Key Points
After TLI, donor blood stem cells first engraft in irradiated marrow and gradually redistribute into unexposed sites.
Long-term donor cell engraftment requires the presence of regulatory host cells that promote host stem-cell cycling.
Abstract
Total lymphoid irradiation (TLI) with antithymocyte globulin (ATG) is a unique regimen that prepares recipients for allogeneic hematopoietic cell transplantation by targeting lymph nodes, while sparing large areas of the bone marrow. TLI is reported to increase the frequency of CD4+CD25+FoxP3+ T-regulatory cells (Treg) relative to conventional T cells. In this study, barriers to hematopoietic stem cell (HSC) engraftment following this nonmyeloablative conditioning were evaluated. TLI/ATG resulted in profound lymphoablation but endogenous host HSC remained. Initial donor HSC engraftment occurred only in radiation exposed marrow sites, but gradually distributed to bone marrow outside the radiation field. Sustained donor engraftment required host lymphoid cells insofar as lymphocyte deficient Rag2γc−/− recipients had unstable engraftment compared with wild-type. TLI/ATG treated wild-type recipients had increased proportions of Treg that were associated with increased HSC frequency and proliferation. In contrast, Rag2γc−/− recipients who lacked Treg did not. Adoptive transfer of Treg into Rag2γc−/− recipients resulted in increased cell cycling of endogenous HSC. Thus, we hypothesize that Treg influence donor engraftment post-TLI/ATG by increasing HSC cell cycling, thereby promoting the exit of host HSC from the marrow niche. Our study highlights the unique dynamics of donor hematopoiesis following TLI/ATG, and the effect of Treg on HSC activity.
Introduction
In the past decade, different methods have been developed to reduce the toxicity of allogeneic hematopoietic cell transplantations (HCTs), and thus allow a broader patient population to benefit from this powerful cellular therapy. Total lymphoid irradiation (TLI) has emerged as a distinct way to prepare cancer patients to accept allografts, resulting in reduced regimen-related toxicity and acute graft-versus-host disease, and hence markedly reduced morbidity and mortality following HCT.1 Moreover, the use of TLI has been successfully extended to solid organ transplants for the purpose of immune tolerance induction.2,3
The basic principle of TLI is irradiation targeted to the lymph nodes (LNs), spleen, and thymus, delivered in multiple small fractions daily over several weeks, and given in combination with immunotherapy with antithymocyte globulin or serum (ATG/S).4-7 Lymphoablation by TLI/ATG alters the host’s immune profile to favor regulatory populations, as natural killer T (NKT) cells are more resistant to radiation than non-NKT cells due their high levels of antiapoptotic genes.8,9 Via secretion of noninflammatory cytokines, including IL-4, NKT cells promote the expansion of CD4+CD25+FoxP3+ T-regulatory cells (Treg) which act to ameliorate acute graft-versus-host disease.10
The radiation fields in TLI encompass the major lymphoid organs, while the long bones of the legs, pelvis, and skull are not exposed. Recipients of TLI reconstitute blood formation without cell rescue, and thus it is a nonmyeloablative treatment. Clinical studies have shown that following TLI/ATG, sustained donor engraftment can be problematic, particularly if patients have not received chemotherapy prior to this treatment.2,3 Engraftment resistance in other nonmyeloablative settings is typically caused by the persistence of host immune cells present at the time of graft infusion. The most prominent effectors of the host’s immune barrier are T and natural killer (NK) cells, with NK cells playing the major role in rejecting major histocompatibility complex (MHC)-disparate grafts.11-15 Mature donor T cells contained in a graft are thought to aid in overcoming engraftment resistance by eradicating residual host cells. Moreover, host hematopoietic stem cells (HSCs) that compete for “niche space” within the bone marrow (BM) must be reduced, and/or removed. In unconditioned hosts, most HSCs are quiescent,16,17 and only occasionally proliferate and leave the HSC-niche to circulate.18,19 Conditioning by conventional total body irradiation (TBI) or chemotherapy opens up abundant HSC niches, allowing donor HSC engraftment.20 However, in TLI/ATG, most of the BM is shielded from radiation; therefore, the question of where donor hematopoiesis is established and how is it sustained remains unclear.
Here, we studied the interactions between host immune cells, niche-space barriers, and donor HSC engraftment following TLI/ATG. Because non-HSC cells contained in an allograft can aid in overcoming host resistance, we used a reductionist approach of transplanting purified HSC to study only the barriers enforced by the host. We demonstrate that successful engraftment and long-term persistence of donor HSC following TLI depend on host regulatory cells. Our data suggest that host Treg promote engraftment by driving host HSCs into cycle, thereby opening niche space, and thus lead us to hypothesize that Treg play an important role in controlling the dynamics of early hematopoiesis post-HCT.
Methods
Mice
C57BL/6 (B6) mice (H-2b, Thy1.1, B6.CD45.1, B6.CD45.2, luciferase expressing transgenic B6.luc+, and B6.GFP) and AKR/b mice (H-2b) were HSC donors for B6 (B6.CD45.2 or B6 Thy1.2 CD45.1; albino B6 [Thy1.2; CD45.2]; B6 Jα18−/− [Thy1.2; CD45.1]; B6.Rag2cγc−/−), BALB.B (H-2b, Thy1.2, CD45.2, CD229.1+), and BALB/c (H-2d, Thy1.2, CD45.2, CD229.1+) recipient mice. B10.D2 (H-2d, Thy1.1, CD45.2) were used as donors for BALB/c mice. All studies were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
HSC isolation
BM was flushed into Hanks balanced salt solution/2% fetal bovine serum enriched for c-Kit+ (3C11) cells by magnetic column separation. “KTLS-HSCs” were selected by fluorescence-activated cell sorting (FACS) for c-Kit+ Thy1.1lo-int Sca-1+ Linneg cells, as described.21
TLI, TBI, and HSC transplantation
Lead jigs used for TLI in mice exposed the thymus, spleen, cervical (cerv), mediastinal, and mesenteric (mes) LN. TLI/ATG conditioning began on day −23 prior to HCT, and involved 17 doses of 240 cGy (4080 cGy) irradiation plus 3 doses of ATG. Rabbit ATG (Accurate Laboratories, New York, NY) was injected into recipients intraperitoneally at a dose of 0.2 mg in 0.5 mL saline on day −12, −10, and −8 (see experimental schema in supplemental Figure 1A on the Blood Web site).22 TBI was delivered in split doses (2 × 400 to BALB and 2 × 475 cGy to B6, separated by 3 hours) on day 0, and 5000 to 10 000 KTLS-HSC were infused IV within 3 hours after the second TBI session and 24 hours after the end of TLI. Whole BM (WBM) control groups received 1 × 107 WBM cells. For reference, the radiation fields for human TLI are shown in supplemental Figure 1B.
Flow cytometry
Tissues were manually processed into single cell suspensions. BM was obtained by flushing long leg bones with phosphate-buffered saline/2% bovine calf serum, or by crushing 5 representative vertebrae, exposed to radiation. Details on staining procedure and clones of antibodies are provided in the supplemental Methods.
Assessment of engraftment by in vivo bioluminescence imaging
In-vivo bioluminescence imaging (BLI) of recipients was performed as described by Edinger et al.23
Statistical analysis
Statistical differences and their P values for groups were assessed by a 2-tailed Student t test using Microsoft Excel and GraphPad Prism 4.0 software. Column-bar diagrams display the mean and SEM for each group, and were created using GraphPad Prism 4.0 software. For all tests, P < .05 was considered significant.
Results
TLI/ATG is highly lymphoablative
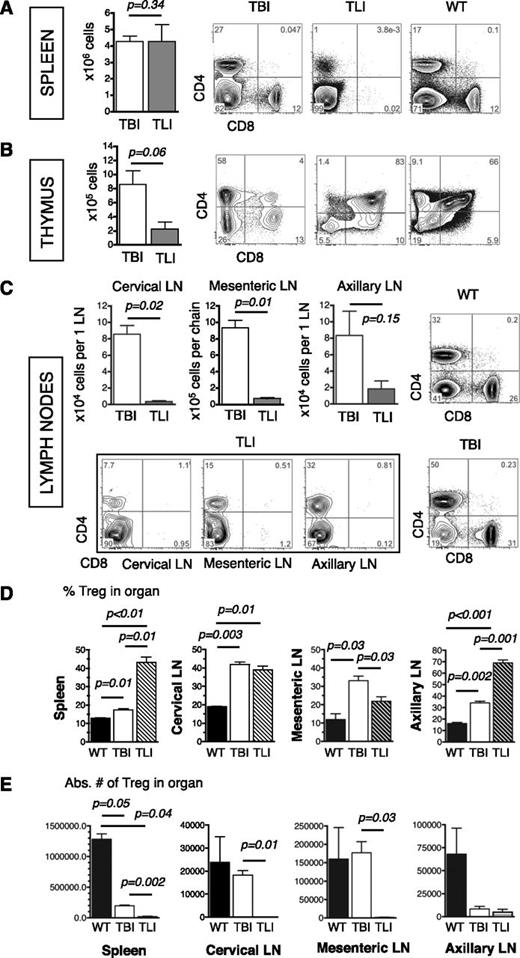
Because host lymphoid cells are key mediators of resistance to allogeneic grafts, the effects of targeting LN as performed in TLI/ATG vs lethal TBI on this immune barrier were compared. At 24 hours after the last radiation dose, absolute splenocyte counts in both conditioning groups were similar (Figure 1A) and 10- to 16-fold lower than in wild-type (WT) mice (supplemental Figure 2). However, depletion of splenic CD4+ and CD8+ T cells was more complete following TLI/ATG compared with TBI. Cell reduction of thymuses was more severe after TLI/ATG (∼80-fold) than TBI (10-fold) compared with WT mice (supplemental Figure 2), respectively, and showed markedly different patterns in both groups. Although lethal TBI spared a percentage of CD4+ and CD8+ single-positive (SP) thymocytes and reduced the proportion of immature CD4+ and CD8+ double-positive (DP) cells, TLI/ATG eliminated most SP cells but spared more DP thymocytes (Figure 1B). Of all lymphoid tissues, LN sustained the largest differential effect of TLI/ATG vs TBI. LN hypocellularity post-TLI/ATG was extreme, resulting in 5- to 20-fold lower absolute cell counts than after TBI, and 40- to 50-fold lower numbers than in WT LN (Figure 1C, top; supplemental Figure 2). TLI/ATG resulted in the elimination of nearly all CD8+ cells and a marked reduction of CD4+ T cells, while the proportions of CD4+ and CD8+ T cells following TBI were increased. LN location determined the degree of CD4+ T-cell depletion after TLI/ATG. Near complete depletion was observed in cervLN and mesLN that were exposed to radiation during TLI (Figure 1C). Although axillary (ax) LNs were not directly exposed to radiation (supplemental Figure 1A), they demonstrated a marked cellular reduction suggesting that lymphoid cell depletion also occurred as a consequence of lymphocyte migration away from these organs. Thus, compared with lethal TBI, TLI/ATG resulted in a greater overall reduction in T-cell content.
TLI/ATG effectively ablates lymphoid organs. Absolute cell counts of lymphoid organs of mice at 24 hours after lethal TBI, and 24 hours after the last TLI dose; FACS plots of tissues from representative animals. (A) Bar graph shows the absolute spleen cell counts revealing no significant difference between TBI and TLI/ATG-conditioned groups. FACS plots show CD4+ and CD8+ T cells in spleens of TBI vs TLI/ATG-conditioned and unconditioned WT mice. (B) Bar graph shows the absolute cell counts per thymus with lower numbers of thymocytes in TLI-treated vs TBI-treated animals. FACS plots show the shifts of single and positive CD4+ and CD8+ T-cell populations in conditioned vs unconditioned mice. (C) Bar graphs show the absolute cell numbers of cervLN (top left), mesLN (top, second from left), and axLN (top, second from right) revealing lower cellularity following TLI/ATG as compared with TBI. FACS plots demonstrate CD4+ and CD8+ depletion in LN in WT mice (top right), after TLI/ATG (bottom 3 left; cervical, mesenteric and axillary LN), and TBI-conditioning (bottom right). (D) Compiled data on the proportion of CD4+CD25+FoxP3+ Treg of all CD4+ cells and (E) their absolute numbers in spleen (left), cervLN (second from left), mesLN (second from right), and axLN (right) in untreated (black solid), TBI-conditioned (white solid), and TLI/ATG-treated (striped) mice are shown. N = 3 to 7 animals per experimental group. Only statistically significant P values are displayed.
TLI/ATG effectively ablates lymphoid organs. Absolute cell counts of lymphoid organs of mice at 24 hours after lethal TBI, and 24 hours after the last TLI dose; FACS plots of tissues from representative animals. (A) Bar graph shows the absolute spleen cell counts revealing no significant difference between TBI and TLI/ATG-conditioned groups. FACS plots show CD4+ and CD8+ T cells in spleens of TBI vs TLI/ATG-conditioned and unconditioned WT mice. (B) Bar graph shows the absolute cell counts per thymus with lower numbers of thymocytes in TLI-treated vs TBI-treated animals. FACS plots show the shifts of single and positive CD4+ and CD8+ T-cell populations in conditioned vs unconditioned mice. (C) Bar graphs show the absolute cell numbers of cervLN (top left), mesLN (top, second from left), and axLN (top, second from right) revealing lower cellularity following TLI/ATG as compared with TBI. FACS plots demonstrate CD4+ and CD8+ depletion in LN in WT mice (top right), after TLI/ATG (bottom 3 left; cervical, mesenteric and axillary LN), and TBI-conditioning (bottom right). (D) Compiled data on the proportion of CD4+CD25+FoxP3+ Treg of all CD4+ cells and (E) their absolute numbers in spleen (left), cervLN (second from left), mesLN (second from right), and axLN (right) in untreated (black solid), TBI-conditioned (white solid), and TLI/ATG-treated (striped) mice are shown. N = 3 to 7 animals per experimental group. Only statistically significant P values are displayed.
Regulatory populations have been reported to create the tolerogenic environment unique to TLI/ATG.6,10 LN and spleens of TLI/ATG vs TBI-treated mice were evaluated for content of CD4+CD25+FoxP3+ Treg cells. Although absolute Treg numbers after TLI/ATG were lower compared with TBI-treated and WT mice in all lymphoid organs studied (Figure 1D), their proportion (within all CD4+ cells) was increased in the spleen and axLN. No proportional increase was observed in cervLN and mesLN, compared with TBI-treated and WT mice, but considering their severe hypocellularity, the contribution of these latter LN groups to the animal’s overall Treg content may be negligible (Figure 1E). Similar observations of proportional but not absolute increases in LN following TLI/ATG as compared with WT mice were made for DX5+CD122+ NK cells, whereas there was no measurable increase of NKT cells at 24 hours post-TLI/ATG (supplemental Figure 3).
