Key Points
C elegans specifically recognizes cardiotoxic LCs as toxicants.
This is an innovative model for studying the heart-specific toxicity of amyloidogenic LCs and developing new therapeutic strategies.
Abstract
Poor prognosis and limited therapeutic options characterize immunoglobulin light-chain (AL) amyloidosis with major heart involvement. Reliable experimental models are needed to study light-chain (LC)/heart interactions and to explore strategies for prevention of cardiac damage. We have exploited the nematode Caenorhabditis elegans as a novel tool, because its pharynx is evolutionarily related to the vertebrate heart. Our data demonstrate that the pharyngeal pumping of C elegans is significantly and selectively reduced by LCs from AL patients suffering from cardiomyopathy, but not by amyloid LCs with different organ tropism or nonamyloidogenic LCs from multiple myeloma. This functional alteration is dependent on the LC concentration and results in persistent pharyngeal dysfunction and in a significant reduction of the worms’ lifespan. These manifestations are paralleled by an increase of mitochondrial reactive oxygen species and can be prevented by treatment with antioxidant agents. In conclusion, these data indicate that this nematode-based assay is a promising surrogate model for investigating the heart-specific toxicity of amyloidogenic LCs and for a rapid screening of new therapeutic strategies.
Introduction
Immunoglobulin light-chain (AL) amyloidosis is the most frequent systemic form in Western countries1,2 and is caused by aggregation and deposition of monoclonal immunoglobulin light chains (LCs) produced by a bone marrow plasma cell clone and transported to target organs through the bloodstream. The clinical picture in this form is heterogeneous and depends on which organs are targeted by fibril deposition. Cardiac involvement is present in up to 75% of patients and is the main prognostic determinant, leading to death for chronic heart failure or fatal arrhythmias.3
Chemotherapy, by reducing the circulating amyloidogenic LCs through suppression of the plasma cell clone, leads to extended survival and improved organ function.3,4 However, its benefits to patients with severe heart dysfunction are minimal, because they are too fragile to receive aggressive treatments and do not survive long enough to benefit from any responses to therapy.5
Clinical and experimental observations suggest that in AL amyloidosis, the soluble precursor protein itself may exert direct cardiotoxicity that significantly contributes to organ dysfunction in addition to the damage caused by cardiac infiltration of amyloid fibrils.4-8 Cellular functional alterations, oxidative stress,7-9 and activation of specific signal transduction pathways8 were found as prominent aberrant features in vitro. Possibly, both host-related factors and intrinsic LC characteristics are required to cause organ toxicity. In vitro data suggest that LCs associated with cardiomyopathy have an intrinsic and specific cardiotoxic potential. Although the use of certain LC germline genes has been associated with specific organ targeting,10 including the heart,11 no specific features related to LCs cardiotoxicity have been described yet, and predicting a protein’s ability to target the heart is currently impossible.
The development of experimental models able to reproduce LCs cardiotoxicity remains a major and urgent need to facilitate early diagnosis and investigate the mechanisms of toxicity in order to develop novel therapeutic strategies.
We used Caenorhabditis elegans as in vivo model to investigate the effect of cardiotoxic LCs and clarify the underlying mechanisms of toxicity. C elegans was chosen because its pharynx is considered to be evolutionarily related to the vertebrate heart and its muscle cells have autonomous contractile activity, reminiscent of cardiac myocytes.12 The rhythmic contraction and relaxation of the nematode’s pharyngeal muscle, pharyngeal pumping, is responsible for the ingestion and transport of food from the mouth to the intestine.13 Stress-induced inhibition of feeding was suggested as an important survival mechanism that limits the intake of toxic solutes. In fact, pharyngeal pumping is inhibited by chemical stressors that induce the production of cellular stress proteins.14
We evaluated the effect on pharyngeal pumping of soluble amyloidogenic LCs with different organ tropisms from AL patients and nonamyloidogenic LCs from multiple myeloma (MM) subjects. Only amyloidogenic LCs that are cardiotoxic in patients caused a specific impairment of the pharyngeal pumping rate of C elegans. These manifestations are paralleled by an increase of mitochondrial reactive oxygen species (ROS) and can be prevented by treatment with antioxidant agents. The data indicate that the nematode-based assay is a promising model for investigating heart-specific toxicity of amyloidogenic LCs and for a rapid screening of the potential drugs and novel therapeutic approaches.
Methods
Patient samples
Urine, serum, and bone marrow plasma cells were obtained from patients during routine diagnostic procedures at the Amyloid Research and Treatment Center, Foundation Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo (Pavia, Italy). Acquisition, storage, and use of biological samples were approved by the institutional review board. Written informed consent was received from participants prior to inclusion in the study. The study was conducted in accordance with the Declaration of Helsinki. The presence of tissue amyloid deposits and amyloid organ involvement were defined according to the International Consensus Panel criteria.15,16 LC cardiotoxicity was evaluated on the basis of clinical, instrumental (echocardiography), and biochemical parameters.17 The clinical characteristics of the patients included in the study are reported in Table 1.
