To the editor:
JAK2-V617F is the most frequently observed somatic mutation in patients with myeloproliferative neoplasms (MPNs).1,2 Recently, mutations in CALR were described in patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF) that were negative for JAK2-V617F.3,4 All CALR mutations associated with MPNs described to date are either insertions or deletions and result in a frameshift into the alternative reading frame 1. Familial predisposition to MPNs also frequently leads to somatic JAK2-V617F mutations that are found in the majority of the affected family members.5 Because polycythemia vera (PV), ET, and PMF phenotypes can be observed in these pedigrees, we tested whether mutations in CALR also occur in familial MPNs.
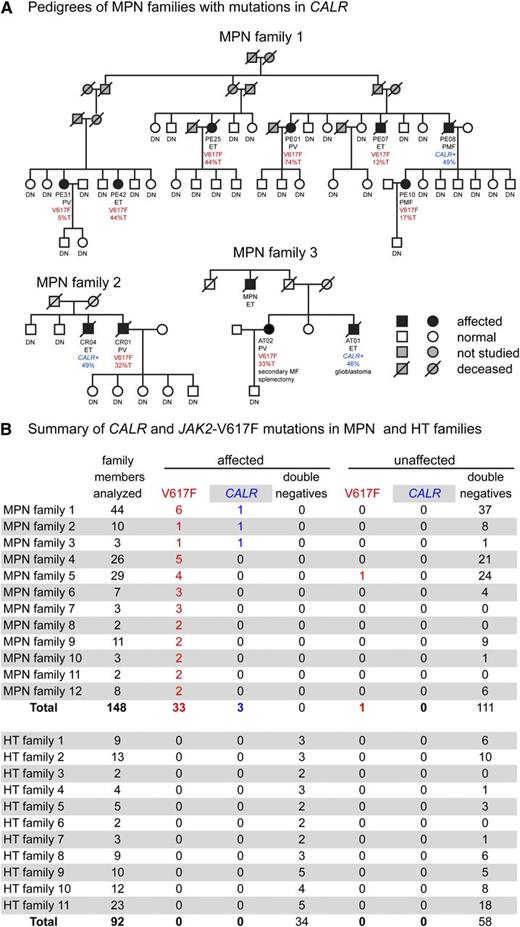
We studied 12 pedigrees with familial MPNs in which at least 1 affected family member carried a somatic JAK2-V617F mutation, and we also examined 11 pedigrees with hereditary thrombocytosis (HT), in which we excluded mutations in the THPO, MPL, and JAK2 genes (data not shown). We found a CALR mutation in 3 of the 12 familial MPN pedigrees (Figure 1A). In each of these 3 pedigrees, 1 affected family member carried a 52-base deletion in exon 9 of the CALR gene (not shown), which represents the most frequent form of the CALR mutations (type 1). One of these patients was initially diagnosed with ET7 and later progressed to PMF (MPN family 1), and the two remaining patients had ET (MPN families 2 and 3). All CALR mutations occurred in patients with an MPN diagnosis, whereas a somatic JAK2-V617F mutation was found in 1 healthy family member with platelets in the upper normal range but otherwise normal blood counts (MPN family 5; data not shown). A similar finding was reported previously.8 We did not detect CALR mutations in any of the 11 families with HT and a total of 44 affected and family members (Figure 1B).
Somatic CALR mutations in familial myeloproliferative diseases. (A) The 3 pedigrees with somatic CALR mutations are shown in detail. Screening for CALR mutations was performed by using the sizing polymerase chain reaction (PCR) assay described by Klampfl et al.3 The JAK2-V617F mutation was screened by allele-specific PCR.6 Percentages in blue represent the CALR mutant allele burden. %T (red), percentage of G>T JAK2-V617F mutant allele burden; CALR+, presence of a 52-base deletion in exon 9 of the CALR gene; DN, double-negative (ie, absence of detectable mutations in CALR exon 9 and JAK2-V617F); V617F, presence of the JAK2-V617F mutation. (B) Summary of families analyzed. Affected and unaffected family members from families with familial predisposition to MPN (upper part) or HT (lower part) were screened for the presence or absence of mutations in CALR.
Somatic CALR mutations in familial myeloproliferative diseases. (A) The 3 pedigrees with somatic CALR mutations are shown in detail. Screening for CALR mutations was performed by using the sizing polymerase chain reaction (PCR) assay described by Klampfl et al.3 The JAK2-V617F mutation was screened by allele-specific PCR.6 Percentages in blue represent the CALR mutant allele burden. %T (red), percentage of G>T JAK2-V617F mutant allele burden; CALR+, presence of a 52-base deletion in exon 9 of the CALR gene; DN, double-negative (ie, absence of detectable mutations in CALR exon 9 and JAK2-V617F); V617F, presence of the JAK2-V617F mutation. (B) Summary of families analyzed. Affected and unaffected family members from families with familial predisposition to MPN (upper part) or HT (lower part) were screened for the presence or absence of mutations in CALR.
In the 12 pedigrees with familial MPNs, all affected family members carried either a somatic JAK2-V617F mutation or a mutation in CALR. In MPN family 1, the underlying predisposition is inherited as an autosomal dominant trait with low penetrance (Figure 1A), whereas in other pedigrees, the mode of transmission is more difficult to determine because too few family members in too few generations were available (eg, MPN families 2 and 3). Two mechanistic models have been proposed regarding how such a germline predisposition may result in MPNs with clonal hematopoiesis.9,10 First, the germline mutation could increase the mutation rate in JAK2 and CALR genes (hypermutability hypothesis), or second, it could synergize with JAK2-V617F or CALR in MPN disease initiation (fertile ground hypothesis). Because the G>T transversion in JAK2-V617F and the 52-base deletion in CALR are mechanistically very different, it seems unlikely that they could be promoted by the same germline mutation through a hypermutability mechanism. Therefore, our findings favor the fertile ground hypothesis of germline predisposition to MPN.
Authorship
Acknowledgments: This work was supported by grants 310000-120724/1 and 32003BB_135712/1 from the Swiss National Science Foundation and KLS-02398-02-2009 from the Swiss Cancer League (R.C.S.).
Contribution: P.L. designed and performed research, analyzed data, and wrote the paper; R.N. performed research and analyzed data; A.A., F.C., and M.M.P.-E. provided clinical data and patient samples and analyzed results; and R.C.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Radek C. Skoda, Department of Biomedicine, Experimental Hematology, University Hospital Basel, Hebelstrasse 20, 4031 Basel, Switzerland; e-mail: radek.skoda@unibas.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal