated PBSC donors vs BM donors. (A) Incidence of reported cancer after donation (excluding nonnelanoma skin cancer). (B) Incidence of reported nonmelanoma skin cancer after donation. (C) Incidence of reported autoimmunity after donation. (D) Incidence of reported thrombosis after donation.
We performed Poisson regression analysis to assess for risk factors associated with the development of cancer (Table 4) or autoimmune disease in this cohort (Table 4). As expected, older donors were more likely to be diagnosed with cancer during the follow-up period. Being overweight but not obese (for cancers other than nonmelanomatous skin cancers) and cytomegalovirus-positive serostatus (for nonmelanomatous skin cancers) were associated with lower cancer rates (Table 4). Factors associated with developing an autoimmune disease included the expected associations of older age, female sex, and nonwhite race (Table 4). Importantly, donating PBSC vs BM was not associated with increased risk for any of these events. There were too few events to perform this analysis for thrombotic complications.
Poisson regression models for reported cancers and autoimmune diseases after donation
| Variable . | n . | Number of events . | Relative risk . | 95% confidence interval . | P . |
|---|---|---|---|---|---|
| Cancer (excluding nonmelanoma skin cancer) | |||||
| Product | |||||
| PBSC | 6768 | 29 | 1.00 | ||
| BM | 2726 | 11 | 0.91 | 0.45-1.82 | .792 |
| Age at donation, years | <.001 | ||||
| 18-39 | 6147 | 12 | 1.00 | ||
| 40-49 | 2413 | 16 | 3.26 | 1.54-6.90 | .002 |
| 50+ | 934 | 12 | 6.42 | 2.87-14.35 | <.001 |
| BMI, kg/m2 | .042 | ||||
| <24.5 | 2850 | 16 | 1.00 | ||
| 25-29.5 | 3703 | 9 | 0.37 | 0.17-0.85 | .019 |
| ≥30 | 2940 | 15 | 0.79 | 0.39-1.59 | .503 |
| Nonmelanoma skin cancer | |||||
| Product | |||||
| PBSC | 6768 | 23 | 1.00 | ||
| BM | 2726 | 11 | 1.20 | 0.58-2.46 | .624 |
| Age at donation, years | <.001 | ||||
| 18-39 | 6147 | 11 | 1.00 | ||
| 40-49 | 2413 | 13 | 2.82 | 1.26-6.30 | .011 |
| 50+ | 934 | 10 | 6.14 | 2.60-14.48 | <.001 |
| Donor cytomegalovirus | |||||
| Negative | 5678 | 27 | 1.00 | ||
| Positive | 3814 | 7 | 0.36 | 0.16-0.84 | .018 |
| Autoimmune diseases | |||||
| Product | |||||
| PBSC | 6768 | 35 | 1.00 | ||
| BM | 2726 | 11 | 0.79 | 0.40-1.55 | .484 |
| Sex | |||||
| Female | 3686 | 29 | 1.00 | ||
| Male | 5808 | 17 | 0.38 | 0.21-0.69 | .001 |
| Race | |||||
| White | 7018 | 41 | 1.00 | ||
| Other | 2476 | 5 | 2.67 | 1.05-6.77 | .039 |
| Age at donation, years | .009 | ||||
| 18-39 | 6147 | 20 | 1.00 | ||
| 40-49 | 2413 | 14 | 1.49 | 0.75-2.95 | .267 |
| 50+ | 934 | 12 | 3.33 | 1.62-6.82 | <.001 |
| Variable . | n . | Number of events . | Relative risk . | 95% confidence interval . | P . |
|---|---|---|---|---|---|
| Cancer (excluding nonmelanoma skin cancer) | |||||
| Product | |||||
| PBSC | 6768 | 29 | 1.00 | ||
| BM | 2726 | 11 | 0.91 | 0.45-1.82 | .792 |
| Age at donation, years | <.001 | ||||
| 18-39 | 6147 | 12 | 1.00 | ||
| 40-49 | 2413 | 16 | 3.26 | 1.54-6.90 | .002 |
| 50+ | 934 | 12 | 6.42 | 2.87-14.35 | <.001 |
| BMI, kg/m2 | .042 | ||||
| <24.5 | 2850 | 16 | 1.00 | ||
| 25-29.5 | 3703 | 9 | 0.37 | 0.17-0.85 | .019 |
| ≥30 | 2940 | 15 | 0.79 | 0.39-1.59 | .503 |
| Nonmelanoma skin cancer | |||||
| Product | |||||
| PBSC | 6768 | 23 | 1.00 | ||
| BM | 2726 | 11 | 1.20 | 0.58-2.46 | .624 |
| Age at donation, years | <.001 | ||||