Clinical and biochemical characteristics of patients at diagnosis of AL amyloidosis or MM
| Code . | Gender, age (y) . | Cardiac stage* . | Diagnosis . | Organs involved† . | Protein source . | Serum λ FLC (mg/L) . | κ/λ FLC ratio . | Proteinuria (g/24 h) . | Creatinine (mg/dL) . | Cardiac parameters . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recombinant . | BJ . | Serum . | NT-pro-BNP (ng/L) . | cTnI (ng/mL) . | IVS (mm) . | PW (mm) . | EF (%) . | |||||||||
| H-1 | M, 54 | III | AL | H, PNS, ST | x | x | 839 | 0.0018 | 0.98 | 0.74 | 1444 | 0.222 | 16.8 | 16 | 70 | |
| H-2 | F, 72 | III | AL | H | x | 769 | 0.02 | 0.23 | 1.58 | 31441 | 0.9 | 16 | 16 | 55 | ||
| H-3 | M, 65 | III | AL | H | x | 252 | 0.09 | 0.18 | 1.76 | 4491 | 0.35 | 16 | 16 | 42 | ||
| H-4 | M, 46 | II | AL | H | x | 185 | 0.08 | 0.35 | 1.50 | 4942 | 0.08 | 18 | 18 | 40 | ||
| H-5 | M, 72 | III | AL | H, K | x | 383 | 0.05 | 8.10 | 2.73 | 21587 | 1.18 | 14.9 | 14.4 | 61 | ||
| H-6 | M, 74 | III | AL | H | x | x | 683 | 0.009 | 0.45 | 0.73 | 4300 | 0.2 | 15 | 15 | 42 | |
| H-7 | M, 45 | III | AL | H | x | x | 477 | 0.01 | 0.33 | 0.98 | 8882 | 0.16 | 19 | 19 | 45 | |
| H-8 | F, 55 | III | AL | H | x | 388 | 0.005 | 0.14 | 0.83 | 5557 | 0.2 | 13 | 14 | 45 | ||
| K-1 | M, 69 | I | AL | K | x | 161 | 0.06 | 5.17 | 0.95 | 106 | 0.02 | 13.3 | 13.3 | 63 | ||
| K-2 | F, 60 | I | AL | K | x | 104 | 0.06 | 4.76 | 0.58 | 57.8 | 0.001 | 9.6 | 10.4 | 64 | ||
| K-3 | M, 68 | I | AL | K | x | 463 | 0.08 | 8.45 | 2.23 | 274 | 0.021 | 11 | 11 | 55 | ||
| K-4 | M, 72 | I | AL | K | x | 320 | 0.03 | 6.76 | 0.90 | 230 | 0.022 | 11.5 | 10 | 60 | ||
| K-5 | F, 63 | I | AL | K | x | 509 | 0.03 | 2.67 | 0.57 | 40.5 | 0.007 | 9.7 | 9.2 | 61 | ||
| K-6 | F, 69 | I | AL | K | x | 228 | 0.08 | 2.33 | 1.39 | 419.5 | 0.005 | 9 | 10 | 60 | ||
| ST-1 | F, 55 | I | AL | ST | x | 1220 | 0.0002 | 0.22 | 0.55 | 271 | 0.025 | 11.6 | 11.6 | 57 | ||
| MM-1 | F, 73 | MM | — | x | 618 | 0.003 | 0.13‡ | 1.20 | 191 | 0.004 | 10.2 | 10.2 | 55 | |||
| MM-2 | F, 71 | MM | — | x | 6130 | 0.001 | 0.52‡ | 2.07 | 42§ | 0.007 | 9 | 9 | 65 | |||
| MM-3 | M, 48 | MM | — | x | 573 | 0.011 | 1.87‡ | 0.84 | 14.5 | 0.003 | 10 | 10.5 | 67 | |||
| MM-4 | M, 65 | MM | — | x | 1140 | 0.001 | 0.12‡ | 0.89 | 201 | n.a. | 11 | 11 | 65 | |||
| MM-5 | M, 37 | MM | — | x | 500 | 0.01 | 0.11‡ | 0.88 | 55 | 0 | 10 | 10 | 64 | |||
| Code . | Gender, age (y) . | Cardiac stage* . | Diagnosis . | Organs involved† . | Protein source . | Serum λ FLC (mg/L) . | κ/λ FLC ratio . | Proteinuria (g/24 h) . | Creatinine (mg/dL) . | Cardiac parameters . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recombinant . | BJ . | Serum . | NT-pro-BNP (ng/L) . | cTnI (ng/mL) . | IVS (mm) . | PW (mm) . | EF (%) . | |||||||||
| H-1 | M, 54 | III | AL | H, PNS, ST | x | x | 839 | 0.0018 | 0.98 | 0.74 | 1444 | 0.222 | 16.8 | 16 | 70 | |
| H-2 | F, 72 | III | AL | H | x | 769 | 0.02 | 0.23 | 1.58 | 31441 | 0.9 | 16 | 16 | 55 | ||
| H-3 | M, 65 | III | AL | H | x | 252 | 0.09 | 0.18 | 1.76 | 4491 | 0.35 | 16 | 16 | 42 | ||
| H-4 | M, 46 | II | AL | H | x | 185 | 0.08 | 0.35 | 1.50 | 4942 | 0.08 | 18 | 18 | 40 | ||
| H-5 | M, 72 | III | AL | H, K | x | 383 | 0.05 | 8.10 | 2.73 | 21587 | 1.18 | 14.9 | 14.4 | 61 | ||
| H-6 | M, 74 | III | AL | H | x | x | 683 | 0.009 | 0.45 | 0.73 | 4300 | 0.2 | 15 | 15 | 42 | |
| H-7 | M, 45 | III | AL | H | x | x | 477 | 0.01 | 0.33 | 0.98 | 8882 | 0.16 | 19 | 19 | 45 | |
| H-8 | F, 55 | III | AL | H | x | 388 | 0.005 | 0.14 | 0.83 | 5557 | 0.2 | 13 | 14 | 45 | ||
| K-1 | M, 69 | I | AL | K | x | 161 | 0.06 | 5.17 | 0.95 | 106 | 0.02 | 13.3 | 13.3 | 63 | ||
| K-2 | F, 60 | I | AL | K | x | 104 | 0.06 | 4.76 | 0.58 | 57.8 | 0.001 | 9.6 | 10.4 | 64 | ||
| K-3 | M, 68 | I | AL | K | x | 463 | 0.08 | 8.45 | 2.23 | 274 | 0.021 | 11 | 11 | 55 | ||
| K-4 | M, 72 | I | AL | K | x | 320 | 0.03 | 6.76 | 0.90 | 230 | 0.022 | 11.5 | 10 | 60 | ||
| K-5 | F, 63 | I | AL | K | x | 509 | 0.03 | 2.67 | 0.57 | 40.5 | 0.007 | 9.7 | 9.2 | 61 | ||
| K-6 | F, 69 | I | AL | K | x | 228 | 0.08 | 2.33 | 1.39 | 419.5 | 0.005 | 9 | 10 | 60 | ||
| ST-1 | F, 55 | I | AL | ST | x | 1220 | 0.0002 | 0.22 | 0.55 | 271 | 0.025 | 11.6 | 11.6 | 57 | ||
| MM-1 | F, 73 | MM | — | x | 618 | 0.003 | 0.13‡ | 1.20 | 191 | 0.004 | 10.2 | 10.2 | 55 | |||
| MM-2 | F, 71 | MM | — | x | 6130 | 0.001 | 0.52‡ | 2.07 | 42§ | 0.007 | 9 | 9 | 65 | |||
| MM-3 | M, 48 | MM | — | x | 573 | 0.011 | 1.87‡ | 0.84 | 14.5 | 0.003 | 10 | 10.5 | 67 | |||
| MM-4 | M, 65 | MM | — | x | 1140 | 0.001 | 0.12‡ | 0.89 | 201 | n.a. | 11 | 11 | 65 | |||
| MM-5 | M, 37 | MM | — | x | 500 | 0.01 | 0.11‡ | 0.88 | 55 | 0 | 10 | 10 | 64 | |||
Reference ranges: serum λ FLC <26.3 mg/L, κ/λ ratio 0.26 to 1.65, serum creatinine <1.18 mg/dL (men) and <1.02 mg/dL (women), NT-pro-BNP5 <332 ng/L, BNP <50 ng/L, and cTnI <0.04 ng/mL.
BNP, brain natriuretic peptide; cTnI, cardiac troponin I; EF, ejection fraction; F, female; FLC, free light chains; H, heart; IVS, interventricular septum; K, kidney; M, male; n.a., not available; PNS, peripheral nervous system; PW, posterior wall; ST, soft tissues.
Entirely constituted by BJ proteins.