Nonimmune barriers remain after TLI/ATG
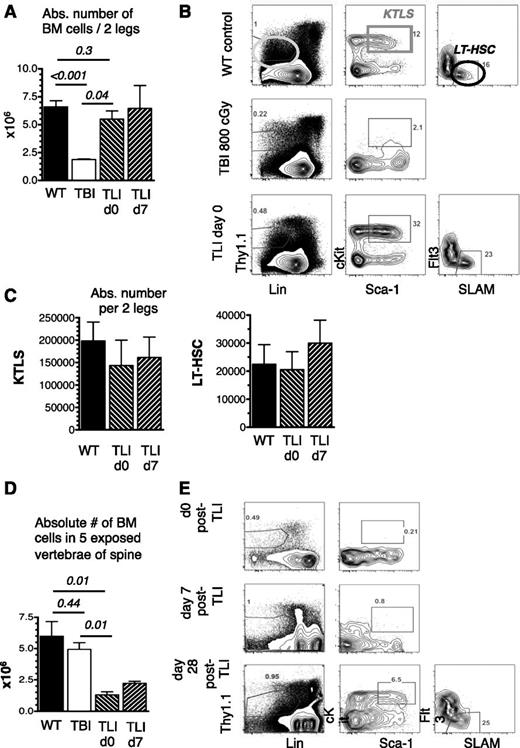
Endogenous HSC residing within marrow niches are thought to constitute the major nonimmune barrier to allogeneic HSC engraftment. Since TLI/ATG spares large portions of the marrow, we studied BM compartments contained within (5 representative vertebrae) or outside (leg bones) the radiation field for persistence of host HSC. Absolute cell numbers per 2 legs (femurs and tibias) taken on day 0 (24 hours after the last radiation dose) and day +7 from TLI/ATG-conditioned mice were compared with WT and TBI-treated mice. Figure 2A shows absolute BM cell numbers of the legs were equivalent in WT- and TLI/ATG-treated mice, whereas TBI significantly decreased cellularity.
Nonimmune barriers remain after TLI/ATG. BM cells of 2 legs and 5 vertebrae of the spine from untreated WT mice, 24 hours after lethal TBI and 24 hours and 7 days after TLI/ATG were counted and analyzed by FACS for HSC-content. (A) Compiled data on absolute cell numbers per 2 legs following TBI, compared with WT and TLI/ATG-treated mice. (B) Representative FACS plots of a WT mouse (top panel) displaying the gating strategy for LinnegThy1.1low (left), cKit+Sca-1high KTLS-HSC (middle), and LT-HSC (right; KTLS-Flt3-SLAM+); a TBI-treated mouse (middle panel) lacking the KTLS-HSC population; and a TLI/ATG-conditioned mouse (bottom panel) with HSC and progenitor phenotypes resembling WT marrow. (C) Compiled data on calculated absolute numbers of KTLS-HSC (left) and LT-HSC (right) per 2 legs were similar in WT and TLI/ATG-treated mice. Due to the lack of phenotypic KTLS-HSC after TBI conditioning, no absolute numbers for this group are displayed. (D) Compiled absolute cell counts in 5 vertebrae of the spine revealing lower cell numbers in TLI/ATG-treated mice compared with TBI and WT controls, with hypocellularity persisting until day +7 post-TLI/ATG. (E) FACS analysis from vertebral BM of representative TLI/ATG-conditioned mice showing absence of the KTLS-HSC population at 24 hours (top) and day +7 post-TLI/ATG (middle), but reconstitution of phenotypically normal KTLS-HSC and LT-HSC populations by day +28 (bottom). N = 4 to 8 animals per experimental group.
Nonimmune barriers remain after TLI/ATG. BM cells of 2 legs and 5 vertebrae of the spine from untreated WT mice, 24 hours after lethal TBI and 24 hours and 7 days after TLI/ATG were counted and analyzed by FACS for HSC-content. (A) Compiled data on absolute cell numbers per 2 legs following TBI, compared with WT and TLI/ATG-treated mice. (B) Representative FACS plots of a WT mouse (top panel) displaying the gating strategy for LinnegThy1.1low (left), cKit+Sca-1high KTLS-HSC (middle), and LT-HSC (right; KTLS-Flt3-SLAM+); a TBI-treated mouse (middle panel) lacking the KTLS-HSC population; and a TLI/ATG-conditioned mouse (bottom panel) with HSC and progenitor phenotypes resembling WT marrow. (C) Compiled data on calculated absolute numbers of KTLS-HSC (left) and LT-HSC (right) per 2 legs were similar in WT and TLI/ATG-treated mice. Due to the lack of phenotypic KTLS-HSC after TBI conditioning, no absolute numbers for this group are displayed. (D) Compiled absolute cell counts in 5 vertebrae of the spine revealing lower cell numbers in TLI/ATG-treated mice compared with TBI and WT controls, with hypocellularity persisting until day +7 post-TLI/ATG. (E) FACS analysis from vertebral BM of representative TLI/ATG-conditioned mice showing absence of the KTLS-HSC population at 24 hours (top) and day +7 post-TLI/ATG (middle), but reconstitution of phenotypically normal KTLS-HSC and LT-HSC populations by day +28 (bottom). N = 4 to 8 animals per experimental group.
Marrow from the legs was examined for HSC, specifically “KTLS-HSC” and long-term (LT)-HSC content. Figure 2B (top) shows delineation of the “KTLS-HSC” (Linneg Thy1.1low cKit+ Sca-1high) population into multipotent progenitor (MPP) (Flt3+), short-term [ST] (SLAM- Flt3-), and LT (SLAM+ Flt3-) HSC fractions in WT mice. Twenty-four hours after 950 cGy lethal TBI, nearly all KTLS-HSC were eliminated in the BM (Figure 2B, middle). In contrast, after TLI/ATG, both KTLS and LT-HSC were preserved (Figure 2B, bottom) and there was no significant change in their absolute numbers per 2 legs compared with WT controls, neither on day 0 nor on day +7 postradiation (Figure 2C).
Cellular contents of the vertebrae following TLI/ATG were markedly different from the legs. TLI/ATG resulted in significant reduction in vertebral cell counts compared with TBI and WT controls (Figure 2D). Following TBI, marrow ablation of vertebrae was less marked compared with the legs, most likely due to ongoing apoptosis and different dynamics of cell death during this early postradiation phase. In TLI/ATG-conditioned mice, KTLS and LT-HSC were undetectable in vertebral BM on day 0 through day +7 postradiation, but were reconstituted by day +28 (Figure 2E).
Donor engraftment across different genetic barriers after TLI/ATG vs TBI
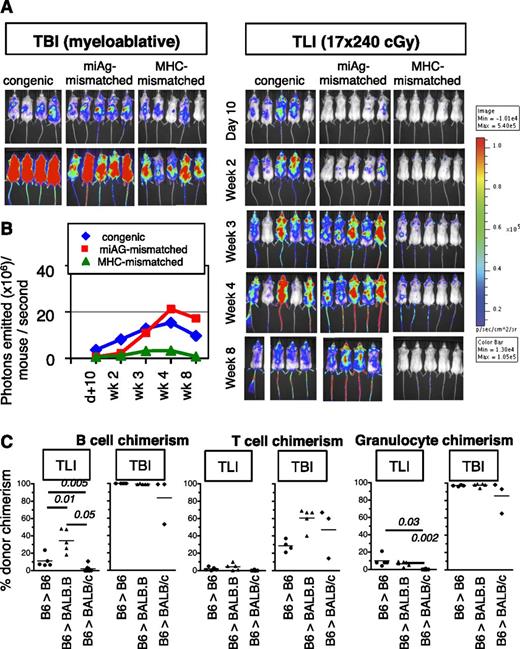
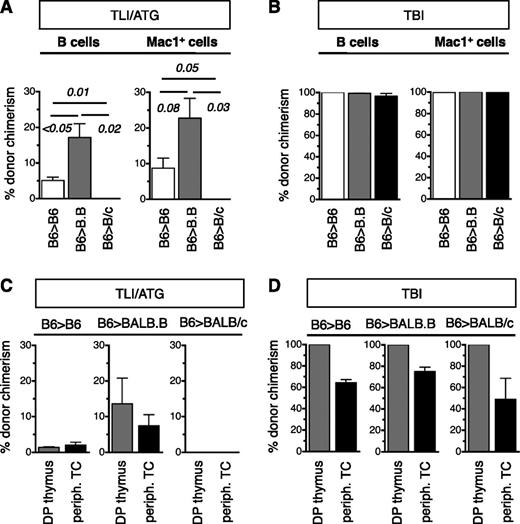
Having established the cell-depletive effects of TLI/ATG and TBI, KTLS-HSC transplantation across different genetic strain combinations was performed to compare sites and tempo of donor-cell engraftment by BLI (Figure 3A). A total of 10 000 KTLS-HSC from transgenic B6.luc+ mice was infused into lethally TBI- or TLI/ATG-conditioned congenic albino B6 (H-2b), minor antigen (miAg)-mismatched BALB.B (H-2b), or MHC-mismatched BALB/c (H-2d) recipients. TBI-treated recipients showed a rapid BLI-signal increase within 2 weeks post-HCT, and robust engraftment in all strain combinations. Genetic differences influenced engraftment dynamics, as donor cell expansion was the fastest in congenic B6 and slowest in MHC-mismatched BALB/c recipients. Only the first 2 weeks are displayed in Figure 3A for TBI-treated mice, since after week 2 BLI images were oversaturated when displayed together with TLI/ATG groups. In TLI/ATG-conditioned mice, patterns of engraftment were very different, with B6 recipients of congenic B6.luc+ HSC engrafting more rapidly than miAg-mismatched BALB.B mice. However, over time, a higher degree of donor cell expansion was achieved in allogeneic miAg-mismatched BALB.B recipients (Figure 3B). BLI revealed in both groups that donor HSC homed to and expanded in the areas of the spine that were exposed to radiation during TLI/ATG. In contrast, MHC-mismatched BALB/c recipients had only transient low levels of donor-cell engraftment that were lost by 2 months post-HCT.
