Key Points
Distinct T-cell patterns characterize the acute and chronic forms of cutaneous GVHD.
Increased TSLP expression is an indicator of acute cutaneous GVHD development.
Abstract
Graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic stem cell transplantation (HCT) and can present in an acute (aGVHD), a chronic lichenoid (clGVHD), and a chronic sclerotic form (csGVHD). It is unclear whether similar or different pathomechanisms lead to these distinct clinical presentations. To address this issue, we collected lesional skin biopsies from aGVHD (n = 25), clGVHD (n = 17), and csGVHD (n = 7) patients as well as serial nonlesional biopsies from HCT recipients (prior to or post-HCT) (n = 14) and subjected them to phenotypic and functional analyses. Our results revealed striking differences between aGVHD and clGVHD. In aGVHD, we found a clear predominance of T helper (Th)2 cytokines/chemokines and, surprisingly, of interleukin (IL)-22 messenger RNA as well as an increase of IL-22–producing CD4+ T cells. Thymic stromal lymphopoietin, a cytokine skewing the immune response toward a Th2 direction, was elevated at day 20 to 30 post-HCT in the skin of patients who later developed aGVHD. In sharp contrast to aGVHD, the immune response occurring in clGVHD showed a mixed Th1/Th17 signature with upregulated Th1/Th17 cytokine/chemokine transcripts and elevated numbers of interferon-γ– and IL-17–producing CD8+ T cells. Our findings shed new light on the T-cell responses involved in the different manifestations of cutaneous GVHD and identify molecular signatures indicating the development of the disease.
Introduction
Graft-versus-host disease (GVHD) remains a major complication of allogeneic hematopoietic stem cell transplantation (HCT) and limits its broader application.1 GVHD is classified into an acute (aGVHD) and chronic (cGVHD) form1,2 based on distinct clinical manifestations.3
In both aGVHD and cGVHD, skin is the most commonly affected target.1 Acute cutaneous GVHD often precedes the involvement of other organs and presents as a maculopapular rash resembling a drug rash and, in an advanced stage, toxic epidermal necrolysis.4 The most characteristic histopathological finding of aGVHD is dyskeratotic epidermal keratinocytes surrounded by lymphocytes, a phenomenon called “satellitosis.”4,5
Chronic cutaneous GVHD shows at the beginning the signs of lichenoid tissue inflammation (chronic lichenoid GVHD [clGVHD]) and later exhibits scleroderma-like features (chronic sclerotic GVHD [csGVHD]).3 Histopathologically, clGVHD displays striking similarities with lichen planus (LP), as evidenced by a prominent band-like lymphocytic infiltrate in the dermis and an epidermal hyperplasia.4,6 In contrast, thickened collagen bundles in the dermis are the most prominent feature of csGVHD lesions.4,6
Concerning the pathogenesis of aGVHD, our basic understanding of the disease as a 3-step process is based on experimental animal models. According to these, the activation of host antigen-presenting cells (APCs), induced by the HCT conditioning, is followed by proliferation and migration of donor T cells toward target sites. In the final “effector phase,” natural killer (NK) cells and CD8+ T cells are presumably involved in mediating organ damage.1,7 The role of T helper (Th)1-induced cytotoxic T lymphocytes as effector cells of aGVHD has been challenged by numerous mouse studies demonstrating an involvement of not only Th17-12 but also Th28,9,12,13 and Th17 cells.8,11,14,15 Studies in aGVHD patients investigating either circulating12,16-21 or skin-localized17-19,22 T-cell subsets have yielded conflicting results. In aGVHD skin, either increased17,22 or decreased numbers18,19 of Th17 cells were reported to occur.
Based on animal studies, chronic GVHD has initially been thought to result from a Th2-type response.23 More recently, data from both murine24,25 and human17,20,26-28 studies have suggested an involvement of Th1 and Th17 cells as well as an altered regulatory T-cell homeostasis. An increasing number of reports also suggest a key role of B cells.29
As for csGVHD, the role of T cells remains to be elucidated.30 In mice, transforming growth factor-β has been shown to be central for the development of fibrosis in csGVHD skin.31
Increasing attention has focused on the role of chemokines and their potential as a therapeutic target in both acute and chronic GVHD.32 The use of agents interfering with these molecules has shown promising results in animal models of aGVHD.32 The first study in patients showed a decreased incidence of visceral injuries when a CCR5-blocking agent was added to aGVHD prophylaxis.33 Meanwhile, acute cutaneous GVHD still occurred at the expected rates, suggesting that distinct chemokines mediate the homing of effector cells to the skin.
Taken together, it is unclear which exact mechanisms are operative in the T-cell immunopathogenesis of the different types of cutaneous GVHD and which molecules are involved in lymphocyte recruitment to the skin. In this study, we searched for cellular and molecular patterns that would allow us to distinguish between the different forms of cutaneous GVHD and draw conclusions about the pathomechanisms involved.
Patients, materials, and methods
Study design
A total of 65 HCT recipients treated in the Bone Marrow Transplantation Unit of Internal Medicine I of Vienna’s Medical University between 2007 and 2013 were enrolled in the study.
Lesional skin biopsies were obtained from patients suffering from aGVHD (n = 25), clGVHD (n = 17), and csGVHD (n = 7). Fourteen HCT recipients underwent serial skin biopsies at different time points prior and after HCT (before and after conditioning, day 20-30 post-HCT, day 100-120 post-HCT), one of which was also included in the aGVHD patients group. Peripheral blood samples were obtained from 5 of the included GVHD patients (aGVHD: n = 3, clGVHD: n = 2) as well as from 3 additional HCT recipients (day 20-25 post-HCT). Skin samples of healthy controls (HCs) (n = 22) obtained as discarded tissue from abdominoplasty as well as blood samples obtained from healthy volunteers (n = 11) served as control groups.
The study was approved by the Ethics Committee of the Medical University of Vienna (EK 607/07). All study subjects gave written informed consent in accordance with the Declaration of Helsinki and participated voluntarily.
Patient characteristics
Using the National Institutes of Health diagnostic criteria,3 25 patients with aGVHD, 17 patients suffering from clGVHD, and 7 persons manifesting csGVHD were included in this study. At the time of biopsy, none of the aGVHD patients was under corticosteroid therapy. Seven of the 14 HCT recipients undergoing serial skin biopsies developed aGVHD (5 women, 2 men, median age 46.7 ± 11.0 years) and 7 did not (5 women, 2 men, median age 49.0 ± 6.7 years). Patient characteristics are summarized in Table 1, details of the individual patients are provided in supplemental Table 1 (available on the Blood Web site).