BNP (ng/L).
Human monoclonal amyloidogenic cardiotoxic LCs (H), amyloidogenic noncardiotoxic LCs, and nonamyloidogenic LCs from MM patients without amyloidosis were isolated from 24-hour urine collected from patients (Bence Jones [BJ]) and/or from serum (s) or produced, as full length recombinant proteins (r), in a bacterial system (see supplemental Methods available on the Blood Web site). The homogeneity of all the purified LCs was assessed by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blot. In addition, mass spectrometry analysis, physicochemical studies, and the examination of the oligomerization state were performed on prototypic proteins, ie, recombinant cardiotoxic (H3-r) and noncardiotoxic (K3-r) amyloidogenic proteins and on BJ proteins purified from urine of a patient suffering from AL cardiomyopathy (H6-BJ) and from a patient with MM (MM2-BJ) (supplemental Methods).18 The biochemical characteristics of these proteins (eg, size, molecular mass, and calculated isoelectric point) are reported in supplemental Figure 1. All LCs included in the study were λ isotype, which represents ∼75% of amyloidogenic LCs.19
C elegans experiments
Bristol N2 strain, from the Caenorhabditis elegans Genetic Center (CGC; University of Minnesota), was propagated at 20°C on solid nematode growth medium (NGM) seeded with Escherichia coli OP50 (from CGC) for food.20 To prepare age-synchronized animals, nematodes were transferred to fresh NGM plates on reaching maturity at 3 days of age and allowed to lay eggs overnight. Isolated hatchlings from the synchronized eggs (day 1) were cultured on fresh NGM plates at 20°C. For pumping-rate assays, nematodes (L3-L4 larval stage) were collected with M9 buffer, centrifuged, and washed twice with 5 mM phosphate-buffered saline (PBS) (pH 7.4) to eliminate bacteria. Incubation of the worms with LCs was performed in the absence of E coli to avoid any potential interference between bacteria and the LCs.14,21 Worms were incubated with 1 to 200 μg/mL BJ or recombinant LCs (100 worms/100 μL) in 10 mM PBS (pH 7.4) or with 50 μg/mL free LCs from patient serum in 5 mM PBS (pH 7.4) (100 worms/100 μL). Control worms were incubated with 10 mM PBS (pH 7.4) (vehicle) only. The effect of eluate obtained from incubation of anti-free-LC antibodies with serum of healthy donors was also considered. After 2 hours, worms were transferred onto NGM plates seeded with OP50 E coli. The pharyngeal pumping rate, measured by counting the number of times the terminal bulb of the pharynx contracted over a 1-minute interval, was scored from 2 hours up to 48 hours later. The pumping rate of worms treated with the eluate obtained from serum of healthy donors was not different from that of vehicle-treated ones (data not shown). For this reason, 10 mM PBS (pH 7.4) was used as vehicle for all the experiments. To visualize the effect of LCs on the pumping rate, the feeding assay was performed by monitoring the ability of worms to ingest multifluorescent beads (supplemental Methods).
The effect of LC administration on pharyngeal cell viability was determined using the cell-impermeable dye propidium iodide, which can be fed to C elegans (supplemental Methods).
In selected experiments, worms were fed for 2 hours with 100 μg/mL of H6-BJ alone or with the prototypic antioxidants N-acetyl-cysteine (NAC; 0.1-20 mM, Sigma-Aldrich) and l-ascorbic acid (5-568 μM, Sigma-Aldrich) in 5 mM PBS (pH 7.4). The effect of tetracycline hydrochloride (TETRA; 5-100 μM, Sigma-Aldrich) and epigallocathechin gallate (EGCG; 0.1-1000 μM, kindly provided by Indena), in 5 mM PBS (pH 7.4), both known to possess antioxidant properties, was also considered. Higher doses of drugs proved toxic for nematodes. The effect of 0.1 mM H2O2 for 30 minutes (min) was also investigated. Worms were then transferred onto fresh NGM plates seeded with E coli in the presence of the same drug concentration and the pharyngeal pumping rate was scored after 20 hours. Worms were also exposed to the drugs alone or to vehicle in the same conditions.
For lifespan experiments, L3 larval stage worms were fed for 2 hours with: (1) 100 μg/mL of MM2-BJ or 100 μg/mL H6-BJ, (2) 100 μg/mL H6-BJ plus the optimal dose of an antioxidant compound (5 mM NAC, 284 µM ascorbic acid, 50 µM TETRA, or 100 µM EGCG), or (3) the optimal dose of antioxidant compound alone. Nematodes were then transferred onto fresh NGM plates seeded with E coli in the presence of the same drug concentration. Control worms were exposed, under the same conditions, to vehicle alone. After 20 hours, nematodes were transferred to fresh NGM plates seeded with bacteria and the number of live worms was scored (considered as day 0). To avoid overlapping generations, the worms were then transferred every day, in the absence of fluorodeoxyuridine, on NGM plates seeded with E coli in the presence or absence of 5 mM NAC, 284 µM ascorbic acid, 50 µM TETRA, or 100 µM EGCG until they stopped laying eggs. The number of live worms was determined for each consecutive day until all worms were dead.22
Mitochondrial production of ROS
The effect of LCs on mitochondrial oxidant burden was evaluated by feeding worms with MitoSOX Red (Molecular Probes).23,24 Worms (L3-L4 larval stage) were incubated for 2 hours with 100 µg/mL of MM2-BJ, H6-BJ, H3-r, or K3-r, as described above. Negative control worms were fed vehicle alone, whereas positive controls were fed 0.1 mM H2O2 to induce oxidant stress. To investigate the effect of antioxidant on mitochondrial ROS production, worms were incubated for 2 hours with 100 µg/mL H6-BJ or for 30 min with 0.1 mM H2O2, in the absence or presence of 5 mM NAC, 284 μM l-ascorbic acid, 50 μM TETRA, or 100 μM EGCG. The effect of drug alone was also determined. Nematodes were then transferred to NGM plates seeded with fresh bacteria as food and 10 µM MitoSOX Red. After 20 hours, nematodes were transferred to fresh NGM plates seeded with OP50 and left for 1 hour, so that residual dye could be washed out from the pharynx lumen. Nematodes were paralyzed with 1 mM levamisole, transferred to tubes containing 1 mL of M9 plus 1 mM levamisole, centrifuged, and fixed in 4% paraformaldehyde in 5 mM PBS (pH 7.4) for 24 hours at 4°C. Worms were then mounted on slides for microscopy and were observed by epifluorescence using an inverted fluorescent microscope (IX-71 Olympus) equipped with a charge-coupled device camera.
Statistical analysis
The data were analyzed using GraphPad Prism 4.0 software by an independent Student t test and 1-way analysis of variance (ANOVA) and Bonferroni post-test analysis. Vehicle and drug effects were compared using an independent Student t test, and the 50% inhibition/inhibitory concentration (IC50) was determined using Prism version 4.0 for Windows (GraphPad Software). A P value < .05 was considered statistically significant.