| 18-39 | 6147 | 11 | 1.00 | ||
| 40-49 | 2413 | 13 | 2.82 | 1.26-6.30 | .011 |
| 50+ | 934 | 10 | 6.14 | 2.60-14.48 | <.001 |
| Donor cytomegalovirus | |||||
| Negative | 5678 | 27 | 1.00 | ||
| Positive | 3814 | 7 | 0.36 | 0.16-0.84 | .018 |
| Autoimmune diseases | |||||
| Product | |||||
| PBSC | 6768 | 35 | 1.00 | ||
| BM | 2726 | 11 | 0.79 | 0.40-1.55 | .484 |
| Sex | |||||
| Female | 3686 | 29 | 1.00 | ||
| Male | 5808 | 17 | 0.38 | 0.21-0.69 | .001 |
| Race | |||||
| White | 7018 | 41 | 1.00 | ||
| Other | 2476 | 5 | 2.67 | 1.05-6.77 | .039 |
| Age at donation, years | .009 | ||||
| 18-39 | 6147 | 20 | 1.00 | ||
| 40-49 | 2413 | 14 | 1.49 | 0.75-2.95 | .267 |
| 50+ | 934 | 12 | 3.33 | 1.62-6.82 | <.001 |
Supplemental Table 2 shows all of the cancers reported by BM and PBSC donors. We found no evidence that donation, with or without exposure to G-CSF, increased the risk for hematologic malignancies. One patient from this cohort of nearly 10 000 donors developed acute myeloid leukemia (a PBSC donor). There were no cases of myelodysplastic syndrome reported. Other hematological malignancies included 1 BM donor with lymphoma and 1 PBSC donor with multiple myeloma. We performed a direct comparison of the cancer incidence in this cohort with SEER data to test whether our donor populations were at increased risk for any nonhematologic malignancy. As shown in Table 5, when normalized to donor age, the expected numbers of cancers during the period of follow-up in our populations of both BM and PBSC donors were significantly lower than those expected (BM and PBSC donors, respectively: standardized incidence ratio, 0.55 and 0.60; P = .045 and .004; Table 5). These results indicate that NMDP donors are actually healthier with less cancer risk than the general population, regardless of exposure to G-CSF.
Comparison of observed number of cancers (excluding nonmelanoma skin cancer) with expected rates in the general population
| Result . | BM . | PB . |
|---|---|---|
| Observed number of cancers | 11 | 29 |
| Expected number of cancers | 19.89 | 47.95 |
| Standardized incidence ratio (observed/expected) | 0.55 | 0.60 |
| 95% confidence interval | 0.28-0.99 | 0.41-0.87 |
| P value | .045 | .004 |
| Result . | BM . | PB . |
|---|---|---|
| Observed number of cancers | 11 | 29 |
| Expected number of cancers | 19.89 | 47.95 |
| Standardized incidence ratio (observed/expected) | 0.55 | 0.60 |
| 95% confidence interval | 0.28-0.99 | 0.41-0.87 |
| P value | .045 | .004 |
Discussion
Although the reported incidence of SAEs in prior reports of unrelated BM1,11-13 and PBSC8,13-15 donation has been low (∼1%), concern about rare, serious events occurring as part of these procedures has persisted. Many earlier studies have inadequately addressed this concern because of differing and sometimes subjective definitions of SAEs and a lack of prospective enrollment and monitoring, leading to possible underreporting. This study was designed to provide a more accurate and definitive assessment of the rare risks donors face of experiencing clinically significant AEs, and to directly compare concurrently enrolled BM and PBSC donations using uniform definitions and prospective reporting mechanisms. Each SAE reported was followed-up closely by a medical monitor at the NMDP, and detailed descriptive information was obtained to follow-up donors through resolution of the event and beyond.
The SAE rate of 2.38% noted in BM donors by applying the FDA definition is many-fold higher than other reports. The strict FDA SAE definition includes unplanned hospitalization for expected events such as fainting, pain, or nausea. This category of SAE is important to follow within a given center to understand their practice; however, it is difficult to compare across registries, as a number of centers and registries routinely hospitalize donors for a period of time, and thus would not report these events as an SAE. With this issue in mind, we also analyzed SAEs, excluding the hospitalization for expected events category. This more limited analysis found an incidence of SAEs that was comparable with published retrospective data.13 Our results indicate that it is important for centers and registries to track patients for both expected and unexpected complications that result in hospitalization so that interventions to further decrease such complications can be instituted and monitored for success.
An analysis of the BMT CTN 0201 trial comparing the health-related quality of life experiences of 332 BM and PBSC donors was recently published.16 BM donors reported more short-term physical AEs; however, they also reported better psychological status. After 3 weeks, there was no difference between BM and PBSC donors in rate or extent of recovery. This result seems to differ from our conclusion that SAEs and chronic conditions are more common in BM donors. The likely reason for this disparity is that the events we report are quite rare (persistent symptoms occur in 0.06% and 0.5% of PBSC and BM donors, respectively) and are thus unlikely to be detected in a study of 332 donors. Our observation regarding BM donation risk should be viewed with the understanding that more than 99% of BM or PBSC donors will not experience an SAE, and therefore, even if BM donation carries a threefold increase in risk for an SAE compared with PBSC donation, it is still considered a safe and appropriate procedure.
Patterns in the types of SAEs occurring with BM and PBSC suggest targets for interventions to decrease risks for these events. Events occurring with BM donation were generally anesthesia complications, local mechanical damage, or local infection. Older age, female sex, prolonged OR time, and spinal anesthesia have been associated with these complications in this and previous NMDP studies.1 Avoidance of prolonged OR time and spinal anesthesia in donors, along with caution when performing BM harvest in older patients, should be routinely practiced. Local or systemic approaches to minimizing the risks for nerve damage leading to chronic pain or neuropathy are potential targets for future study.
SAEs in PBSC donors were often inflammatory in nature or CVC complications. The use of CVCs should be minimized. An unusual complication of marked, transient hematuria was reported in 3 donors during G-CSF therapy. Although definitive diagnosis of why this occurred was not reached with these donors, biopsy-proven acute glomerulonephritis has been reported in a donor on day 5 of G-CSF therapy.17 It appears that G-CSF is associated with the rare occurrence of significant renal inflammation. Detailed long-term follow-up of the very rare donors experiencing this complication is still needed to understand whether there is long-term risk for renal dysfunction when this occurs.
Concern about the possibility of an increase in cancer risk resulting from the short course of G-CSF given for PBSC donation has been a worry because of reports of increased risk for acute myeloid leukemia/myelodysplastic syndrome in Kostmann patients receiving G-CSF,18 an increase in secondary malignancies in patients with breast cancer receiving G-CSF as part of adjuvant chemotherapy,19 anecdotal reports of donors developing myeloid malignancies,20,21 and laboratory reports of persistent aneuploidy in normal donors after G-CSF use.22 The aneuploidy observation has been adequately addressed in other studies that did not demonstrate any persistent increase in chromosomal changes in pluripotent stem cells,22-24 nor any differences between healthy donors treated with G-CSF and age- and sex-matched control patients followed-up for 1 year.23 These reports dampened, but did not eliminate, the concern raised by the earlier observations of persistent aneuploidy after G-CSF treatment. A number of small studies with detailed follow-up of donors,24-26 and larger retrospective studies13,14,27,28 have provided reassurance by failing to demonstrating evidence of increased risk for cancer in donors resulting from G-CSF. A major strength of the data we report here is the large number of donors prospectively followed-up for long periods. The virtually identical cancer incidence between donors receiving and not receiving G-CSF and the lower incidence of cancer in NMDP donors receiving G-CSF compared with the general population provide reassurance that the risk for cancer, hematologic or otherwise, is likely not increased after G-CSF mobilized PBSC donation.