Patient characteristics
| . | GVHD patients . | HCT recipients undergoing serial skin biopsies . | |||
|---|---|---|---|---|---|
| aGVHD (n = 25) . | clGVHD (n = 17) . | csGVHD (n = 7) . | no aGVHD (n = 7) . | aGVHD (n = 7) . | |
| Mean age, y + SD | 46.4 ± 13.0 | 44.7 ± 12.6 | 52.0 ± 8.0 | 49.0 ± 6.7 | 46.7 ± 11 |
| Gender recipient | |||||
| Female | 13 | 10 | 4 | 5 | 5 |
| Male | 12 | 7 | 3 | 2 | 2 |
| Diagnosis prior to HCT | |||||
| AML | 10 | 6 | 2 | 1 | 1 |
| CML | 1 | 2 | 1 | ||
| HL | 1 | ||||
| NHL | 5 | 2 | 1 | 1 | 1 |
| sAML | 3 | 2 | |||
| ALL | 3 | 6 | 1 | 2 | 2 |
| MDS | 2 | 1 | 1 | ||
| CLL | 1 | ||||
| PNH | 1 | ||||
| Disease stage prior to HCT | |||||
| CR1 | 8 | 5 | 3 | 4 | 4 |
| CR2 | 7 | 3 | 1 | ||
| CR3 | 1 | 1 | 3 | ||
| CR4 | 1 | ||||
| CR5 | 1 | ||||
| PROG | 1 | 1 | 1 | ||
| REFR | 7 | 5 | 4 | 1 | |
| CP | 1 | ||||
| Stem cell source | |||||
| BM | 2 | 1 | 2 | 1 | |
| PBSC | 23 | 17 | 6 | 5 | 6 |
| Donor type | |||||
| MUD | 15 | 8 | 5 | 2 | 2 |
| MMUD | 6 | 5 | 2 | 2 | |
| SIB | 4 | 4 | 2 | 3 | 3 |
| Gender mismatch (donor/recipient) | |||||
| F/F | 11 | 2 | 2 | 4 | 3 |
| M/F | 2 | 8 | 1 | 1 | 2 |
| M/M | 12 | 4 | 1 | 1 | 2 |
| F/M | 3 | 1 | 1 | ||
| N/A | 2 | ||||
| Conditioning | |||||
| MAC | 2 | ||||
| MAC (TBI) | 9 | 8 | 6 | 3 | 2 |
| RIC | 8 | 6 | 2 | ||
| RIC (TBI) | 6 | 3 | 1 | 2 | 5 |
| GVHD prophylaxis | |||||
| CSA | 1 | 1 | 1 | 1 | |
| CSA MMF | 13 | 6 | 3 | 2 | |
| CSA MTX | 11 | 10 | 6 | 4 | 4 |
| GVHD score skin | (modified Glucksberg criteria35 ) | (NIH consensus criteria3 ) | (NIH consensus criteria3 ) | ||
| Score 1 | 6 | 4 | |||
| Score 2 | 7 | 7 | 1 | ||
| Score 3 | 12 | 6 | 6 | ||
| GVHD overall grade | (modified Glucksberg criteria35 ) | (NIH consensus criteria3 ) | (NIH consensus criteria3 ) | ||
| Grade 1 | 10 | ||||
| Grade 2 | 10 | ||||
| Grade 3 | 2 | ||||
| Grade 4 | 3 | ||||
| Mild | 2 | ||||
| Moderate | 9 | 1 | |||
| Severe | 6 | 6 | |||
| Onset type of cGVHD | |||||
| DN | 4 | 2 | |||
| QUIESC | 9 | 5 | |||
| PROG | 4 | ||||
| . | GVHD patients . | HCT recipients undergoing serial skin biopsies . | |||
|---|---|---|---|---|---|
| aGVHD (n = 25) . | clGVHD (n = 17) . | csGVHD (n = 7) . | no aGVHD (n = 7) . | aGVHD (n = 7) . | |
| Mean age, y + SD | 46.4 ± 13.0 | 44.7 ± 12.6 | 52.0 ± 8.0 | 49.0 ± 6.7 | 46.7 ± 11 |
| Gender recipient | |||||
| Female | 13 | 10 | 4 | 5 | 5 |
| Male | 12 | 7 | 3 | 2 | 2 |
| Diagnosis prior to HCT | |||||
| AML | 10 | 6 | 2 | 1 | 1 |
| CML | 1 | 2 | 1 | ||
| HL | 1 | ||||
| NHL | 5 | 2 | 1 | 1 | 1 |
| sAML | 3 | 2 | |||
| ALL | 3 | 6 | 1 | 2 | 2 |
| MDS | 2 | 1 | 1 | ||
| CLL | 1 | ||||
| PNH | 1 | ||||
| Disease stage prior to HCT | |||||
| CR1 | 8 | 5 | 3 | 4 | 4 |
| CR2 | 7 | 3 | 1 | ||
| CR3 | 1 | 1 | 3 | ||
| CR4 | 1 | ||||
| CR5 | 1 | ||||
| PROG | 1 | 1 | 1 | ||
| REFR | 7 | 5 | 4 | 1 | |
| CP | 1 | ||||
| Stem cell source | |||||
| BM | 2 | 1 | 2 | 1 | |
| PBSC | 23 | 17 | 6 | 5 | 6 |
| Donor type | |||||
| MUD | 15 | 8 | 5 | 2 | 2 |
| MMUD | 6 | 5 | 2 | 2 | |
| SIB | 4 | 4 | 2 | 3 | 3 |
| Gender mismatch (donor/recipient) | |||||
| F/F | 11 | 2 | 2 | 4 | 3 |
| M/F | 2 | 8 | 1 | 1 | 2 |
| M/M | 12 | 4 | 1 | 1 | 2 |
| F/M | 3 | 1 | 1 | ||
| N/A | 2 | ||||
| Conditioning | |||||
| MAC | 2 | ||||
| MAC (TBI) | 9 | 8 | 6 | 3 | 2 |
| RIC | 8 | 6 | 2 | ||
| RIC (TBI) | 6 | 3 | 1 | 2 | 5 |
| GVHD prophylaxis | |||||
| CSA | 1 | 1 | 1 | 1 | |
| CSA MMF | 13 | 6 | 3 | 2 | |
| CSA MTX | 11 | 10 | 6 | 4 | 4 |
| GVHD score skin | (modified Glucksberg criteria35 ) | (NIH consensus criteria3 ) | (NIH consensus criteria3 ) | ||
| Score 1 | 6 | 4 | |||
| Score 2 | 7 | 7 | 1 | ||
| Score 3 | 12 | 6 | 6 | ||
| GVHD overall grade | (modified Glucksberg criteria35 ) | (NIH consensus criteria3 ) | (NIH consensus criteria3 ) | ||
| Grade 1 | 10 | ||||
| Grade 2 | 10 | ||||
| Grade 3 | 2 | ||||
| Grade 4 | 3 | ||||
| Mild | 2 | ||||
| Moderate | 9 | 1 | |||
| Severe | 6 | 6 | |||
| Onset type of cGVHD | |||||
| DN | 4 | 2 | |||
| QUIESC | 9 | 5 | |||
| PROG | 4 | ||||
ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; BM, bone marrow; CLL, chronic lymphoid leukemia; CML, chronic myeloid leukemia; CP, chronic phase; CR1/CR2/CR3/CR4/CR5, first/second/third/fourth/fifth complete remission; CSA, cyclosporin A; DN, de novo; F, female; HL, Hodgkin lymphoma; M, male; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MMUD, mismatched unrelated donor; MTX, methotrexate; MUD, matched unrelated donor; N/A, not available; NHL, non-Hodgkin lymphoma; PBSC, peripheral blood stem cell; PNH, paroxysmal nocturnal hemoglobinuria; PROG, progressive; QUIESC, quiescent; REFR, refractory; RIC, reduced intensity conditioning; sAML, secondary AML; SIB, sibling; TBI, total body irradiation.