Donor HSC engraftment across genetic barriers after TLI/ATG vs TBI. (A) A total of 10 000 KTLS-HSC from B6.luc+ mice (H2b) were infused into albino B6 (H-2b), BALB.B (H-2b), and BALB/c (H-2d) mice after myeloablative TBI (left; 950 cGy for B6, 800 cGy for BALB) or TLI/ATG (right; 17 × 240 cGy). Engraftment and the degree of donor cell expansion are indicated by bioluminescent signal intensity, showing more rapid and stronger luc+-donor cell expansion in TBI compared with TLI/ATG-conditioned recipients. Beyond 2 weeks post-HCT, images in the TBI group were oversaturated and are thus not shown. (B) Median expansion of luc+ donor B6.HSC grafts as quantified in emitted photons over total body area in congenic, miAg-mismatched, and MHC-mismatched recipients at serial time points (day +10, weeks 2, 3, 4, and 8) post-TLI/ATG and -HCT. (C) Percent donor chimerism of blood B220+ B cells (left), CD3+/TCRαβ+ T cells (middle), and CD11b+ (= Mac1+) myeloid cells (right) at 1 month post-HCT using TLI/ATG vs TBI conditioning, revealing mixed chimerism for all lineages in TLI/ATG-conditioned congenic and miAg-mismatched, but no engraftment in MHC-mismatched recipients; and full donor-chimerism in B cell and myeloid lineages after TBI conditioning but mixed chimerism for T cells. N = 5 to 7 animals per experimental group.
Donor HSC engraftment across genetic barriers after TLI/ATG vs TBI. (A) A total of 10 000 KTLS-HSC from B6.luc+ mice (H2b) were infused into albino B6 (H-2b), BALB.B (H-2b), and BALB/c (H-2d) mice after myeloablative TBI (left; 950 cGy for B6, 800 cGy for BALB) or TLI/ATG (right; 17 × 240 cGy). Engraftment and the degree of donor cell expansion are indicated by bioluminescent signal intensity, showing more rapid and stronger luc+-donor cell expansion in TBI compared with TLI/ATG-conditioned recipients. Beyond 2 weeks post-HCT, images in the TBI group were oversaturated and are thus not shown. (B) Median expansion of luc+ donor B6.HSC grafts as quantified in emitted photons over total body area in congenic, miAg-mismatched, and MHC-mismatched recipients at serial time points (day +10, weeks 2, 3, 4, and 8) post-TLI/ATG and -HCT. (C) Percent donor chimerism of blood B220+ B cells (left), CD3+/TCRαβ+ T cells (middle), and CD11b+ (= Mac1+) myeloid cells (right) at 1 month post-HCT using TLI/ATG vs TBI conditioning, revealing mixed chimerism for all lineages in TLI/ATG-conditioned congenic and miAg-mismatched, but no engraftment in MHC-mismatched recipients; and full donor-chimerism in B cell and myeloid lineages after TBI conditioning but mixed chimerism for T cells. N = 5 to 7 animals per experimental group.
Blood chimerism analysis by FACS verified the presence of donor cells in congenic and miAg-mismatched TLI/ATG-treated recipients (Figure 3C). No donor hematopoiesis was established in MHC-mismatched BALB/c recipients. After TLI/ATG, engrafted congenic and miAg-mismatched hosts had mixed chimerism in all lineages, while lethal TBI resulted in near 100% donor chimerism in B and myeloid cells in all strain combinations. Only T cells remained mixed, with chimerism levels significantly higher compared with TLI/ATG (Figure 3C).
Because TLI/ATG is nonmyeloablative, we compared levels of donor blood chimerism to those achieved after sublethal, nonmyeloablative TBI of BALB/c mice transplanted with miAg-mismatched B10.D2 HSC. Even at an attenuated dose of 400 cGy, TBI donor chimerism levels were substantially higher in all lineages compared with TLI (supplemental Figure 4). The use of WBM (instead of selected HSC grafts) modestly improved the levels of donor chimerism following TLI. The addition of ATG to TLI did not influence engraftment (supplemental Figure 5).
Engraftment of HSC differs in distinct marrow sites after TLI/ATG
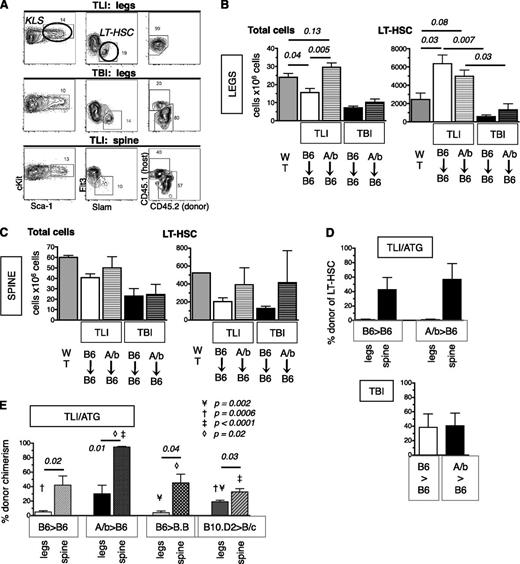
Our BLI studies suggested that following TLI/ATG, donor HSC initially engraft only in BM sites exposed to radiation. To confirm these patterns and determine if HSC migrate to and engraft in shielded BM sites, B6 (Thy1.2, CD45.1) mice underwent TLI/ATG or sublethal TBI followed by infusion of 10 000 congenic B6 (Thy1.1, CD45.2), or miAg-mismatched AKR/b (H-2b, Thy1.1, CD45.2) KTLS-HSC. Recipient BM of vertebrae vs legs was assessed at 2 weeks post-HCT for cellularity, absolute LT-HSC content, and chimerism. In TBI-treated mice, absolute numbers of LT-HSC were markedly reduced in the BM of the legs (Figure 4A-B) and spine (Figure 4A,C), whereas in TLI/ATG-treated mice, absolute LT-HSC numbers were lower in the irradiated vertebrae (Figure 4C) but substantially increased in the shielded legs compared with WT controls (Figure 4B). After TBI conditioning, chimerism of LT-HSC was mixed in both BM sites (mean ∼40% donor). In contrast, after TLI/ATG, although the vertebrae contained both donor and host LT-HSC (mean donor levels = 43% to 57%), all of the LT-HSCs were of host type in the legs at 2 weeks post-HCT (Figure 4A,D).