Likewise, concerns about G-CSF use stimulating autoimmunity or increasing risk for thrombosis have lingered, spurred on by case reports.29,30 Rare cases of death reported within 30 days of harvest or PBSC collection (estimated to occur in <1/10 000) have involved pulmonary embolism (after surgery, antithrombin III deficiency in the family) and cardiac events.13 This comparative study is reassuring in showing a similar incidence of postcollection, new-onset autoimmunity and thrombotic events with and without G-CSF therapy. It should be noted that donors with known autoimmune disease are generally medically deferred from PBSC donation, potentially biasing this population.
In conclusion, we demonstrate that unexpected SAEs are very rare, occurring in less than 1% of collection procedures, with those undergoing BM donation being at higher risk than PBSC donors. Reassuringly, life-threatening events occurred in only about 1/1000 donors, and no fatalities occurred in this NMDP donor cohort. BM donors were more likely than PBSC donors to have lingering symptoms after the procedure, but most of the time, these symptoms consisted of mild localized pain. We saw no evidence of an increased risk for cancer of any kind, autoimmunity, or thrombosis in donors of PBSC who received G-CSF compared with BM donors, and the incidence of cancer in NMDP donors receiving G-CSF was well below the expected incidence in the general population. Although this provides reassurance for unrelated donors, further studies of the related donor population are needed to clarify the risk profiles of donation, especially in very young donors and those older than 60 years.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This project has been supported by funding from the National Marrow Donor Program and the Health Resources and Services Administration (contract number HHSH234200637020C and HHSH250201200024C) to the National Marrow Donor Program. The views expressed in this article do not reflect the official policy or position of the Health Resources and Services Administration or the National Marrow Donor Program.
M.A.P. was supported by the National Heart, Lung, and Blood Institute (NHLBI; R01 HL085707).
Statistical design and analysis for this study was supported by the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the NHLBI, and the National Institute of Allergy and Infectious Diseases; Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; Contract HHSH250201200016C with Health Resources and Services Administration; grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Actinium Pharmaceuticals; Allos Therapeutics, Inc; Amgen, Inc; anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Chimerix, Inc; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc; Gamida Cell Teva Joint Venture Ltd; Genentech, Inc; Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc; Roswell Park Cancer Institute; HistoGenetics, Inc; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc; Millennium: The Takeda Oncology Co; Milliman USA, Inc; Miltenyi Biotec, Inc; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc; Osiris Therapeutics, Inc; Otsuka America Pharmaceutical, Inc; Perkin Elmer, Inc; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-τ Pharmaceuticals; Soligenix, Inc; St. Baldrick’s Foundation; StemCyte; A Global Cord Blood Therapeutics Co; Stemsoft Software, Inc; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc; THERAKOS, Inc; University of Minnesota; University of Utah; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US government.
Authorship
Contribution: M.A.P. and D.L.C. designed the research, participated in the adjudication of SAEs, analyzed and interpreted the data, wrote the manuscript, and had responsibility for the entire manuscript as an accurate, verifiable report; P.C. designed the research, prepared the data file, performed the statistical analysis, analyzed and interpreted the data, and wrote the manuscript; B.R.L. designed the research, performed the statistical analysis, analyzed and interpreted the data, and wrote the manuscript; W.H.N., J.E.L., and J.P.M. participated in the adjudication of SAEs, analyzed and interpreted the data, and wrote the manuscript; and B.E.S., P.V.O., and N.S.M. analyzed and interpreted the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Pulsipher, University of Utah School of Medicine, Division of Hematology/Blood and Marrow Transplant, 30 North 1900 East, Room 5C402, Salt Lake City, UT 84132; e-mail: michael.pulsipher@hsc.utah.edu.