Skin samples
Skin punch biopsies (6 mm in diameter) were taken under local anesthesia. One-half or one-third was embedded in formalin and used for conventional hematoxylin/eosin histopathological analysis. The residual tissue material was divided in half, one for TRIzol (Invitrogen Life Technologies, Vienna, Austria) lysis (real-time reverse transcription-polymerase chain reaction [RT-PCR]) and one for embedding in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek Europe B.V., Alphen aan der Rijn, The Netherlands) (immunohistology) and subsequent deep-freezing in liquid nitrogen. Tissue specimens were stored at −20°C (OCT) and −80°C (TRIzol) until further processing.
For the fluorescence-activated cell sorting (FACS) analyses of infiltrating cells, one and a half of two 6-mm skin punch biopsies were used per patient (aGVHD: n = 3, clGVHD: n = 2); the rest was used for histopathological work-up. Biopsies were transported to the laboratory in phosphate-buffered saline (Gibco Life Technologies, Lofer, Austria) and directly processed. Subcutaneous fat was removed, and the remaining tissue was minced with a scalpel and digested at 37°C with type 4 collagenase (Worthington, Lakewood, NJ) and deoxyribonuclease I from bovine pancreas (Sigma-Aldrich, Vienna, Austria) dissolved in RPMI-1640 (Gibco Life Technologies). The cell suspension was collected and filtered through a 70-μm cell strainer (BD Falcon, Franklin Lakes, NJ).
Peripheral blood samples
Blood drawn from HCT recipients (aGVHD: n = 3, clGVHD: n = 2) and HCs (n = 6) was collected in BD Vacutainer CPT tubes (BD Biosciences, Vienna, Austria) and processed according to the manufacturer’s guidelines.
IF and IH stainings
For both immunofluorescence (IF) and immunohistochemistry (IH) stainings, OCT-embedded tissue was cut into 5-µm sections and mounted on capillary gap microscope slides (Dako, Glostrup, Denmark). After 20 minutes of air-drying, the cryostat sections were fixed in ice-cold acetone (Sigma-Aldrich) for 10 minutes and stored at –20°C. A detailed description of IF and IH stainings is provided in the supplemental Materials and methods and Table 2.
Details and combinations of antibodies used for IF stainings
| Cell type . | Antibodies used for IF stainings . | ||||||
|---|---|---|---|---|---|---|---|
| Specificity . | Labeling . | *Sec. labeling . | Clone . | Isotype . | Dilution . | Manufacturer (location) . | |
| Leukocyte | CD45 | Purified | *A488 | 2D1 | Mouse IgG1 κ | 1:25 | BD Biosciences (Vienna, Austria) |
| T cell | CD3 | Purified | *A488 | UCHT1 | Mouse IgG1 κ | 1:150 | Beckman Coulter (Vienna, Austria) |
| CD4+/CD8+ | CD4 | Purified | *A568 | MT310 | Mouse IgG1 | 1:400 | Dako (Vienna, Austria) |
| CD8 | Purified | *A647 | C8/144B | Mouse IgG1 κ | 1:100 | Dako | |
| Regulatory T cell | CD3 | Purified | *A488 | UCHT1 | Mouse IgG1 κ | 1:150 | Beckman Coulter |
| CD25 | FITC | *A568 | BC96 | Mouse IgG1 κ | 1:10 | BioLegend (California, CA) | |
| Foxp3 | Purified | *A647 | 236A/E7 | Mouse IgG1 | 1:300 | eBioscience (Vienna, Austria) | |
| NK T cell | CD3 | Purified | *A488 | UCHT1 | Mouse IgG1 κ | 1:150 | Beckman Coulter |
| CD1d | Purified | *A568 | NOR3.2 | Mouse IgG1 | 1:50 | BioSource International (Camarillo, CA) | |
| NK cell | CD3 | Purified | *A488 | UCHT1 | Mouse IgG1 κ | 1:150 | Beckman Coulter |
| CD56 (Leu-19) | Purified | *A568 | MY31 | Mouse IgG1 κ | 1:100 | BD Biosciences | |
| B cell | CD19 | Purified | *A488 | J3-119 | Mouse IgG1 | 1:150 | Beckman Coulter |
| LC | CD1a | FITC | *A488 | HI149 | Mouse IgG1 κ | 1:50 | BD Pharmingen (Vienna, Austria) |
| CD207 (Langerin) | Purified | *A568 | DCGM4 | Mouse IgG1 | 1:1000 | Beckman Coulter | |
| pDC | CD303 (BDCA-2) | Purified | *A568 | 104C12.08 | Mouse IgG1 | 1:200 | Dendritics (Lyon, France) |
| CD123 (IL-3Ra) | Purified | *A488 | 9F5 | Mouse IgG1 | 1:100 | BD Pharmingen | |
| CD1c+ DC | CD1c (BDCA-1) | Purified | *A568 | M241 | Mouse IgG1 κ | 1:1000 | Ancell (Bayport, MN) |
| HLA-DR | APC | *A488 | G46-6 | Mouse IgG2a κ | 1:400 | BD Biosciences | |
| Macrophage | CD68 | Purified | *A568 | KP1 | Mouse IgG1 | 1:400 | Dako |
| CD163 | FITC | *A488 | 5C6-FAT | Mouse IgG1 | 1:100 | Acris (Herford, Germany) | |
| CD11c | Purified | *A647 | KB90 | Mouse IgG1 | 1:100 | Dako | |
| Neutrophil | CD15 | FITC | (no labeling) | 80H5 | Mouse IgM | 1:200 | Beckman Coulter |
| HLA-DR | APC | *A568 | G46-6 | Mouse IgG2a κ | 1:400 | BD Biosciences | |
| Eosinophil | MBP | Purified | *A488 | BMK13 | Mouse IgG1 | 1:400 | Research Diagnostics, Inc (Flanders, NJ) |
| Basophil | CD123 (IL-3Ra) | Purified | *A488 | 9F5 | Mouse IgG1 | 1:100 | BD Pharmingen |
| CD203c (ENPP-3) | Purified | *A568 | NP4D6 | Mouse IgG1 | 1:400 | BioLegend | |
| Mast cell | CD117 (c-Kit) | Purified | *A488 | 95C3 | Mouse IgG1 | 1:100 | An der Grub (Vienna, Austria) |
| CD203c (ENPP-3) | Purified | *A568 | NP4D6 | Mouse IgG1 | 1:400 | BioLegend | |
| Cell type . | Antibodies used for IF stainings . | ||||||
|---|---|---|---|---|---|---|---|
| Specificity . | Labeling . | *Sec. labeling . | Clone . | Isotype . | Dilution . | Manufacturer (location) . | |
| Leukocyte | CD45 | Purified | *A488 | 2D1 | Mouse IgG1 κ | 1:25 | BD Biosciences (Vienna, Austria) |
| T cell | CD3 | Purified | *A488 | UCHT1 | Mouse IgG1 κ | 1:150 | Beckman Coulter (Vienna, Austria) |
| CD4+/CD8+ | CD4 | Purified | *A568 | MT310 | Mouse IgG1 | 1:400 | Dako (Vienna, Austria) |
| CD8 | Purified | *A647 | C8/144B | Mouse IgG1 κ | 1:100 | Dako | |
| Regulatory T cell | CD3 | Purified | *A488 | UCHT1 | Mouse IgG1 κ | 1:150 | Beckman Coulter |
| CD25 | FITC | *A568 | BC96 | Mouse IgG1 κ | 1:10 | BioLegend (California, CA) | |
| Foxp3 | Purified | *A647 | 236A/E7 | Mouse IgG1 | 1:300 | eBioscience (Vienna, Austria) | |
| NK T cell | CD3 | Purified | *A488 | UCHT1 | Mouse IgG1 κ | 1:150 | Beckman Coulter |
| CD1d | Purified | *A568 | NOR3.2 | Mouse IgG1 | 1:50 | BioSource International (Camarillo, CA) | |
| NK cell | CD3 | Purified | *A488 | UCHT1 | Mouse IgG1 κ | 1:150 | Beckman Coulter |
| CD56 (Leu-19) | Purified | *A568 | MY31 | Mouse IgG1 κ | 1:100 | BD Biosciences | |
| B cell | CD19 | Purified | *A488 | J3-119 | Mouse IgG1 | 1:150 | Beckman Coulter |
| LC | CD1a | FITC | *A488 | HI149 | Mouse IgG1 κ | 1:50 | BD Pharmingen (Vienna, Austria) |
| CD207 (Langerin) | Purified | *A568 | DCGM4 | Mouse IgG1 | 1:1000 | Beckman Coulter | |
| pDC | CD303 (BDCA-2) | Purified | *A568 | 104C12.08 | Mouse IgG1 | 1:200 | Dendritics (Lyon, France) |
| CD123 (IL-3Ra) | Purified | *A488 | 9F5 | Mouse IgG1 | 1:100 | BD Pharmingen | |
| CD1c+ DC | CD1c (BDCA-1) | Purified | *A568 | M241 | Mouse IgG1 κ | 1:1000 | Ancell (Bayport, MN) |
| HLA-DR | APC | *A488 | G46-6 | Mouse IgG2a κ | 1:400 | BD Biosciences | |
| Macrophage | CD68 | Purified | *A568 | KP1 | Mouse IgG1 | 1:400 | Dako |
| CD163 | FITC | *A488 | 5C6-FAT | Mouse IgG1 | 1:100 | Acris (Herford, Germany) | |
| CD11c | Purified | *A647 | KB90 | Mouse IgG1 | 1:100 | Dako | |
| Neutrophil | CD15 | FITC | (no labeling) | 80H5 | Mouse IgM | 1:200 | Beckman Coulter |
| HLA-DR | APC | *A568 | G46-6 | Mouse IgG2a κ | 1:400 | BD Biosciences | |
| Eosinophil | MBP | Purified | *A488 | BMK13 | Mouse IgG1 | 1:400 | Research Diagnostics, Inc (Flanders, NJ) |
| Basophil | CD123 (IL-3Ra) | Purified | *A488 | 9F5 | Mouse IgG1 | 1:100 | BD Pharmingen |
| CD203c (ENPP-3) | Purified | *A568 | NP4D6 | Mouse IgG1 | 1:400 | BioLegend | |
| Mast cell | CD117 (c-Kit) | Purified | *A488 | 95C3 | Mouse IgG1 | 1:100 | An der Grub (Vienna, Austria) |
| CD203c (ENPP-3) | Purified | *A568 | NP4D6 | Mouse IgG1 | 1:400 | BioLegend | |
Details of antibodies used for IF single/double/triple staining procedures and the antibody combinations used for the indicated cell types. Antibodies were, if indicated, labeled with the listed dyes (secondary labeling). *A488, antibody labeled with a Zenon Alexa Fluor 488 labeling kit; *A568, antibody labeled with Alexa Fluor 568; *A647, antibody labeled with Alexa Fluor 647); DC, dendritic cell; MBP, major basic protein; Sec., secondary.
Evaluation of IF results
Using a rectangular grid, biopsy specimens were read in a blinded fashion at ×400 magnification by 2 independent investigators using a fluorescence microscope (ocular 10×/25; Axiophot, Zeiss, Oberkochen, Germany) equipped with a filter for double stainings. Confocal IF microscopy (LSM 510, Zeiss) was used to evaluate triple stainings. The mean inter-observer coefficient of variation was <10%. Skin-infiltrating cells were separately quantified in the epidermis and dermis. Labeled cells were enumerated per visual field and expressed as the number of cells ± standard deviation (SD) per either millimeter basement membrane (epidermis) or square millimeter (dermis). Only cells clearly positive for the antigen of interest were counted.34
Evaluation of IH staining for TSLP
To analyze the intensity of the thymic stromal lymphopoietin (TSLP) staining, the slides were scanned using the digital HistoFAXS imaging system (TissueGnostics GmbH, Vienna, Austria). The mean intensity of TSLP expression was measured using HistoQuest imaging analysis software (TissueGnostics GmbH). All images were acquired and analyzed using identical hardware and software settings.
Real-time RT-PCR
TRIzol-fixed frozen samples were thawed on ice and then homogenized with a FastPrep-24 homogenizer (MP Biomedicals, Solon, OH) for RNA isolation according to the manufacturer’s instructions. The RNA concentration was determined with a NanoDrop ND-100 spectrophotometer (Peqlab Biotechnology, Erlangen, Germany). DNA was removed by TURBO DNase (Applied Biosystems, Foster City, CA) treatment as described by the manufacturer. A High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used to perform reverse transcription of RNA to cDNA according to the standard protocol for a 20-μL reaction on a Thermocycler Perkin Elmer 480 (Perkin Elmer, Waltham, MA). The reactions were amplified and quantified by a TaqMan Universal PCR Master Mix (Applied Biosystems) on an Applied Biosystems ABI PRISM 7700. The following thermal cycler conditions were used: 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of 15 seconds at 95°C followed by 60 seconds at 60°C. The housekeeping human β2-microglobulin (β2m) gene was used to normalize each sample and each gene. The TaqMan Gene Expression Assays (all Applied Biosystems) listed in supplemental Table 2 were used for the primers and probes for the TaqMan RT-PCR assays; the data were analyzed and samples were quantified by software provided with the Applied Biosystems ABI PRISM 7700 (Sequence Detection Systems, version 1.7) and calculated using the comparative CT method with β2m as reference gene relative to normal skin.
FACS
Peripheral blood mononuclear cells and cell suspensions obtained from collagenase digestion of skin specimens were adjusted to a concentration of 1 × 106 cells/mL and then stimulated for 4 hours at 37°C with 25 ng/mL phorbolmyristate acetate and 2 µg/mL ionomycin in the presence of 10 µg/mL brefeldin A (all Sigma-Aldrich) dissolved in RPMI-1640 (Gibco Life Technologies). Cells treated with brefeldin A only served as a control. After washing with magnetic-activated cell sorting buffer, the cells were fixed and permeabilized according to the manufacturer’s protocol (BioLegend, San Diego, CA). All FACS stainings were performed after fixation (antibodies listed in supplemental Table 3). Seven-color flow cytometry analyses were performed on a FACS Aria (BD Biosciences) and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). All stainings were adapted to isotype-matched controls and gated accordingly.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software (Graphpad, San Diego, CA). For comparison of means between more than 2 groups (aGVHD, clGVHD, csGVHD, HC), analysis of variance one-way analysis with Tukey post-test was used for all experiments. Unpaired Student t test was applied to compare patients who had undergone serial skin biopsies (those who later developed aGVHD vs those who did not). A P value < .05 was considered statistically significant.
Results
Clinical and histopathological diagnosis of GVHD
In all patients, the diagnosis of GVHD was based on the National Institutes of Health consensus criteria3 and confirmed by histopathology (supplemental Figure 1). Clinical grading of aGVHD was based on modified Glucksberg criteria,35 histopathological grading on Lerner criteria5 (Table 1; supplemental Table 1). For cGVHD, clinical grading was performed according to the National Institutes of Health consensus.3
Composition of the cellular infiltrate in the different forms of cutaneous GVHD
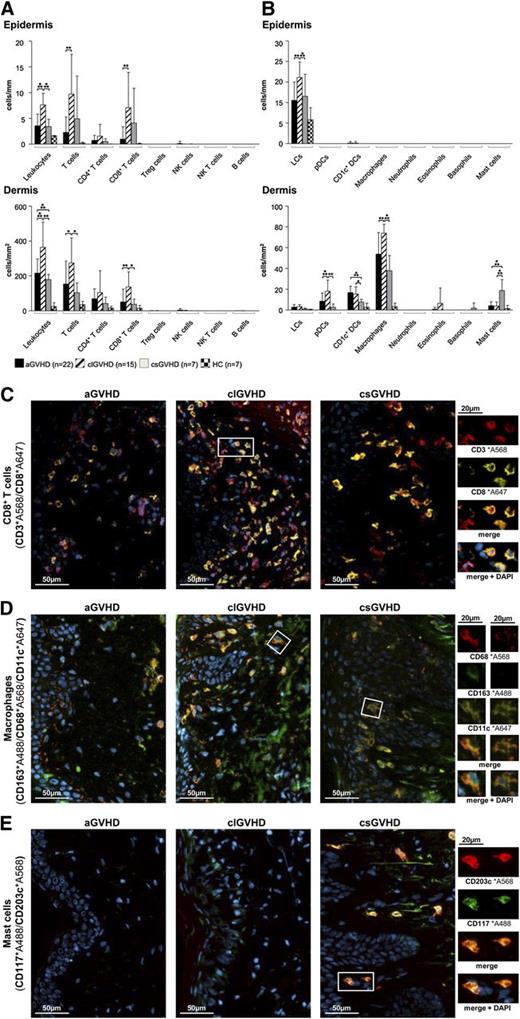
In all forms of GVHD, IF stainings of frozen skin sections revealed the infiltrate in both the epidermis and the dermis to be dominated by T cells (Figure 1A), whereas CD19+ B cells, NK cells (CD3−CD56+), granulocytes and mast cells (CD117+CD203c+) (Figure 1B) were almost absent. In aGVHD, the increase of CD3+ cells affected both the CD4+ and CD8+ (Figure 1C) subsets but not T cells with a “regulatory” phenotype (CD3+CD25+FoxP3+) (Figure 1A; supplemental Figure 2D) and NK T cells (CD3+CD1d+). With regard to APCs (Figure 1B), the most prominent populations in the dermis were macrophages (CD163+CD68+) (Figure 1D) and CD1c+ DCs (supplemental Figure 2A), while Langerhans cells (CD207+CD1a+) (supplemental Figure 2B) predominated in the epidermis.
The composition of the cellular infiltrate differs between the diverse types of cutaneous GVHD. (A-B) Quantitative in situ analysis of the cellular infiltrate within aGVHD, clGVHD, and csGVHD skin lesions and normal skin from HCs. Single and multicolor IF stainings were performed with the following markers: pan-leukocytes: CD45+; T cells: CD3+; CD4+ T cells: CD3+CD4+; CD8+ T cells: CD3+CD8+; regulatory T (Treg) cells: CD3+CD25+Foxp3+; NK cells: CD56+CD3−; NK T cells: CD3+CD1d+; B cells: CD19+; Langerhans cells (LCs): CD207+CD1a+; plasmacytoid DCs (pDCs): BDCA2+CD123+; CD1c+ DCs: CD1c+HLA-DR+; macrophages: CD163+CD68+; neutrophils: CD15+HLA-DR−; eosinophils: MBP+; basophils: CD123+CD203c+; and mast cells: CD117+CD203c+. Data are given either as absolute numbers of positive cells ± SD per mm (epidermis) or per mm2 (dermis). *P < .05, **P < .01, ***P < .001. A significance in differences between the various groups is shown only for the various GVHD manifestations. (C-E) Representative images of IF multicolor stainings of CD8+ T cells (CD3*A568/CD8*A647) (C), macrophages (CD163*A488/CD68*A568/CD11c*A647) (D), and mast cells (CD117*A488/CD203c*A568) (E) merged with 4′,6-diamidino-2-phenylindole (DAPI) in an aGVHD, clGVHD, and csGVHD patient. The framed cells are shown in a larger magnification on the right. The epidermis is, if shown, located on the left in all images. Slides were scanned using a TissueFAXS imaging system (TissueGnostics GmbH) equipped with a Zeiss Axio Imager.Z1 microscope (Carl Zeiss Inc., Jena, Germany) with filters detecting DAPI, green fluorescent protein, Cy3, and Cy5 fluorochromes. Images were taken with Zeiss LD Plan-Neofluar objectives (primary objective ×20/0.4, ocular objective ×10) at room temperature using a PCO PixelFly camera (Zeiss), exported from the TissueQuest software (TissueGnostics GmbH) as tiff images, and processed in Adobe Photoshop CS5 (Adobe Systems, San Jose, CA).
The composition of the cellular infiltrate differs between the diverse types of cutaneous GVHD. (A-B) Quantitative in situ analysis of the cellular infiltrate within aGVHD, clGVHD, and csGVHD skin lesions and normal skin from HCs. Single and multicolor IF stainings were performed with the following markers: pan-leukocytes: CD45+; T cells: CD3+; CD4+ T cells: CD3+CD4+; CD8+ T cells: CD3+CD8+; regulatory T (Treg) cells: CD3+CD25+Foxp3+; NK cells: CD56+CD3−; NK T cells: CD3+CD1d+; B cells: CD19+; Langerhans cells (LCs): CD207+CD1a+; plasmacytoid DCs (pDCs): BDCA2+CD123+; CD1c+ DCs: CD1c+HLA-DR+; macrophages: CD163+CD68+; neutrophils: CD15+HLA-DR−; eosinophils: MBP+; basophils: CD123+CD203c+; and mast cells: CD117+CD203c+. Data are given either as absolute numbers of positive cells ± SD per mm (epidermis) or per mm2 (dermis). *P < .05, **P < .01, ***P < .001. A significance in differences between the various groups is shown only for the various GVHD manifestations. (C-E) Representative images of IF multicolor stainings of CD8+ T cells (CD3*A568/CD8*A647) (C), macrophages (CD163*A488/CD68*A568/CD11c*A647) (D), and mast cells (CD117*A488/CD203c*A568) (E) merged with 4′,6-diamidino-2-phenylindole (DAPI) in an aGVHD, clGVHD, and csGVHD patient. The framed cells are shown in a larger magnification on the right. The epidermis is, if shown, located on the left in all images. Slides were scanned using a TissueFAXS imaging system (TissueGnostics GmbH) equipped with a Zeiss Axio Imager.Z1 microscope (Carl Zeiss Inc., Jena, Germany) with filters detecting DAPI, green fluorescent protein, Cy3, and Cy5 fluorochromes. Images were taken with Zeiss LD Plan-Neofluar objectives (primary objective ×20/0.4, ocular objective ×10) at room temperature using a PCO PixelFly camera (Zeiss), exported from the TissueQuest software (TissueGnostics GmbH) as tiff images, and processed in Adobe Photoshop CS5 (Adobe Systems, San Jose, CA).
In clGVHD, the prominent band-like infiltrate consisted mainly of T lymphocytes, which, in majority, displayed a CD8+ phenotype (Figure 1A,C). The distribution of APCs in clGVHD lesions was comparable with that seen in aGVHD (Figure 1B), with macrophages (Figure 1D) dominating the dermis and Langerhans cells the epidermis (supplemental Figure 2B).
csGVHD lesions harbored, besides CD4+ and CD8+ T cells, substantial numbers of mast cells (Figure 1B,E).
Upregulation of different cytokines and chemokines in acute and chronic cutaneous GVHD
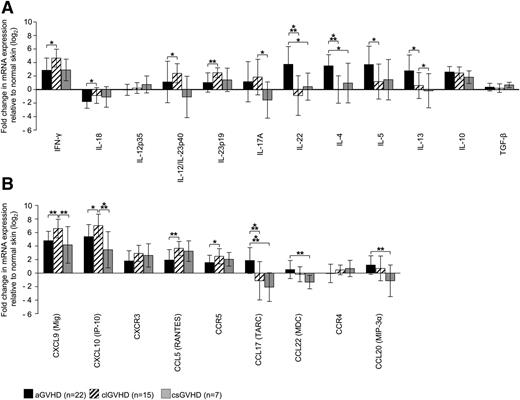
To get a clue as to the cytokine and chemokine patterns in GVHD skin lesions, we measured the expression of these molecules by quantitative RT-PCR and quantified it relative to samples from HC (Figure 2A-B).
Distinct expression of T-cell cytokines and chemokine ligands/receptors in GVHD skin lesions. (A-B) Quantitative real-time RT-PCR was performed after TRIzol lysis of lesional skin biopsies of aGVHD, clGVHD, and csGVHD patients. Data are normalized to β2m of each specimen and represent the mean ± SD of fold change (log2) in mRNA expression relative to normal skin (n = 10). *P < .05, **P < .01, ***P < .001.
Distinct expression of T-cell cytokines and chemokine ligands/receptors in GVHD skin lesions. (A-B) Quantitative real-time RT-PCR was performed after TRIzol lysis of lesional skin biopsies of aGVHD, clGVHD, and csGVHD patients. Data are normalized to β2m of each specimen and represent the mean ± SD of fold change (log2) in mRNA expression relative to normal skin (n = 10). *P < .05, **P < .01, ***P < .001.
Our analysis revealed striking differences between the different types of GVHD. In addition to an increase of interferon (IFN)-γ and interleukin (IL)-10 mRNA, we found IL-4, IL-5, and IL-13 transcripts to be highly upregulated in aGVHD compared with the other forms of the disease (Figure 2A). This strong “Th2 signature” was paralleled by an enhancement of the Th2 chemokines CCL17 and CCL22, which, in contrast, were downregulated in clGVHD and csGVHD (Figure 2B). Strikingly, we observed an overexpression of IL-22, but not IL-17 mRNA, in aGVHD, which was not the case in the chronic forms of GVHD.
In sharp contrast to the situation in aGVHD, we found a mixed “Th1/Th17 signature” in clGVHD skin. This was evidenced by a robust upregulation of IFN-γ as well as of Th1 (IL-12/IL-23p40) and Th17 (IL-23p19/p40) cytokine transcripts compared with the other forms of GVHD (Figure 2A). The Th1 signature was also manifested in an increase of chemokines associated with Th1-mediated immune responses (CXCL9, CXCL10) (Figure 2B). In csGVHD, Th1 signals (IFN-γ, CXCL9, CXCL10, CCL5) predominated (Figure 2A-B).
Expression patterns of cytotoxic molecules in cutaneous GVHD
In all forms of GVHD, transcripts of granzyme A and B as well as of perforin were strongly upregulated and contrasted with a downregulation of inducible nitric oxide synthase and TNF-related apoptosis-inducing ligand (supplemental Figure 3A-B). Fas ligand (FasL) mRNA was particularly enhanced in clGVHD and TRAIL receptor 2, 3, and 4 mRNA was upregulated in csGVHD.
Distinct cytokine profiles of T cells in aGVHD and clGVHD skin lesions
To determine the cellular source of those effector cytokines that were differentially regulated at the transcriptional level, we stimulated leukocytes that had been freshly isolated from lesional skin biopsies (aGVHD: n = 3, clGVHD: n = 2) with phorbolmyristate acetate/ionomycin and analyzed CD4+ and CD8+ T cells for their expression of IFN-γ, IL-4, IL-17, and IL-22 (Figure 3A-C). In accordance with our RT-PCR data, biopsies from aGVHD, but not those from clGVHD and HC, contained increased numbers of IL-22 single-positive CD4+ (Th22) T cells. Whereas IL-17/IL-22 double-positive T cells were almost absent in both aGVHD and clGVHD lesions, we found an increased proportion of IL-17 single-producing CD8+ T cells in clGVHD compared with aGVHD and HC (Figure 3B-C).
CD4+and CD8+T cells isolated from aGVHD and clGVHD skin lesions differ in their cytokine profiles. Cells isolated from collagenase-digested skin biopsies of aGVHD (n = 3) or clGVHD (n = 2) patients as well as of HCs (n = 5) were stimulated for 4 hours with phorbolmyristate acetate/ionomycin. Samples that had been incubated with brefeldin A only (data not shown) served as negative controls. Thereafter, cells were stained for CD3, CD4, CD8, IFN-γ, IL-4, IL-17, and IL-22 and analyzed with 7-color flow cytometry; gates were set based on isotype-matched controls. (A) FACS gating strategy. (B) CD4+ T cells in aGVHD skin produce higher amounts of IL-22 than in clGVHD. Data are given as percentages ± SD of CD4+ and CD8+ T cells that stained positive for IL-22, IL-17/IL-22, IL17, IL-4, or IFN-ү. (C) Representative FACS plots of cytokine expression (IFN-γ, IL-4, IL-17, and IL-22) detected in CD4+ and CD8+ T cells by intracellular flow cytometry. *P < .05, **P < .01.
CD4+and CD8+T cells isolated from aGVHD and clGVHD skin lesions differ in their cytokine profiles. Cells isolated from collagenase-digested skin biopsies of aGVHD (n = 3) or clGVHD (n = 2) patients as well as of HCs (n = 5) were stimulated for 4 hours with phorbolmyristate acetate/ionomycin. Samples that had been incubated with brefeldin A only (data not shown) served as negative controls. Thereafter, cells were stained for CD3, CD4, CD8, IFN-γ, IL-4, IL-17, and IL-22 and analyzed with 7-color flow cytometry; gates were set based on isotype-matched controls. (A) FACS gating strategy. (B) CD4+ T cells in aGVHD skin produce higher amounts of IL-22 than in clGVHD. Data are given as percentages ± SD of CD4+ and CD8+ T cells that stained positive for IL-22, IL-17/IL-22, IL17, IL-4, or IFN-ү. (C) Representative FACS plots of cytokine expression (IFN-γ, IL-4, IL-17, and IL-22) detected in CD4+ and CD8+ T cells by intracellular flow cytometry. *P < .05, **P < .01.
To determine whether potential effector cells of GVHD are also present in the peripheral blood, we analyzed, in parallel with T cells isolated from skin, the intracellular cytokine pattern of circulating CD4+ and CD8+ T lymphocytes. As can be seen in supplemental Figure 4A-B, the proportion of IFN-γ–producing CD8+ T cells in the peripheral blood was increased in both aGVHD and clGVHD patients in comparison with HC. In contrast to the situation in skin, there were no significant differences in cytokine profiles of circulating T cells between aGVHD and clGVHD.
TSLP as an indicator of acute cutaneous GVHD development
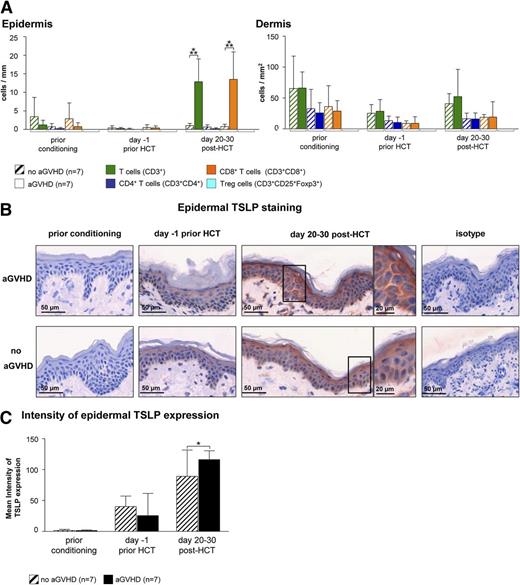
In an attempt to identify a cellular or molecular pattern predicting the occurrence of cutaneous aGVHD, we obtained serial skin biopsies of HCT recipients before the onset of aGVHD. To assess changes of the cellular infiltrate over the time course of HCT, we performed IF stainings with a broad panel of antibodies (Table 2). This analysis revealed an increase of CD8+ T cells (Figure 4A) but no other cell subset (supplemental Figure 5) at day 20 to 30 post-HCT in the skin of patients who, 5 to 26 days later, developed acute cutaneous GVHD compared with those who did not. As aGVHD showed a Th2 signature, we immunohistochemically quantified the expression of TSLP, a keratinocyte-derived cytokine skewing the immune response toward the Th2 direction. As can be seen in Figure 4B-C, the mean intensity of the cytoplasmic TSLP staining in epidermal keratinocytes measured by HistoFAXS on day 20 to 30 post-HCT was significantly higher in the epidermis of patients who later developed aGVHD compared with those who did not (Figure 4B-C).
Epidermal TSLP expression and the presence of CD8+T cells indicate the occurrence of aGVHD at day 20 to 30 after HCT. (A) IF stainings of T cells (T cells: CD3+; CD4+ T cells: CD3+CD4+; CD8+ T cells: CD3+CD8+; and regulatory T cells: CD3+CD25+Foxp3+) in normal-appearing skin of HCT recipients at different time points prior to and after HCT. Patients who later developed aGVHD (n = 7) were compared with those who did not (no aGVHD, n = 7). Data are given either as absolute numbers of positive cells ± SD per mm (epidermis) or per mm2 (dermis). (B) Epidermal IH taining of TSLP at different time points prior to or after HCT (prior conditioning, day −1 prior HCT, day +20-30 post-HCT) in a patient who later developed acute cutaneous GVHD compared with a patient who did not. The framed cells are shown in a larger magnification on the right-hand side of the figure. Slides were scanned using a TissueFAXS imaging system equipped with a Zeiss Axio Observer.Z1 microscope (Zeiss). Images were taken with a LD Plan-Neofluar objective (primary objective ×20/0.4, ocular objective ×10) using a PixeLINK PL-B623CF color digital camera (Zeiss), exported from the HistoQuest software (TissueGnostics GmbH) as tiff images, and processed in Adobe Photoshop CS5 (Adobe Systems). (C) The mean intensity of TSLP expression was evaluated using HistoQuest imaging analysis software (TissueGnostics GmBbH). All images were acquired and analyzed using identical hardware and software settings. *P < .05, ***P < .001.
Epidermal TSLP expression and the presence of CD8+T cells indicate the occurrence of aGVHD at day 20 to 30 after HCT. (A) IF stainings of T cells (T cells: CD3+; CD4+ T cells: CD3+CD4+; CD8+ T cells: CD3+CD8+; and regulatory T cells: CD3+CD25+Foxp3+) in normal-appearing skin of HCT recipients at different time points prior to and after HCT. Patients who later developed aGVHD (n = 7) were compared with those who did not (no aGVHD, n = 7). Data are given either as absolute numbers of positive cells ± SD per mm (epidermis) or per mm2 (dermis). (B) Epidermal IH taining of TSLP at different time points prior to or after HCT (prior conditioning, day −1 prior HCT, day +20-30 post-HCT) in a patient who later developed acute cutaneous GVHD compared with a patient who did not. The framed cells are shown in a larger magnification on the right-hand side of the figure. Slides were scanned using a TissueFAXS imaging system equipped with a Zeiss Axio Observer.Z1 microscope (Zeiss). Images were taken with a LD Plan-Neofluar objective (primary objective ×20/0.4, ocular objective ×10) using a PixeLINK PL-B623CF color digital camera (Zeiss), exported from the HistoQuest software (TissueGnostics GmbH) as tiff images, and processed in Adobe Photoshop CS5 (Adobe Systems). (C) The mean intensity of TSLP expression was evaluated using HistoQuest imaging analysis software (TissueGnostics GmBbH). All images were acquired and analyzed using identical hardware and software settings. *P < .05, ***P < .001.
To determine whether TSLP can also function as a T-cell chemoattractant, we isolated T cells from peripheral blood of HCT recipients at day 20 to 25 post-HCT as well as of HC and performed transwell migration assays. We observed that in both groups, T cells migrated toward TSLP to similar extents as toward the T-cell chemoattractants CCL17 and CCL27 (supplemental Figure 6A-B).
Discussion
Mechanisms involved in the initiation and propagation of the different forms of GVHD are a main focus of HCT research but remain controversially discussed. Our results shed new light on this issue by identifying distinct molecular patterns of acute and chronic GVHD. In human skin, aGVHD is predominated by a Th2 signature with an upregulation of Th2 cytokines (IL-4, IL-13) and chemokines (CCL17) as well as an infiltration of lesions by IL-4 and IL-22 single-producing CD4+ T cells. In contrast, a mixed Th1/Th17 response seems to be operative in chronic lichenoid cutaneous GVHD, as evidenced by a predominance of Th1 (IFN-γ, IL-12/IL-23p40) and Th17 cytokines (IL-17, IL-23p19) and Th1 chemokines (CCL5, CXCL9, CXCL10) and a relative increase of IL-17 and IFN-γ single-producing CD8+ T cells. csGVHD also displays a Th1 signature, shows an abundance of mast cells, and exhibits a higher expression of the TRAIL-receptors TRAIL-R2/-R3/-R4 than clGVHD.
aGVHD has long been thought to result from an alloantigen-specific cytotoxic type 1 T-cell response of the donor against the host.1,7 This paradigm has been challenged by controversial reports on the composition and functional properties of T cells infiltrating aGVHD lesions in mice.8-15 Apart from differences in immunopathomechanisms between the various aGVHD target organs,8-10,15 there exist discrepancies as to the involvement of certain T-cell subsets in different models of murine GVHD.12 In humans, associations between aGVHD and increased numbers of Th1, Th2, or Th17 cells in the peripheral blood have been reported by some investigators,16,17 but not by others.18,19 We also did not find any correlation between a particular CD4+ T cell subset and aGVHD in the circulation of affected patients but detected a characteristic Th2 mRNA pattern in skin lesions and could confirm this observation by intracellular cytokine stainings of T cells in a limited number of patients (aGVHD: n = 3, clGVHD: n = 2). The Th2 cytokine-producing CD4+ T cells infiltrating aGVHD could conceivably be recruited via the Th2-chemokine ligand CCL17 (TARC),36 which we found to be highly upregulated.
Similar to what has been reported for atopic dermatitis,37 we observed a predominance of not only Th2 cells but also of Th22 cells in aGVHD. Our results partly differ from those obtained by Broady et al,19 who reported that human aGVHD skin contains fewer Th22 cells than normal skin. This discrepancy may be due to differences in the experimental design. Whereas Broady et al19 cultured the punch biopsies for 3 weeks before stimulating the emigrated T cells and measuring their cytokine production, we analyzed the T cells directly after obtaining the biopsy to avoid culture-induced artifacts.
In skin, IL-22 is known to act on keratinocytes by modulating their differentiation and inducing the expression of proinflammatory mediators such as IL-20, certain metalloproteinases, as well as β-defensin 3 and cathelicidin.38 The chemotactic effect that these antimicrobial peptides exert on T cells39 could be one of the mechanisms by which IL-22 contributes to tissue inflammation in cutaneous aGVHD. Given the dampening effects of IL-22 in murine acute intestinal GVHD40 and reports on the context-specific immunoregulatory properties of IL-22,38 one should also entertain the possibility that overproduction of IL-22 in acute cutaneous GVHD is a compensating attempt aimed at preventing and/or reducing keratinocyte injury caused by as-yet–unknown mechanisms.
To gain a deeper understanding of acute cutaneous GVHD, we searched for molecular and cellular signatures indicating the development of the disease. At day 20 to 30 post-HCT, we found an increased expression of TSLP in the skin of patients who later developed aGVHD, but not in those who did not. TSLP is a keratinocyte-derived cytokine that acts through multiple pathways to promote Th2 responses in skin, ie, an enhancement of IL-4 production by T cells, of the maturation of CD11c+ DCs, and of the expression of CCL17.41,42 We found all of these features in aGVHD skin and could further demonstrate that TSLP acts as a chemoattractant for T cells. The proinflammatory cytokines TNF-α and IL-1α synergize with Th2 cytokines to induce TSLP production in skin,43 and an excessive release of these inflammatory cytokines and other “danger signals” occurs in damaged host tissues in response to the conditioning prior to HCT.1 Taken together, these arguments support, but do not prove, an involvement of TSLP in the initiation of aGVHD and point to a role of TSLP in predicting acute cutaneous GVHD development. Further studies with a larger number of patients are needed to prove or disprove the validity of this assumption.
Much less is known about the pathomechanisms underlying clGVHD, which, based on animal models,2,30 has initially been described as a Th2-mediated disease process.23 As for cutaneous clGVHD in humans, studies by us and others17,20 show a major upregulation of Th1/Th17 cytokines and an increase of IFN-γ+ and IL-17A+ CD8+ T cells. This finding strongly suggests that the immune response occurring in clGVHD skin lesions is of a mixed Th1/Th17 type. Lethal keratinocyte injury, a striking histopathological feature that clGVHD shares with cutaneous LP,4,6 has been attributed to Fas/FasL interactions and the release of cytotoxic molecules by CD8+ T cells.44 According to our data, one could hypothesize that CD8+ T cells contribute to keratinocyte damage directly via the release of granzyme A/B and perforin, by the up-regulation of FasL and, indirectly, by the production of IFN-γ, an important promoter of cytotoxic immune responses.45 TRAIL, another death-inducing ligand, was unchanged relative to normal skin.
Furthermore, the interaction of the Th1 chemokine-receptor CXCR3 on CD8+ T cells with its ligands CXCL9 and CXCL10 on keratinocytes is considered to be a central element of LP pathogenesis.46 Interestingly enough, these 2 chemokines were found to be overexpressed in epidermal areas of clGVHD skin that were infiltrated by CXCR3+CD8+ T cells.47 Our studies confirm these data,47-49 ie, an upregulation of chemokines of the CXCR3 family, and further demonstrate an overexpression of CCL5 and CCR5. We would therefore propose that both the CXCR3 and CCR5 pathways are critically involved in the attraction of pathogenic lymphocytes to clGVHD skin.
In conclusion, the present study provides new insights into the diverse pathomechanisms operative in the acute and chronic forms of cutaneous GVHD. Our findings allow to more accurately distinguish aGVHD from clGVHD based on different cellular and molecular patterns. The analysis of TSLP expression in skin (before disease onset) could be helpful in identifying HCT recipients at risk of developing acute cutaneous GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Frieder Koszik for helpful discussions and critically reading the manuscript.
This work was supported by funds from the Austrian Federal Reserve bank (Anniversary Fund, project no. 13926).
Authorship
Contribution: M.C.B. designed and performed research, collected data, and drafted the manuscript; I.K. and W.B. performed research and collected data; Z.K., W.R., P.K., P.P., and R.K. provided tissue material and contributed vital reagents; H.G. provided tissue material and clinical data, designed research, and edited the manuscript; G. Stingl designed research and drafted the manuscript; and G. Stary designed research, collected data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Georg Stary, Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases (DIAID), Medical University of Vienna, Waehringer Guertel 18–20, 1090 Vienna, Austria; e-mail: georg.stary@meduniwien.ac.at.