Additional details are provided in supplemental Methods.
Results
Cardiotoxic LCs specifically impair pharyngeal pumping in C elegans
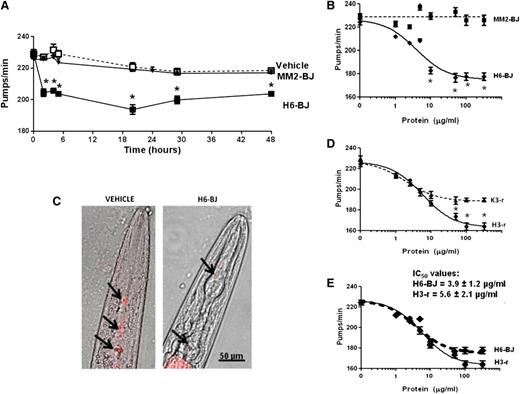
We first monitored the time-dependent effect of LC administration to C elegans by using 2 representative BJ proteins, an amyloidogenic cardiotoxic protein (H6-BJ) and a nonamyloidogenic one (MM2-BJ), from a patient with MM (Table 1 and supplemental Figures 1-5). These proteins derive from 2 unrelated germline genes (IGLV6-57 and IGLV3-19) and share only 74% amino acid identity. Their size, molecular mass, and isoelectric point are shown in supplemental Figure 1. H6-BJ and MM2-BJ were present in solution as small monomers or dimers, or at most trimers, and fibrils or higher aggregates were not present (supplemental Figures 3-4).
Proteins were administered to worms for 2 hours at 100 µg/mL, which is the representative concentration of circulating levels of free LCs in AL patients, and were then plated on NGM plates seeded with OP50 E coli. These experimental conditions did not affect the physiological pharyngeal contraction of worms14 ; in fact, the pumping rate of C elegans incubated with vehicle, without bacteria (235.0 ± 3.6 pumps/min, n = 30), as determined 2 hours after plating, was not different from untreated ones fed OP50 seeded on the plate for the same time (225.4 ± 5.1 pumps/min, n = 30).
The pharyngeal pumping of LC-fed nematodes was scored at different times after plating worms on NGM plates seeded with bacteria. After 2 hours, the pumping rate of H6-BJ–fed worms was already significantly reduced compared with vehicle-fed ones, and a comparable inhibition was observed for up to 48 hours (Figure 1A). This reduction in pumping motion was not accompanied by a modification of the worms’ viability. Feeding worms with the nonamyloidogenic MM2-BJ did not cause reduction in the pumping rate or affect the viability for every interval considered (Figure 1A). Therefore, cardiotoxic LCs caused a significant and prolonged reduction in pharyngeal contraction and a time point of 20 hours after plating was selected.
Characterization of the effects of selected LC on the pumping rate. (A) Time-dependent effect of the amyloidogenic cardiotoxic protein (H6-BJ) and the nonamyloidogenic one (MM2-BJ) on the pumping rate of worms. Proteins, in 10 mM PBS (pH 7.4), were administered to worms at 100 µg/mL. Control worms received vehicle alone (Vehicle). Nematodes (100 worms/100 µL) were incubated with LCs for 2 hours in the absence of OP50 E coli and then plated on NGM plates seeded with bacteria. The pharyngeal pumping was scored at different times after plating (2-48 hours). Data are expressed as the mean ± standard error (SE) (n = 20 worms/group). *P < .01 vs vehicle and MM2-BJ, Student t test. (B) Dose-response effect of 1 to 200 µg/mL of H6-BJ and MM2-BJ. Mean ± SE (n = 40 worms/group). *P < .01 vs MM2-BJ, Student t test. (C) Effect of H6-BJ on the feeding behavior. Feeding assay was performed by monitoring the ability of worms to ingest multifluorescent beads. H6-BJ protein (100 µg/mL) in 10 mM PBS (pH 7.4) or vehicle alone (Vehicle) were administered to worms. Representative images, obtained from the overlay of a contrast phase and epifluorescence, indicated the presence of fluorescent beads (black arrows) in the pharynx of control worms, but not in those fed H6-BJ protein. (D) Dose-response effect of 1 to 200 µg/mL of recombinant cardiotoxic (H3-r) or noncardiotoxic (K3-r) proteins. Mean ± SE (n = 30 worms/group). *P < .01 vs K3-r, Student t test. (E) Comparison of the dose-response curves obtained for H3-r and H6-BJ proteins. IC50 values ± standard deviation were reported. The 2 proteins, at 100 µg/mL, similarly inhibited the pumping rate of worms (from 235.0 ± 3.6 pumps/min of vehicle to 177.6 ± 3.2 pumps/min and 170.4 ± 2.8 pumps/min for H6-BJ and H3-r, respectively).
Characterization of the effects of selected LC on the pumping rate. (A) Time-dependent effect of the amyloidogenic cardiotoxic protein (H6-BJ) and the nonamyloidogenic one (MM2-BJ) on the pumping rate of worms. Proteins, in 10 mM PBS (pH 7.4), were administered to worms at 100 µg/mL. Control worms received vehicle alone (Vehicle). Nematodes (100 worms/100 µL) were incubated with LCs for 2 hours in the absence of OP50 E coli and then plated on NGM plates seeded with bacteria. The pharyngeal pumping was scored at different times after plating (2-48 hours). Data are expressed as the mean ± standard error (SE) (n = 20 worms/group). *P < .01 vs vehicle and MM2-BJ, Student t test. (B) Dose-response effect of 1 to 200 µg/mL of H6-BJ and MM2-BJ. Mean ± SE (n = 40 worms/group). *P < .01 vs MM2-BJ, Student t test. (C) Effect of H6-BJ on the feeding behavior. Feeding assay was performed by monitoring the ability of worms to ingest multifluorescent beads. H6-BJ protein (100 µg/mL) in 10 mM PBS (pH 7.4) or vehicle alone (Vehicle) were administered to worms. Representative images, obtained from the overlay of a contrast phase and epifluorescence, indicated the presence of fluorescent beads (black arrows) in the pharynx of control worms, but not in those fed H6-BJ protein. (D) Dose-response effect of 1 to 200 µg/mL of recombinant cardiotoxic (H3-r) or noncardiotoxic (K3-r) proteins. Mean ± SE (n = 30 worms/group). *P < .01 vs K3-r, Student t test. (E) Comparison of the dose-response curves obtained for H3-r and H6-BJ proteins. IC50 values ± standard deviation were reported. The 2 proteins, at 100 µg/mL, similarly inhibited the pumping rate of worms (from 235.0 ± 3.6 pumps/min of vehicle to 177.6 ± 3.2 pumps/min and 170.4 ± 2.8 pumps/min for H6-BJ and H3-r, respectively).