Engraftment of HSC differs in distinct marrow sites after TLI/ATG. Thy1.2.CD45.1 B6 mice underwent TLI/ATG or sublethal TBI (475 cGy) and were injected IV with 10 000 congenic Thy1.1.CD45.2 B6 or miAg-mismatched AKR/b (Thy1.1. CD45.2) KTLS-HSC. At 2 weeks (A-D) and 3 months (E) post-HCT, the level of donor chimerism of HSC in the BM of the legs vs the vertebrae was assessed. (A) Representative FACS plots and gating schema reveals no evidence of engrafted donor LT-HSC in the legs after TLI/ATG (top), and mixed chimerism after TBI (middle). After TLI/ATG, donor and host LT-HSC were detectable in the BM of irradiated spine (bottom). (B) Compiled data on absolute cell counts per 2 legs in WT mice (dark gray); TLI/ATG-treated B6 recipients of B6-HSC (solid white); AKR/B (A/b)-HSC (white with stripes); TBI-conditioned B6 recipients of B6-HSC (solid black); and AKR/B-HSC (black with stripes). AKR/b grafts resulted in higher cell counts in B6 recipients compared with B6 grafts (left). Absolute numbers of LT-HSC per 2 legs in WT mice; TLI/ATG-treated B6 recipients of B6-HSC and AKR/B-HSC; and TBI-conditioned B6 recipients of B6-HSC and AKR/b-HSC (right). (C) Compiled absolute cell counts per 5 vertebrae from spines of WT mice, TLI/ATG vs TBI-treated B6 recipients of B6-HSC and AKR/b-HSC, respectively, revealing insignificant differences between groups (left). Absolute numbers of LT-HSC per 5 vertebrae of WT mice, TLI/ATG vs TBI-treated B6 recipients of B6-HSC and AKR/b-HSC showing no significant differences (right). (D) Compiled data on percent donor chimerism in TLI/ATG-treated (top) and TBI-treated (bottom) B6 recipients of B6-HSC or AKR/B-HSC in legs and spine, respectively. (E) Percent donor chimerism at 3 months post-HCT in TLI/ATG-treated B6 (H2b) recipients of B6-HSC (H2b), B6 recipients of AKR/b-HSC (H-2b), BALB.B (B.B; H-2b) recipients of B6 HSC, and BALB/c (B/c; H-2d) recipients of B10.D2 HSC (H-2b) in legs (solid) and spines (striped pattern), respectively. N = 3 to 6 animals per experimental group.
Engraftment of HSC differs in distinct marrow sites after TLI/ATG. Thy1.2.CD45.1 B6 mice underwent TLI/ATG or sublethal TBI (475 cGy) and were injected IV with 10 000 congenic Thy1.1.CD45.2 B6 or miAg-mismatched AKR/b (Thy1.1. CD45.2) KTLS-HSC. At 2 weeks (A-D) and 3 months (E) post-HCT, the level of donor chimerism of HSC in the BM of the legs vs the vertebrae was assessed. (A) Representative FACS plots and gating schema reveals no evidence of engrafted donor LT-HSC in the legs after TLI/ATG (top), and mixed chimerism after TBI (middle). After TLI/ATG, donor and host LT-HSC were detectable in the BM of irradiated spine (bottom). (B) Compiled data on absolute cell counts per 2 legs in WT mice (dark gray); TLI/ATG-treated B6 recipients of B6-HSC (solid white); AKR/B (A/b)-HSC (white with stripes); TBI-conditioned B6 recipients of B6-HSC (solid black); and AKR/B-HSC (black with stripes). AKR/b grafts resulted in higher cell counts in B6 recipients compared with B6 grafts (left). Absolute numbers of LT-HSC per 2 legs in WT mice; TLI/ATG-treated B6 recipients of B6-HSC and AKR/B-HSC; and TBI-conditioned B6 recipients of B6-HSC and AKR/b-HSC (right). (C) Compiled absolute cell counts per 5 vertebrae from spines of WT mice, TLI/ATG vs TBI-treated B6 recipients of B6-HSC and AKR/b-HSC, respectively, revealing insignificant differences between groups (left). Absolute numbers of LT-HSC per 5 vertebrae of WT mice, TLI/ATG vs TBI-treated B6 recipients of B6-HSC and AKR/b-HSC showing no significant differences (right). (D) Compiled data on percent donor chimerism in TLI/ATG-treated (top) and TBI-treated (bottom) B6 recipients of B6-HSC or AKR/B-HSC in legs and spine, respectively. (E) Percent donor chimerism at 3 months post-HCT in TLI/ATG-treated B6 (H2b) recipients of B6-HSC (H2b), B6 recipients of AKR/b-HSC (H-2b), BALB.B (B.B; H-2b) recipients of B6 HSC, and BALB/c (B/c; H-2d) recipients of B10.D2 HSC (H-2b) in legs (solid) and spines (striped pattern), respectively. N = 3 to 6 animals per experimental group.
At 3 months post-TLI/ATG and HCT, robust engraftment was identified in the spine. Moreover, low levels of donor HSC chimerism were detectable in the legs of B6 recipients given congenic (B6.45.1) or miAg-mismatched (AKR/b) grafts (Figure 4E), indicating that HSC migrate and eventually engraft in other sites. Because miAg-mismatched AKR/b HSC engrafted in the legs of B6 recipients at levels higher than the genetically more compatible congenic HSC (mean = 32% and 5% donor chimerism, respectively), we asked if in the setting of TLI/ATG conditioning allogeneic HSC might have an advantage over congenic HSC grafts. Comparative transplantation experiments using miAg-mismatched B6 into BALB.B (H-2b) and B10.D2 into BALB/c (H-2d) pairs revealed mean levels of donor chimerism in the legs of 4% and 19%, respectively. These results corroborate our prior findings that cell autonomous differences in HSC potency exist between mouse strains,24 and that congenic grafts do not necessarily have a competitive advantage over allogeneic grafts.
LT engraftment and the origins of T cells after TLI/ATG
Assessment of recipient blood at 3 months revealed that all lineages were mixed after TLI/ATG in congenic and miAg-mismatched recipients (Figure 5A), whereas after sublethal TBI, B cells and myeloid cells were mostly donor derived (Figure 5B). In contrast, the T-cell pool remained of mixed origins after TLI/ATG and TBI (Figure 5C). To determine the source of these T cells, immature DP thymocytes were studied and compared with peripheral mature T cells. Within the thymus of TLI/ATG-treated mice, donor cells engrafted at low levels in the DP fraction in congenic and miAg-mismatched recipients, indicating that newly produced T cells developed from both donor and remaining host HSC. The primitive origins of the T-cell lineage correlated with the mixed chimerism of LT-HSC in the BM (Figure 5C). In contrast, although TBI-treated mice had mixed blood T-cell chimerism, their DP thymocytes were of donor origin only, indicating that new T cells arose from donor HSC, while T cells of host origin were from postthymic T cells that had survived the radiation conditioning (Figure 5D).
LT engraftment patterns and the origins of T cells are different after TLI/ATG vs TBI conditioning. A total of 10 000 KTLS-HSC from B6 (H-2b) donors was infused into B6 (H-2b), BALB.B (H-2b), and BALB/c (H-2d) recipients. (A) Compiled data of percent donor chimerism achieved in the blood for B cells (left) and Mac1+ (CD11b+) myeloid cells (right) at 3 months post-HCT following TLI/ATG conditioning. There was no engraftment in MHC-mismatched BALB/c recipients. (B) Compiled percent donor chimerism achieved in the blood for B cells (left) and Mac1+ (CD11b+) myeloid cells (right) at 3 months post-HCT following TBI conditioning (950 cGy for B6; 800 cGy for BALB), showing full donor chimerism in both lineages in all groups. (C) Bar graphs display percent donor chimerism of CD4+CD8+ immature thymic T cells and peripheral blood TCRβ+ T cells with low level of donor chimerism in both populations in B6 and BALB.B, but not BALB/c recipients following TLI/ATG conditioning. (D) Thymic T cells were largely donor derived across all groups, and peripheral blood T cells were mixed chimeric following TBI. N = 4 to 8 animals per experimental group.
LT engraftment patterns and the origins of T cells are different after TLI/ATG vs TBI conditioning. A total of 10 000 KTLS-HSC from B6 (H-2b) donors was infused into B6 (H-2b), BALB.B (H-2b), and BALB/c (H-2d) recipients. (A) Compiled data of percent donor chimerism achieved in the blood for B cells (left) and Mac1+ (CD11b+) myeloid cells (right) at 3 months post-HCT following TLI/ATG conditioning. There was no engraftment in MHC-mismatched BALB/c recipients. (B) Compiled percent donor chimerism achieved in the blood for B cells (left) and Mac1+ (CD11b+) myeloid cells (right) at 3 months post-HCT following TBI conditioning (950 cGy for B6; 800 cGy for BALB), showing full donor chimerism in both lineages in all groups. (C) Bar graphs display percent donor chimerism of CD4+CD8+ immature thymic T cells and peripheral blood TCRβ+ T cells with low level of donor chimerism in both populations in B6 and BALB.B, but not BALB/c recipients following TLI/ATG conditioning. (D) Thymic T cells were largely donor derived across all groups, and peripheral blood T cells were mixed chimeric following TBI. N = 4 to 8 animals per experimental group.
Regulatory host cells are required for donor cell engraftment
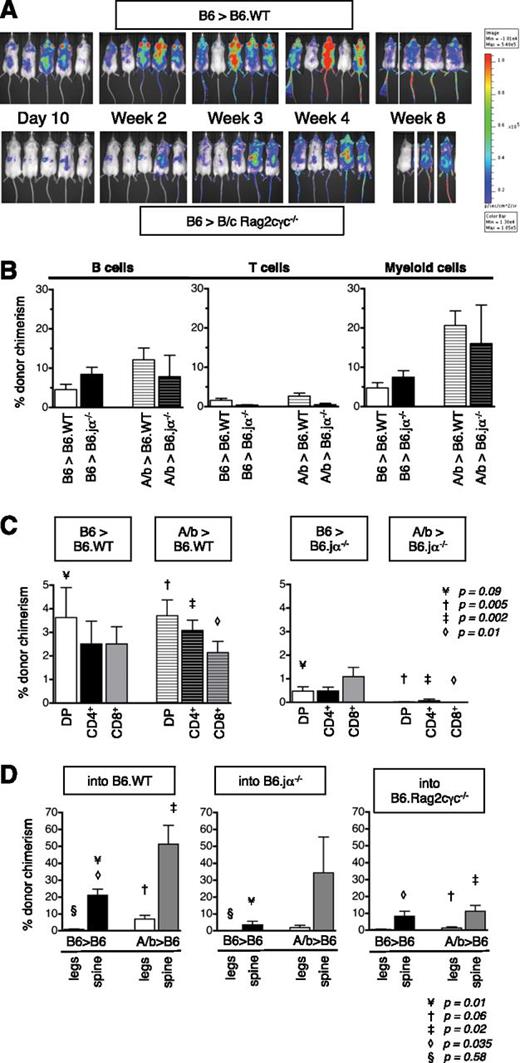
To study in isolation the effect of TLI/ATG conditioning on spatial barriers to donor HSC engraftment, Rag2γc−/− recipients that lack T, B, and NK cell immune barriers were used. Transplantation of HSC from B6.luc+ donors into BALB/c Rag2γc−/− recipients unexpectedly revealed by BLI that, despite an initial wave of donor cell expansion, no stable long-term engraftment was achieved (Figure 6A). Most animals (4 of 5) lost their graft and subsequently died (between weeks 8 and 12), presumably due to their high susceptibility to infections. This lack of engraftment suggested that following conditioning with TLI/ATG, host lymphoid populations are needed to support donor-cell engraftment. To more specifically characterize such regulatory cell effects, B6 Jα18−/− recipients were studied. These mice have a defect in the invariant Vα14Jα18 TCRα chain, and thus produce no functional invariant NKT cells. Following TLI/ATG transplantations of HSC grafts from congenic B6 or miAg-mismatched AKR/b donors, chimerism analysis of mature blood cells revealed no significant differences between WT and Ja18−/− recipients (Figure 6B). However, in Ja18−/− recipients of both congenic and allogeneic grafts, the proportion of donor-derived immature DP T cells was substantially lower compared with WT recipients. In thymuses of WT recipients, donor cells comprised 1% to 5% of the T-lineage cells at 6 weeks post-HCT, whereas thymic donor cell engraftment in Jα18−/− recipients was either absent or reached 1% at best (Figure 6C).
Regulatory host cells are required for donor cell engraftment. Engraftment of 10 000 HSC following TLI/ATG was studied in 3 immune-deficient strains BALB/c Rag2γc−/−, B6 Rag2γc−/−, and B6.Jα18−/− and compared with WT recipients. (A) BLI used to monitor homing and expansion of B6.luc+ HSC revealed. In BALB/c Rag2γc−/− recipients, engraftment was slow and inconsistent, and 4 of 5 mice lost their graft (2 dead before week 8, 2 died between weeks 8 and 12). Images were compared with WT albino B6. (B) Compiled data on percent donor engraftment in the blood B cells (left graph), T cells (middle graph), CD11b+ (Mac1+) myeloid lineages (right graph) in WT B6 (white solid and white striped bars), and B6.Jα18−/− (black solid and black striped bars) recipients of congenic B6 or miAg-mismatched AKR/b (A/b) KTLS-HSC at 6 weeks post-HCT showing similar levels of mixed chimerism in WT and B6.Jα18−/− recipients. (C) Compiled levels of donor chimerism within immature CD4+CD8+ DP and more mature CD4+ and CD8+ SP thymic T-cell populations revealed higher proportions of donor cells in WT B6 (left) compared with B6.Jα18−/− (right) recipients of B6 and AKR/b-HSC grafts. Statistical significance was reached for differences between WT B6 and B6.Jα18−/− recipients of AKR/b but not B6.HSC grafts in DP (¥P = .09) and SP populations (‡: CD4+, P = .002; ◇: CD8+, P = .01). (D) Compiled levels of donor chimerism within LT-HSC in BM of legs and spine in WT B6 (left), B6.Jα18−/− (middle), and B6.Rag2γc−/− (right) recipients of congenic B6 vs miAg-mismatched AKR/b HSC grafts at 6 weeks post-TLI/ATG, and HCT showing lower proportions of donor LT-HSC in spines and legs of B6.Jα18−/− compared with WT recipients, and the lowest proportions in B6.Rag2γc−/− recipients, regardless of graft type. Statistical significance was reached in the following subsets: B6 donor: WT vs B6.Jα18−/− recipients (spine ¥: P = .01); WT vs B6.Rag2γc−/− recipient: (spine ◇: P = .035). AKR/b donor: WT vs B6.Rag2γc−/− recipients: (spine ‡: P = .02). N = 5 animals per experimental group.
Regulatory host cells are required for donor cell engraftment. Engraftment of 10 000 HSC following TLI/ATG was studied in 3 immune-deficient strains BALB/c Rag2γc−/−, B6 Rag2γc−/−, and B6.Jα18−/− and compared with WT recipients. (A) BLI used to monitor homing and expansion of B6.luc+ HSC revealed. In BALB/c Rag2γc−/− recipients, engraftment was slow and inconsistent, and 4 of 5 mice lost their graft (2 dead before week 8, 2 died between weeks 8 and 12). Images were compared with WT albino B6. (B) Compiled data on percent donor engraftment in the blood B cells (left graph), T cells (middle graph), CD11b+ (Mac1+) myeloid lineages (right graph) in WT B6 (white solid and white striped bars), and B6.Jα18−/− (black solid and black striped bars) recipients of congenic B6 or miAg-mismatched AKR/b (A/b) KTLS-HSC at 6 weeks post-HCT showing similar levels of mixed chimerism in WT and B6.Jα18−/− recipients. (C) Compiled levels of donor chimerism within immature CD4+CD8+ DP and more mature CD4+ and CD8+ SP thymic T-cell populations revealed higher proportions of donor cells in WT B6 (left) compared with B6.Jα18−/− (right) recipients of B6 and AKR/b-HSC grafts. Statistical significance was reached for differences between WT B6 and B6.Jα18−/− recipients of AKR/b but not B6.HSC grafts in DP (¥P = .09) and SP populations (‡: CD4+, P = .002; ◇: CD8+, P = .01). (D) Compiled levels of donor chimerism within LT-HSC in BM of legs and spine in WT B6 (left), B6.Jα18−/− (middle), and B6.Rag2γc−/− (right) recipients of congenic B6 vs miAg-mismatched AKR/b HSC grafts at 6 weeks post-TLI/ATG, and HCT showing lower proportions of donor LT-HSC in spines and legs of B6.Jα18−/− compared with WT recipients, and the lowest proportions in B6.Rag2γc−/− recipients, regardless of graft type. Statistical significance was reached in the following subsets: B6 donor: WT vs B6.Jα18−/− recipients (spine ¥: P = .01); WT vs B6.Rag2γc−/− recipient: (spine ◇: P = .035). AKR/b donor: WT vs B6.Rag2γc−/− recipients: (spine ‡: P = .02). N = 5 animals per experimental group.
The most pronounced difference in donor cell engraftment between the WT and knockout strains was observed in LT-HSC. The proportion of donor-derived LT-HSC was lower in spines and legs of invariant NKT-deficient mice compared with WT mice, and even more reduced in the B6.Rag2cγc−/− recipients, regardless of whether or not they received a congenic B6 or miAg-mismatched AKR/b HSC-graft (Figure 6D). The inferior engraftment in the lymphocyte knockout mice suggests that regulatory lymphoid cells modulate the host environment, permitting durable engraftment of donor HSC following conditioning with TLI/ATG.
Treg regulate HSC activity
To understand how regulatory cells modify the host BM environment to facilitate donor HSC engraftment following TLI/ATG, we hypothesized Treg might positively influence host HSC proliferative activity, thereby opening-up niche space. In contrast to TBI or chemotherapy (which permit donor cell engraftment by eliminating host HSC), following TLI/ATG most of the marrow sites (except parts of the spine exposed to radiation) remain occupied and only become available when host HSC cycle and leave the niche. Our hypothesis was based on our related studies of nonmyeloablative TBI, wherein we observed that transplantation of HSC plus Treg resulted in significantly faster hematopoietic recovery compared with control recipients of either HSC or HSC plus CD4conv T cells. Supplemental Figure 6 shows the percent B- and T-cell numbers at 4 and 6 weeks post-HCT for these groups, respectively, illustrating this accelerated recovery in recipients of HSC cotransplanted with Treg.
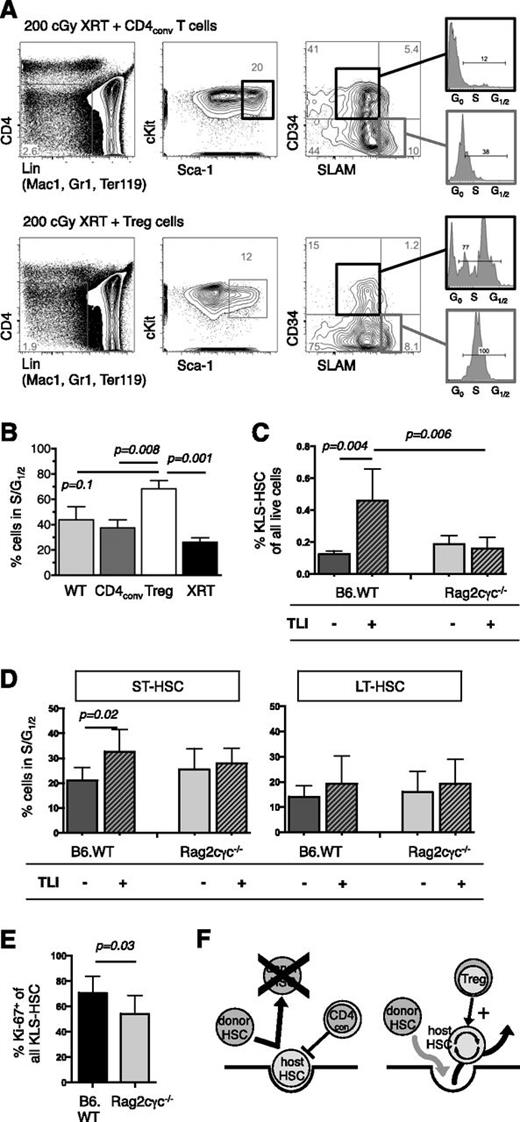
To study the effect of Treg on HSC cycling, FACS-purified B6 Treg vs CD4conv cells were infused into congenic B6.Rag2γc−/− mice, the latter of which lack lymphocytes, including regulatory cells. To provide a stimulus for HSC cell cycling, recipients were irradiated with low-dose 200 cGy TBI on the day of T-cell infusion. Eight days after cell infusion, the BM of Treg recipients contained proportionally fewer KTLS, LT-HSC (CD34−SLAM+ KTLS), and MPP (CD34+SLAM− KTLS) compared with CD4conv recipients (Figure 7A) and control-irradiated mice that did not receive a cell infusion. Furthermore, in the Treg recipients, there was a marked increase in S/G1/2 activity in both the LT-HSC and MPP fractions compared with the other groups; and in the MPP fraction, this increased activity reached statistical significance (Figure 7B).
Tregs influence HSC activity. (A) FACS analysis at day +8 of BM from B6.Rag2γc−/− that had received 200 cGy TBI and IV infusion of 5 × 105 CD4conv (top) or 2 × 105 Treg (bottom). FACS plots of representative animals show the gating strategy to delineate HSC populations, with MPP defined by a CD4−Lin− (Mac1, Gr1, Ter119) cKIT+ Sca-1+ CD34+ SLAM− phenotype and their cell cycle activity. (B) Increased cell cycle activity, as determined by S and G1/2 phases were found in MPP of Treg recipients (N = 3 to 5 per group). (C) BM cells of legs from untreated TLI/ATG-treated B6.WT or B6.Rag2γc−/− mice were analyzed by FACS at 30 hours after the final radiation dose. After TLI/ATG conditioning, B6 WT mice had a significantly higher proportion of KLS-HSC compared with baseline, whereas Rag2γc−/− mice had no detectable increase. (D) In B6.WT, but not in B6.Rag2γc−/− mice S/G1/2 cell cycling activity in ST-HSC was significantly increased following TLI/ATG treatment. Cell cycling activity of LT-HSC was similar in both groups. (E) The increased proliferative activity of the HSC compartment was confirmed by higher Ki-67-expression in B6. WT vs B6.Rag2γc−/− mice at the end of TLI/ATG treatment. (C-E) N = 8 to 11 per experimental group. (F) Hypothesis on T-cell–mediated regulation of HSC activity post-HCT: in the presence of CD4conv cells host HSC decrease cell cycling activity and remain in a quiescent state, which leads to occupation of the niche and failure of donor HSC to replace endogenous host HSC. The presence of Treg increases cell cycling of HSC and their egress into circulation, thereby resulting in open niche space that becomes available for donor HSC in a competitive fashion.
Tregs influence HSC activity. (A) FACS analysis at day +8 of BM from B6.Rag2γc−/− that had received 200 cGy TBI and IV infusion of 5 × 105 CD4conv (top) or 2 × 105 Treg (bottom). FACS plots of representative animals show the gating strategy to delineate HSC populations, with MPP defined by a CD4−Lin− (Mac1, Gr1, Ter119) cKIT+ Sca-1+ CD34+ SLAM− phenotype and their cell cycle activity. (B) Increased cell cycle activity, as determined by S and G1/2 phases were found in MPP of Treg recipients (N = 3 to 5 per group). (C) BM cells of legs from untreated TLI/ATG-treated B6.WT or B6.Rag2γc−/− mice were analyzed by FACS at 30 hours after the final radiation dose. After TLI/ATG conditioning, B6 WT mice had a significantly higher proportion of KLS-HSC compared with baseline, whereas Rag2γc−/− mice had no detectable increase. (D) In B6.WT, but not in B6.Rag2γc−/− mice S/G1/2 cell cycling activity in ST-HSC was significantly increased following TLI/ATG treatment. Cell cycling activity of LT-HSC was similar in both groups. (E) The increased proliferative activity of the HSC compartment was confirmed by higher Ki-67-expression in B6. WT vs B6.Rag2γc−/− mice at the end of TLI/ATG treatment. (C-E) N = 8 to 11 per experimental group. (F) Hypothesis on T-cell–mediated regulation of HSC activity post-HCT: in the presence of CD4conv cells host HSC decrease cell cycling activity and remain in a quiescent state, which leads to occupation of the niche and failure of donor HSC to replace endogenous host HSC. The presence of Treg increases cell cycling of HSC and their egress into circulation, thereby resulting in open niche space that becomes available for donor HSC in a competitive fashion.
HSC cycling was next assessed in WT B6 vs B6.Rag2γc−/− at 24 hours following TLI/ATG conditioning. As shown in Figure 7C, WT B6 mice had significantly higher proportions of cKit+ Lin− Sca-1+ (KLS)-HSC in the marrow of the legs, whereas this increase in proportion was not observed in Rag2γc−/− mice. Furthermore, in WT (but not B6.Rag2γc−/−) mice, there was a significant increase in S/G1/2 activity in the ST-HSC fraction compared with untreated groups (Figure 7D). Of note, at the baseline, the proportions of phenotypic HSC and HSC cell-cycle activity were similar in WT compared with Rag2γc−/− mice (Figure 7D). Analysis by Ki-67 staining further confirmed that at the end of TLI/ATG treatment, there was increased proliferative activity of the HSC compartment in WT but not in Rag2γc−/− mice (Figure 7E). Together, these data support our hypothesis that regulatory cells can promote HSC cell cycling by direct and/or indirect effects and stimulate hematopoiesis, which in turn alters HSC niche availability (Figure 7F). Our findings further imply that this Treg activity on HSC may be an important factor in the achievement of long-term stable donor cell engraftment following TLI/ATG conditioning.
Discussion
TLI/ATG is a unique strategy to prepare patients for allogeneic HCT.1,25 Unlike other regimens, TLI/ATG creates an environment of tolerance, which, together with its favorable toxicity profile, has allowed us to extend the application of HCT beyond its standard indications of hematologic malignancies to combined hematopoietic cell plus kidney allotransplants, with cessation of pharmacologic immunosuppression.2,3 Skewing of the host environment to favor the survival of regulatory lymphoid cells is the reported mechanism by which TLI/ATG exerts its tolerogenic effect. Hence, TLI/ATG with allogeneic HCT also represents a viable treatment option to improve or even cure autoimmune diseases by redirecting the malfunctioning immune system.26,27
One distinguishing feature of TLI/ATG compared with other conditioning regimens is the greater variability in the levels of donor engraftment. Heavily pretreated cancer patients who receive unmanipulated mobilized peripheral blood grafts from HLA-identical donors, routinely achieve full donor chimerism.25 However, recipients who have undergone more limited treatments prior to allografting can demonstrate low chimerism levels and even late graft loss. Our studies of combined kidney transplantation and HCT constitute an extreme example of the latter group, because recipients had not received cytoreductive agents prior to transplant and were infused with T-cell–reduced mobilized peripheral blood. Accordingly, two-thirds of those patients experienced only transient and mostly mixed hematopoietic donor/host chimerism.3
How TLI/ATG permits hematopoietic cell engraftment has not been previously studied. We show here that compared with lethal TBI, TLI/ATG resulted in significantly more lymphocyte depletion in the blood and lymphoid organs, yet engraftment was superior in TBI-treated mice. Lack of available marrow niche space appeared to be the principle reason for the lower engraftment levels in TLI/ATG-treated recipients. Early after TLI/ATG conditioning, donor HSC were identified only in the radiation-exposed areas of the spine, while in the shielded legs increased numbers of exclusively host-HSC were present. This increase in LT-HSC in the legs suggests either that a global decrease in HSC triggered their compensatory proliferation, or that under repetitive low doses of radiation, LT-HSC in the spine relocate to protected marrow areas. Over time, in WT (immunocompetent) recipient mice but not those lacking lymphoid populations, donor HSC were observed at low levels in the shielded BM areas, a finding that is consistent with the idea that HSC leave their niches, circulate, and enter empty niches at distant sites. As we have previously noted in studies of competitive reconstitution, HSC of certain strains such as AKR, demonstrate cell autonomous superiority in their capability to engraft and outcompete other strains.24
Although lymphoid cells are generally thought to be a barrier to donor HSC, we identified a paradoxical role for host lymphocytes in supporting HSC engraftment. Under most conditioning regimens, such as low-dose TBI, lymphocyte-deficient Rag2γc−/− mice engraft more readily compared with WT mice.24 However, in this study, following TLI/ATG Rag2γc−/− recipients had significantly worse and unpredictable engraftment leading us to hypothesize that Treg have a role in the promotion of donor-HSC–derived hematopoiesis. The idea that regulatory cells, and Treg in particular, can facilitate donor HSC engraftment is supported by recent studies of Fujisaki et al, who showed that host Treg colocalize with and promote the survival of allogeneic HSC in the marrow niche, whereas Treg depletion results in the loss of these sequestered HSC.28 In our study, TLI/ATG-treated hosts were highly lympho-depleted but maintained Treg, and niche space competition was the major obstacle to engraftment. Our studies support the idea that beyond immune protection, surviving host Treg facilitate donor HSC engraftment by promoting cell cycling of endogenous HSC, thereby making available open niche space for incoming donor HSC.
Our finding that Treg can have an impact on HSC activity in this way has not been previously reported. Currently, we can only speculate on the mechanism by which Treg influences HSC cycling activity. It is known that the BM physiologically contains a high proportion of Treg and that LT-HSC express endoglin (CD105), which is a part of the receptor of TGF-β, the main cytokine secreted by Treg.29,30 We are currently testing if Treg exert their effect by direct cell contact vs indirect mechanisms.
In summary, here we studied how hematopoietic engraftment is achieved following TLI/ATG conditioning. While niche space creation in areas exposed to radiation allows donor cell engraftment and expansion early post-HCT, it is regulatory host cells that sustain the ability of donor cells to redistribute throughout the body. Hence, we propose the novel idea that the activities of BM Treg cells include the modulation of hematopoiesis. A better understanding of the effects of conditioning treatments on HSC dynamics and the surviving cells that modulate their activity provides both insight into the basic biology of engraftment, and will help guide the development of improved clinical protocols in the future.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO1 HL087240 and PO1 HL075462), the California Institute of Regenerative Medicine (RM 01733), and the Snyder and Steinhart-Reed Foundations. We are grateful for advice and help from the Stanford Shared FACS facility, and to Dr Hye-Sook Kwon and Dr Richard Hoppe for their review and comments on the manuscript.
Authorship
Contribution: A.M.S.M., J.P., N.J.K., C.B., R.M.K., M.F., and P.Z. performed experiments; A.M.S.M., H.E.K.K., and M.F. analyzed results and made the figures; H.E.K.K. and R.S.N. gave critical advice and discussion on the design of the experiments, and edited the manuscript; and A.M.S.M. and J.A.S. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Judith A. Shizuru, Stanford University School of Medicine, Department of Medicine, Division of Blood and Marrow Transplantation, 259 W. Campus Dr, CCSR, Stanford, CA 94305-5623; e-mail: jshizuru@stanford.edu.