The inhibition to the pumping rate caused by H6-BJ was dose dependent in the range of concentrations from 2.5 to 200 µg/mL, whereas MM2-BJ was not effective (Figure 1B). The effect of H6-BJ was significant from 10 µg/mL and reached the maximum at 100 µg/mL (Figure 1B). The pumping impairment caused by this protein concentration was visualized by evaluating the ability of worms to ingest fluorescent beads (Figure 1C and supplemental Methods). In the pharynx of H6-BJ–fed worms, a strong reduction of the steady-state fluorescent signal was observed compared with vehicle-fed animals (Figure 1C), indicative of a pumping impairment.
To further exclude the chance that the different toxicity observed in the above-described experiments could be attributed only to extensive differences in the LC primary sequences, we also compared the effects of 2 recombinant proteins derived from patients with severe amyloid cardiomyopathy (H3-r) or overt nephrotic syndrome and renal insufficiency (K3-r) (Table 1). These 2 selected LCs derived from the rearrangement of the same IGLV1-44 germline gene, associated with a fivefold increase in the odds of dominant heart involvement in AL amyloidosis,11 and have ∼94% amino acid identity. Although both proteins proved able to inhibit the pumping rate, H3-r was more effective than K3-r at concentrations above 10 µg/mL (Figure 1D).
Notably, urinary and recombinant cardiotoxic LC had very similar effects on C elegans pharynx, as indicated by the shape of the dose-response curves and IC50 values obtained (3.9 ± 1.2 µg/mL and 5.6 ± 2.1 µg/mL for H6-BJ and H3-r, respectively; P = .294, Student t test) (Figure 1E).
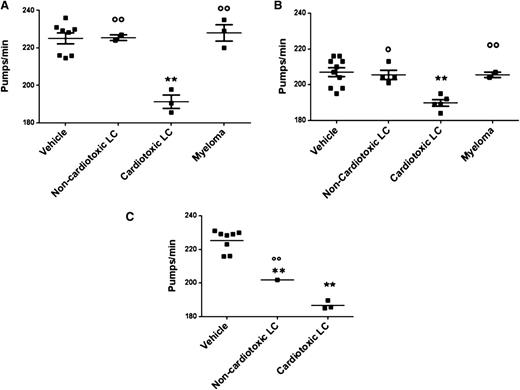
Then, we evaluated the effect of LCs with different organ tropism obtained from 15 AL and 5 MM patients (Table 1). Overall, 23 proteins were tested (8 BJ, 11 serum-free LCs, 4 recombinant-free LCs) (Table 1 and Figure 2). BJ proteins were tested at 100 µg/mL; LCs that are cardiotoxic to patients significantly impaired the pumping rate of nematodes (191.0 ± 3.5 and 225.0 ± 3.0 pumps/min for cardiotoxic LC- and vehicle-fed worms, respectively; P < .001, 1-way ANOVA), whereas no effect was observed with LCs from subjects affected by amyloidosis involving organs other than the heart (eg, kidney and soft tissue) (226.5 ± 1.5 pumps/min, P < .01 vs cardiotoxic LCs, 1-way ANOVA) or with MM (228.2 ± 4.0 pumps/min, P < .01 vs cardiotoxic LCs, 1-way ANOVA) (Figure 2A). The impairment of pharyngeal pumping caused by LCs purified from patient serum was then explored. Despite the lower dosage used (50 µg/mL), due to reduced recovery of LCs from serum,25 the specific effect of cardiotoxic LCs was also observed in this experimental setting (190.1.0 ± 2.0 and 207.2 ± 2.0 pumps/min for cardiotoxic LC- and vehicle-fed worms, respectively; P < .001, 1-way ANOVA) (Figure 2B). Similarly, cardiotoxic LCs (100 µg/mL), produced as recombinant proteins in a bacterial system, caused a significant pumping rate reduction (186.7 ± 1.5 and 225.3 ± 23.0 pumps/min for cardiotoxic LC- and vehicle-fed worms, respectively; P < .001, 1-way ANOVA) (Figure 2C). Overall, these data indicate that cardiotoxic LCs obtained from different patients specifically trigger a pharyngeal dysfunction in C elegans independent of their origin: serum, urine, or recombinant.
Effect on the pumping rate of LC with different organ tropism, purified from different patients’ urine and serum or obtained as recombinant. Effect of (A) BJ LC (100 µg/mL) purified from 8 patients (3 heart AL, 1 kidney AL, 1 soft tissue AL, 3 MM), (B) serum-free LC (50 µg/mL) purified from 11 patients (5 heart AL, 4 kidney AL, 2 MM), and (C) recombinant LC (100 µg/mL) obtained from 4 patients (3 heart AL and 1 kidney AL) on pharyngeal pumping. Nematodes (100 worms/100 µL) were incubated for 2 hours, in the absence of OP50 E coli, with different amyloidogenic noncardiotoxic or cardiotoxic LCs or nonamyloidogenic LCs from patients with MM. The pharyngeal pumping rate was scored 20 hours after plating the worms on NGM agar plates seeded with fresh OP50 E coli as food. Control worms received vehicle alone (Vehicle). Each dot on the scatterplot represents the mean value of pumps per minute obtained for each single protein from 3 independent assays (n = 30 worms/assay). These values were used to calculate the mean ± SE for LCs with the similar organ tropism (horizontal line) and to perform a statistical comparison across the different groups. **P < .001 vs vehicle, °P < .05 and °°P < .01 noncardiotoxic vs cardiotoxic LCs, according to 1-way ANOVA followed by Bonferroni post hoc test.
Effect on the pumping rate of LC with different organ tropism, purified from different patients’ urine and serum or obtained as recombinant. Effect of (A) BJ LC (100 µg/mL) purified from 8 patients (3 heart AL, 1 kidney AL, 1 soft tissue AL, 3 MM), (B) serum-free LC (50 µg/mL) purified from 11 patients (5 heart AL, 4 kidney AL, 2 MM), and (C) recombinant LC (100 µg/mL) obtained from 4 patients (3 heart AL and 1 kidney AL) on pharyngeal pumping. Nematodes (100 worms/100 µL) were incubated for 2 hours, in the absence of OP50 E coli, with different amyloidogenic noncardiotoxic or cardiotoxic LCs or nonamyloidogenic LCs from patients with MM. The pharyngeal pumping rate was scored 20 hours after plating the worms on NGM agar plates seeded with fresh OP50 E coli as food. Control worms received vehicle alone (Vehicle). Each dot on the scatterplot represents the mean value of pumps per minute obtained for each single protein from 3 independent assays (n = 30 worms/assay). These values were used to calculate the mean ± SE for LCs with the similar organ tropism (horizontal line) and to perform a statistical comparison across the different groups. **P < .001 vs vehicle, °P < .05 and °°P < .01 noncardiotoxic vs cardiotoxic LCs, according to 1-way ANOVA followed by Bonferroni post hoc test.
To determine whether the impairment of pumping function caused by cardiotoxic LCs may be related to a reduction of viable cells in the pharynx, worms were fed propidium iodide, a fluorescent dye that enters and stains dead cells (supplemental Methods). In the pharynx region of H6-BJ–fed worms, but not in the control ones, we observed a red-fluorescence–positive signal due to staining of dead cells (supplemental Figure 6B,D). This cell death level was comparable to that observed by exposing worms for 30 min to 10 mM H2O2 (supplemental Figure 6F), which caused a significant impairment of the pharyngeal pumping rate (226.7 ± 2.0 and 190.1 ± 1.0 pumps/min for vehicle- and H2O2-fed worms, respectively; P < .01, 1-way ANOVA, n = 30 worms/group).
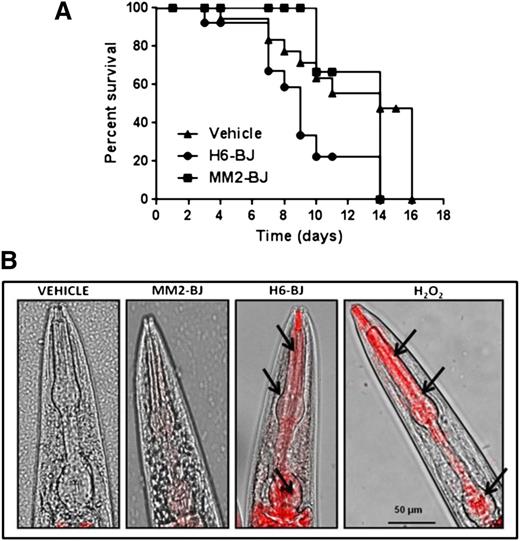
Recent data indicated that soluble LCs caused a marked reduction in the lifespan of zebrafish.26,27 We therefore analyzed whether the pharyngeal dysfunction induced by cardiotoxic LC could affect, in time, nematode survival. The administration of H6-BJ, but not MM2-BJ, significantly reduced the worm’s survival (median survival = 14 days for vehicle and 9 days for H6-BJ, P = .024 vs vehicle, log-rank test; 14 days for MM2-BJ, P = .072 vs H6-BJ and P = .53 vs vehicle, log-rank test) (Figure 3A).
Cardiotoxic LC affects nematode survival and pharyngeal ROS generation. (A) Kaplan-Meier survival curves of worms treated with vehicle, 100 µg/mL of amyloidogenic BJ cardiotoxic protein (H6-BJ), or 100 µg/ml of nonamyloidogenic BJ protein (MM2-BJ). Survival is expressed as a percentage of the initial population (3 independent experiments, n = 30 worms/group). Median survival was 14 days for vehicle, 9 days for H6-BJ (P = .024 vs vehicle, log-rank test), and 14 days for MM2-BJ (P = .072 vs worms treated with H6-BJ and P = .53 vs vehicle, log-rank test). (B) Representative images obtained from the overlay of a contrast phase and MitoSOX fluorescence. N2 worms (at L3-L4 larval stage) were fed 2 hours with vehicle or 100 µg/mL of MM2-BJ or H6-BJ. Positive control worms were fed 0.1 mM hydrogen peroxide (H2O2) for 30 min. Nematodes were then transferred to NGM plates seeded with fresh OP50 E coli and 10 µM MitoSOX Red dye. Original magnification ×40 was used. Arrows indicate the radical superoxide generation in the mitochondria of pharyngeal bulb of C elegans fed cardiotoxic H6-BJ protein or H2O2.
Cardiotoxic LC affects nematode survival and pharyngeal ROS generation. (A) Kaplan-Meier survival curves of worms treated with vehicle, 100 µg/mL of amyloidogenic BJ cardiotoxic protein (H6-BJ), or 100 µg/ml of nonamyloidogenic BJ protein (MM2-BJ). Survival is expressed as a percentage of the initial population (3 independent experiments, n = 30 worms/group). Median survival was 14 days for vehicle, 9 days for H6-BJ (P = .024 vs vehicle, log-rank test), and 14 days for MM2-BJ (P = .072 vs worms treated with H6-BJ and P = .53 vs vehicle, log-rank test). (B) Representative images obtained from the overlay of a contrast phase and MitoSOX fluorescence. N2 worms (at L3-L4 larval stage) were fed 2 hours with vehicle or 100 µg/mL of MM2-BJ or H6-BJ. Positive control worms were fed 0.1 mM hydrogen peroxide (H2O2) for 30 min. Nematodes were then transferred to NGM plates seeded with fresh OP50 E coli and 10 µM MitoSOX Red dye. Original magnification ×40 was used. Arrows indicate the radical superoxide generation in the mitochondria of pharyngeal bulb of C elegans fed cardiotoxic H6-BJ protein or H2O2.
Functional damage caused by cardiotoxic LC is related to their ability to generate oxygen radicals.
We investigated whether the ability of cardiotoxic LCs to reduce the pharyngeal muscular pumping of the nematodes was related to their propensity to form specific, partially folded intermediates, ie, small, soluble oligomeric assemblies. The physicochemical properties and the oligomerization state of the above-described recombinant and BJ proteins were considered (see supplemental Methods). The electrophoretic data, western blot, and mass spectrometry analysis indicated that these LC preparations were highly homogeneous, revealing a molecular mass in agreement with the theoretical one (supplemental Figure 1). Cardiotoxic and noncardiotoxic LCs showed a comparable degree of stability and a similar pattern of secondary structures (supplemental Figure 2). The size and oligomerization state of recombinant and urinary LCs were evaluated by size-exclusion chromatography and dynamic light scattering studies and indicated that similar monomeric and dimeric species were present in both cardiotoxic and noncardiotoxic LC solutions (supplemental Figures 3-4). In addition, LCs with different organ tropism had a similar exposure of hydrophobic regions (supplemental Figure 5). These data indicated that the ability of cardiotoxic LCs to specifically impair the nematode’s pharynx contraction was not related to a peculiar protein stability or assembly or to differences in exposure to hydrophobic regions.
We then explored whether LC toxicity can be related to their propensity to produce ROS,7,8,28 thus triggering an oxidative damage. Oxygen free radicals produced by MM2-BJ, H6-BJ, H3-r, and K3-r were detected by electron paramagnetic resonance (supplemental Methods and supplemental Figure 7), and the spectra obtained indicated that cardiotoxic LCs exhibited a markedly different behavior compared with noncardiotoxic ones. In particular, H6-BJ produced a greater amount of OH and 2OH radical species than MM2-BJ (supplemental Figure 7). Notably, whereas the tiny amount of radicals produced by noncardiotoxic LCs quickly disappeared, cardiotoxic proteins continuously generate oxygen radicals (supplemental Figure 7), which are known stressors, causing the pharyngeal pumping inhibition in C elegans.
To further explore the oxidative stress pathogenic hypothesis, we next investigated mitochondria to clarify whether the stressful condition generated by cardiotoxic LCs in the C elegans pharynx can result in ROS generation within its subcellular compartments. In fact, an excessive production of ROS within mitochondria can induce oxidative damage that may be related to the pharyngeal dysfunction.29 The pharyngeal pumping dysfunction caused by the cardiotoxic H6-BJ and H3-r proteins was accompanied by a significant increase in the fluorescence of MitoSOX, a mitochondria-specific redox-sensitive dye, indicative of an enhanced oxidant burden (Figure 3B and supplemental Figure 8). This oxidant level was comparable to that generated by exposing worms to 0.1 mM H2O2 (Figure 3B), which caused a significant reduction in the pharyngeal pumping rate (228.3 ± 2.0 and 196.1 ± 1.2 pumps/min for vehicle- and H2O2-fed worms, respectively; P < .01, 1-way ANOVA, n = 30 worms/group). No specific MitoSOX fluorescence was observed in the pharynx of worms fed the MM2-BJ protein or vehicle (Figure 3B). Thus, the pharyngeal pumping dysfunction induced by cardiotoxic LCs is associated with enhanced mitochondrial ROS production.
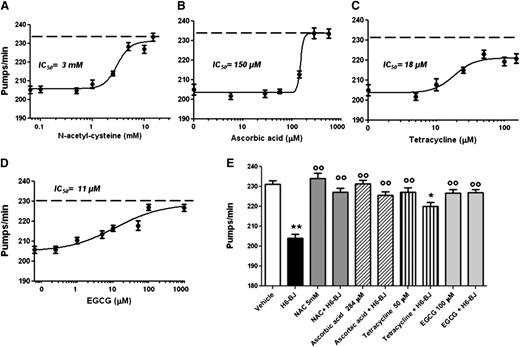
These observations prompted us to explore an alternative approach to abolishing or reducing LC toxicity. The effect of the prototypic antioxidants NAC and ascorbic acid was investigated. We also examined the polyphenolic green tea constituent EGCG and TETRA, both known to possess antioxidant properties.30,31 All these compounds reduced the inhibition of pharyngeal pumping caused by H6-BJ in a dose-dependent manner, although with different potency (Figure 4 A-D). The highest IC50 values were measured for compounds displaying only antioxidant effects (3 ± 1.1 mM and 150 ± 4.5 µM for NAC and ascorbic acid, respectively), whereas EGCG and TETRA had much lower IC50 (11 ± 1.2 µM and 18 ± 1.1 µM for EGCG and TETRA, respectively) (Figure 4). Five mM NAC, 284 µM ascorbic acid, or 100 µM EGCG completely abolished the pharyngeal impairment caused by the H6-BJ, whereas 50 µM TETRA significantly counteracted this inhibition but did not reverse it (Figure 4E). The ability of all these drugs to neutralize the pumping dysfunction caused by cardiotoxic LCs was accompanied by a strong reduction of ROS generation into pharyngeal mitochondria, as indicated by the inhibition of MitoSOX-related fluorescence (supplemental Figure 9). Drugs alone did not affect the pumping rate (Figure 4E) or the increase of pharyngeal mitochondrial oxygen burden (supplemental Figure 9). We also investigated whether ROS scavengers prolonged the lifespan of nematodes fed cardiotoxic LCs. Daily repeated administration of 5 mM NAC or 284 µM ascorbic acid significantly prolonged the survival of H6-BJ–treated worms, completely restoring their natural lifespan (supplemental Figure 10), indicating that the prevention of ROS generation by cardiotoxic LCs can modify the overall toxicity of the proteins. However, these protective effects were not observed with repeated administration of 50 µM TETRA or 100 µM EGCG, even at lower doses, because they were toxic to worms. These findings are in agreement with the reported antihelminthic activity of chronic administration of TETRA and EGCG.32,33
Protective effect of antioxidants, TETRA, and EGCG on the pharyngeal dysfunction caused by cardiotoxic LC. Dose-dependent effect of (A) NAC, (B) ascorbic acid, (C) TETRA, or (D) EGCG. Worms were fed for 2 hours with 100 µg/mL amyloidogenic cardiotoxic protein (H6-BJ) in the absence or presence of increasing concentrations of drugs. Control worms were fed with vehicle alone (dotted line). The pharyngeal pumping rate was scored 20 hours after plating on NGM plates, as described in “Methods.” IC50 value was calculated for each compound. (E) The effect of 5 mM NAC, 284 µM ascorbic acid, 50 µM TETRA, or 100 µM EGCG was determined by incubating worms for 2 hours with 100 µg/mL H6-BJ. The effect of the administration of each compound, alone, at the same concentration and H6-BJ alone was also evaluated. The pharyngeal pumping rate was scored 20 hours after plating on NGM plates, as described in “Methods.” Control worms were fed vehicle alone (vehicle). Mean ± SE (n = 30). **P < .01 and *P < .05 vs vehicle, °°P < .01 vs H6-BJ alone according to 1-way ANOVA followed by Bonferroni post hoc test.
Protective effect of antioxidants, TETRA, and EGCG on the pharyngeal dysfunction caused by cardiotoxic LC. Dose-dependent effect of (A) NAC, (B) ascorbic acid, (C) TETRA, or (D) EGCG. Worms were fed for 2 hours with 100 µg/mL amyloidogenic cardiotoxic protein (H6-BJ) in the absence or presence of increasing concentrations of drugs. Control worms were fed with vehicle alone (dotted line). The pharyngeal pumping rate was scored 20 hours after plating on NGM plates, as described in “Methods.” IC50 value was calculated for each compound. (E) The effect of 5 mM NAC, 284 µM ascorbic acid, 50 µM TETRA, or 100 µM EGCG was determined by incubating worms for 2 hours with 100 µg/mL H6-BJ. The effect of the administration of each compound, alone, at the same concentration and H6-BJ alone was also evaluated. The pharyngeal pumping rate was scored 20 hours after plating on NGM plates, as described in “Methods.” Control worms were fed vehicle alone (vehicle). Mean ± SE (n = 30). **P < .01 and *P < .05 vs vehicle, °°P < .01 vs H6-BJ alone according to 1-way ANOVA followed by Bonferroni post hoc test.
Discussion
Although amyloid deposition is a defining feature of cardiac amyloidosis, the presence of fibrils is not sufficient to explain the cardiotoxicity observed in AL patients.4,5 Clinically, the rapid improvement or worsening of cardiac function, which parallels variations in serum LC concentration in the absence of changes in amyloid load, cannot be explained without attributing a toxic role to prefibrillar, soluble LC species.4,5 The extreme heterogeneity of the clinical presentation, together with the complex interplay of biological, biochemical, and biophysical factors in determining the disease, makes it highly desirable to develop controllable model organisms for the investigation of the pathogenic mechanisms underlying AL in vivo. A transgenic mouse has recently been generated,34 but it does not adequately reproduce AL amyloid cardiomyopathy, thus precluding its use for an efficient study of the mechanisms of heart damage. The toxicity exerted by soluble LCs has been confirmed in in vitro 6-9 and in vivo26,27,34 experiments. Recent data obtained in zebrafish indicated that soluble LC species caused cardiomyopathy as well as a marked dose-dependent reduction in lifespan.26,27
In order to study the pathogenic role of exogenous, soluble LCs in AL cardiomyopathy, we here describe the use of C elegans as a novel animal model. The limitation of this model is its distance from vertebrates, and translation to human pathology requires caution. However, many human stress pathways are replicated in worms, rendering this animal a rapid and versatile system for exploring the mechanisms underlying various complex diseases.35 The suitability of this model was supported by the knowledge that the worm’s pharynx possesses an automatic contractile activity, reminiscent of the vertebrate heart, of which it is considered an ortholog.12,13 We demonstrated that soluble LCs from patients suffering from amyloid cardiomyopathy, but not noncardiotoxic LCs, are specifically recognized as toxicants by the worm’s pharynx. The effect of cardiotoxic LCs was dose dependent and became maximal at concentrations in the range of those commonly found in blood of patients with AL amyloidosis.4,5 Interestingly, damage was already exerted by low concentrations of toxic LCs.
The inhibition of the worm’s pharyngeal pumping persisted up to 48 hours after exposure and was accompanied by cell death, suggesting a profound subversion of pharynx cell function. In time, this resulted in a significant reduction in the worms’ lifespan.
Interestingly, we observed that toxic LCs did not display a peculiar protein folding or propensity to form aggregates of a different size compared with the nontoxic LC species. The lack of differences in oligomerization, secondary structure, stability, and surface hydrophobicity between cardiotoxic and noncardiotoxic LCs lead us to hypothesize that toxicity may proceed through common supersecondary structures linked to the proteins’ primary sequence. Indeed, the nematode assay proved to be very sensitive and specific in this aspect, being capable of discerning the few amino acid differences of 2 recombinant LCs derived from the same germline gene (IGLV1-44), but with different organ tropism and toxicity (Figure 1D, K3-r, from a patient with kidney involvement, and H3-r, from a patient with cardiac dysfunction). This argument is in line with the notion that the LC sequence is a major determinant of specific organ targeting and damage.10,11
It is noteworthy that C elegans has previously been employed as a model to study various amyloidoses.22,36-39 In particular, our group has demonstrated that Aβ1-42 oligomeric intermediates, but not monomers or fibrils, caused a specific impairment of the worm’s pharyngeal function.21,39 The common pattern of damage exerted by cardiotoxic LCs and Aβ oligomers on the worm’s pharyngeal cells supports the hypothesis of shared pathways of toxicity between the 2 proteins. One of these, as described later, may involve mitochondria, which are known early targets of damage in Alzheimer disease40,41 and are particularly abundant in both brain and cardiac muscular cells.
In the zebrafish AL model, the cardiotoxic effect was paralleled by the activation of the oxidative stress pathway,26 confirming the data obtained from other in vitro studies.6-8 Also in our model, dysfunction caused by cardiotoxic LCs was associated with oxidant stress; this, in turn, was related to an increase in the mitochondrial oxidant burden in the pharynx. This organ, similar to the heart, is a mitochondrial-rich tissue, due to mitochondrial crucial role as continuously supplier of energy during the contraction.12,23 Mitochondria are highly sensitive to oxidative damage, and the stressful conditions produced by cardiotoxic LCs in the pharynx, induced by ROS formation, can result in dramatic reduction of ATP availability for cardiomyocyte contraction with loss of organ function. The causal role of oxidative damage in dysfunction is supported by the fact that antioxidant drugs, which counteract ROS-induced damage, prevent the pharyngeal functional impairment caused by cardiotoxic LCs, thus reducing the burden of mitochondrial oxygen radicals. All tested compounds are effective in protecting worms from LC toxicity. From a translational point of view, the data obtained with EGCG and TETRA are of particular interest. These drugs were reported to influence the toxic properties of several amyloidogenic proteins.31,42-47 TETRA, besides its ability to disrupt and/or inhibit amyloid fibril formation, also hinders the oxidative stress by inhibiting ROS generation and stimulating endogenous antioxidant enzymes, such as superoxide dismutase and glutathione peroxidase.31 Preliminary data indicate that EGCG can produce clinical benefits with possible reduction of the cardiac amyloid deposits in patients with AL amyloidosis.48,49 The efficacy of EGCG and TETRA is currently being evaluated in AL patients in phase 2 clinical trials (www.clinicaltrials.gov #NCT01511263 and #NCT01677286, respectively).48,49 Our data indicate that EGCG and TETRA exert a protective effect against LC toxicity. However, at variance with NAC and ascorbic acid, when administered chronically, they did not restore the natural lifespan of C elegans. This is essentially due to the reported peculiar specie-specific toxic effect of both EGCG and TETRA.32,33
Overall, our work shows that C elegans can be considered a “biosensor” suitable for a rapid and inexpensive assessment of the cardiotoxic potential of LCs. Besides allowing the study of the molecular and functional consequences of exposure to LCs, we anticipate its possible use for screening LC cardiotoxicity in basic research and translational applications. As an example, given its sensitivity to small sequence variations (Figure 1D), it may disclose the functional consequences of manipulation of the LC’s primary sequence (directed mutagenesis) or the introduction of posttranslational modifications. This would be of paramount importance for the precise definition of the toxic determinants in LCs. The pharmacologic data show that our assay is also a convenient way to screen the effect of different drugs and to elucidate their molecular mechanisms of action.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ada De Luigi for fluorescence microscopy analysis, Marco Gobbi and Marten Beeg for the critical reading of the manuscript, and Marco Bolis for protein alignment.
C elegans and OP50 E coli were provided by the CGC, funded by the National Institutes of Health Office Research Infrastructure Programs (P40 OD010440). This work was supported by Cariplo Foundation (n 2009-2543 and n 2013-0964), Banca Intesa Sanpaolo (2012-2013), Associazione Italiana per la Ricerca sul Cancro, special program “5 per mille” (N° 9965), the Italian Ministry of Health (GR-2010-2317596), Amyloid Foundation, Fondazione Mintas, Ghislieri College, Pavia, and Fondazione Policlinico San Matteo, Pavia.
Authorship
Contribution: L.D., P.R., F.L., E.d.F., L.C., and E.G. designed and carried out experiments, analyzed results, and wrote the manuscript; M.R., F.F., A.C., and A.d.F. carried out experiments; V.P., G.P. and V.V. helped design experiments and reviewed the manuscript; and G.M. and M.S. designed experiments, interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luisa Diomede, Department of Molecular Biochemistry and Pharmacology, IRCCS-Istituto di Ricerche Farmacologiche “Mario Negri,” Via G. La Masa 19, 20156 Milan, Italy; e-mail: luisa.diomede@marionegri.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal