Key Points
Heparin rechallenge despite prior HIT often induces platelet-activating anti-PF4/heparin antibodies but no faster than seen with typical HIT.
Risk of HIT recurring after heparin rechallenge is low but possible if IgG with heparin-independent platelet-activating properties are made.
Abstract
Heparin reexposure despite a history of previous heparin-induced thrombocytopenia (HIT) can be appropriate if platelet-activating antibodies are no longer detectable. We determined the frequency, timing, and magnitude of the antiplatelet factor 4 (anti-PF4)/heparin immune response (by serotonin-release assay [SRA] and enzyme-immunoassay [EIA]), and the frequency of recurrent HIT in 20 patients with previous HIT reexposed to heparin 4.4 years (mean) post-HIT; 17 patients were given heparin intraoperatively (without postoperative heparin) for cardiac/vascular surgery. One patient developed recurrent HIT beginning 7 days after cardiac surgery, with newly regenerated HIT antibodies exhibiting strong heparin-independent platelet-activating properties. Intraoperative heparin induced EIA seroconversion in 11/17 (65%) patients (immunoglobulin G [IgG]>IgA>IgM) and SRA seroconversion in 8/17 (47%), whereas none of 3 medical patients reexposed to heparin developed seroconversion. Anti-PF4/heparin IgG became detectable at day 7 (median), ie, no sooner than observed in typical-onset HIT. The high proportion of SRA positivity among EIA-seroconverting patients (8/11 [73%]) suggests that patients with previous HIT may be especially predisposed to forming recurrent antibodies with platelet-activating properties. We conclude that among patients with a previous history of HIT who are reexposed to intraoperative (but not postoperative) heparin, the risk of recurrent HIT appears to be low, but is possible if antibodies with strong heparin-independent platelet-activating properties are formed.
Introduction
Heparin-induced thrombocytopenia (HIT) is an immune-mediated prothrombotic adverse drug reaction with a frequency of at least 1% in certain clinical circumstances, such as during unfractionated heparin (UFH) thromboprophylaxis after orthopedic or cardiac surgery.1,2 HIT represents a rather unusual immune response,3,4 including the relatively rapid formation of antiplatelet factor 4 (anti-PF4)/heparin antibodies even upon a first heparin exposure (median time to detectability of immunoglobulin G [IgG] class antibodies, 4-5 days5,6 ) and the transient appearance4 of platelet-activating IgG class antibodies that recognize multimolecular complexes of PF4 bound to heparin.7,8
It remains uncertain what the risk of recurrent HIT is in patients with a previous history of HIT who receive a subsequent reexposure to heparin: to our knowledge, there are only 2 published cases of patients who had 2 well-documented distinct episodes of HIT.9,10 Poetzsch and coworkers11 reported on 10 patients with a previous history of HIT who received a repeat course of UFH for cardiac surgery. Surprisingly, none of the 10 patients formed anti-PF4/heparin antibodies, suggesting that the probability of forming repeat antibodies might be even lower than expected, given that approximately 50% of patients form at least a weak anti-PF4/heparin immune response after cardiac surgery,2,12 and approximately 12% (median of 5 studies [range, 3% to 20%2,12-15 ]) develop a positive platelet activation assay. Two additional studies in hemodialysis patients found no recurrent antibodies or recurrent HIT among 8 patients with previous hemodialysis-associated HIT who, after disappearance of HIT antibodies, resumed anticoagulation either with UFH16 or with low-molecular-weight heparin (LMWH)17 for their long-term hemodialysis.
Assessing the immune response after reexposure to heparin in patients with a history of HIT provides a unique chance to better understand the immunobiology of HIT. In a typical T cell–dependent immune response, a recall rate of nearly 100% should be expected, whereas a frequency of antibody production similar to that observed in prospective studies on HIT should be expected if the immune response in patients with previous HIT is similar to that of the general population undergoing heparin exposure with the same type of surgery. Moreover, specific questions that address features of HIT immunobiology can be addressed: Does anti-PF4/heparin antibody formation occur more rapidly with reexposure vs a first episode of HIT? Does the distribution of immunoglobulin isotypes, or the proportion of anti-PF4/heparin antibodies with platelet-activating antibodies, differ from that observed in other patient populations that form anti-PF4/heparin antibodies?
In our study, we examined the serological profile of patients who underwent a repeat heparin exposure after an episode of well-documented previous HIT. Our primary aim was to study serial postexposure serum samples to determine the frequency of repeat antibody formation, its antibody isotype profile, and its timing. We also determined whether any of our heparin-reexposed patients developed recurrent HIT and, if so, to characterize the serological features of a recurrent HIT immune response.
Methods and patients
Patients
Clinical and laboratory files were reviewed to identify patients with well-documented previous HIT who underwent reexposure to heparin. In most cases, the reexposure had been planned, based upon a strong indication for UFH such as intraoperative administration permitting cardiac or vascular surgery. Approval (10-287-T) was obtained from the Hamilton Integrated Research Ethics Board to test the patients’ serial daily postexposure blood samples for HIT antibodies and to report the data. Patient informed written consent was also obtained for serial blood testing for HIT antibodies after reexposure. This study was conducted in accordance with the Declaration of Helsinki.
Previous HIT
In most cases, the patient’s previous episode of HIT had occurred locally at a Hamilton-area hospital; in this case, we required a compatible clinical picture (thrombocytopenia, timing, thrombosis, and other reason [4Ts] score, ≥4 points,18 scored retrospectively based upon the complete clinical course) as well as a positive platelet serotonin-release assay (SRA)19 as evidence of previous HIT. In other cases, patients had been diagnosed elsewhere with previous HIT (although reexposure occurred at the Hamilton General Hospital) and thus an SRA test may not have been performed; in these circumstances, we required a compatible clinical picture (4Ts score, ≥4 points) with a positive enzyme-immunoassay (EIA). We defined the previous HIT episode as “definite” when the 4Ts score was ≥4 points, the SRA was positive (or EIA strongly positive, ≥2.00 units of optical density [OD], if an SRA result was not available), and review of clinical records indicated no more plausible diagnosis; previous HIT was judged as “probable” when the 4Ts score was ≥4 points, the EIA was positive (with no SRA result available), and no more plausible diagnosis was evident.
Serological investigations
Serological studies were performed using a commercial polyspecific EIA that detects antibodies against PF4/polyvinylsulfonate (PF4 Enhanced, Immucor GTI Diagnostics, Waukesha, WI) and in-house anti-PF4/heparin EIAs of the McMaster Platelet Immunology Laboratory that detects individually antibodies of the 3 major isotypes, IgG, IgA, and IgM20 as well as the SRA.
For assessing the magnitude of the immune responses, we arbitrarily classified an SRA seroconversion (in comparison with a negative SRA preheparin rechallenge) as strong (≥80% peak serotonin release), moderate (50% to 79.9% peak serotonin release), and weak (20% to 49.9% peak release). For the EIAs, we considered as seroconversion any increase in IgG, IgA, or IgM antibodies from negative to a positive result (minimum, 30% increase). If the patient had already tested positive in the baseline (preheparin reexposure) sample, we also considered seroconversion to have occurred if the post-rechallenge OD increased by more than 30% or by at least 0.40 OD units. The EIA seroconversions were regarded as strong if they rose to ≥2.00 OD units, moderate as 1.00 to 1.99 units, and weak as 0.45 to 0.99 units (0.40 as the cutoff for the commercial EIA; 0.45 for the McMaster in-house EIA). We also arbitrarily defined >30% inhibition by high concentrations of heparin (100 U/mL, final) as evidence for heparin dependence of reactivity in the EIA.
Timing of repeat seroconversion in relation to previous timing of onset of HIT
We used the first day of platelet count fall (day of starting heparin = day 0) to determine the presumed date of seroconversion for the episode of previous HIT. This is a conservative estimate of seroconversion because it is known that antibodies usually become detectable a median of 2 days before the beginning of the HIT-related platelet count decline.6 For judging the day of seroconversion after heparin reexposure, we used the earliest seroconversion date if antibodies of more than 1 isotype were formed.
Recurrent HIT
For judging whether a patient developed recurrent HIT, we examined serial platelet counts in relation to seroconversion, judged by both the SRA and isotype-specific anti-PF4/heparin EIAs, testing all available serial postexposure blood samples in relation to the baseline (ie, the sample obtained shortly before heparin reexposure). To classify a patient as having recurrent HIT, both a positive EIA-IgG and platelet-activating antibodies (by SRA) were required that were detectable at the time of a new platelet count fall (minimum, 30%) that was otherwise unexplained by the clinical circumstances; as a general rule, a platelet count fall that occurred in relation to cardiac surgery and before development of a positive SRA was regarded as not being HIT-related. Among the SRA-seroconverting patients, we also evaluated the magnitude of serum-induced platelet activation that occurred at 0 IU/mL UFH (ie, percent serotonin release at buffer control) because thrombocytopenia resulting from HIT antibodies would not be expected to occur in the absence of continuing heparin exposure unless the patient had antibodies that caused substantial platelet activation in the absence of heparin (ie, HIT antibodies with so-called heparin-independent platelet-activating properties).21 If thrombocytopenia occurred in an SRA-seroconverting patient who received postoperative fondaparinux thromboprophylaxis, we tested for in vitro fondaparinux cross-reactivity.
Results
We identified 20 patients with previous HIT (definite, n = 16; probable, n = 4) who underwent repeat reexposure to heparin (UFH, n = 19; LMWH, n = 1), occurring 4.4 years (mean [range, 8 weeks to 13.5 years]) post-HIT diagnosis. We had available 10 samples (median) per patient (last blood sample, median 11 days after reexposure). Table 1 summarizes details for the 20 patients regarding the previous history of HIT, including clinical information (4Ts score, thrombotic events) and serological data. Patients are listed in historical order of their heparin reexposure, with a date range from December 1993 (patient 1) to March 2011 (patient 20).
Prior episodes of HIT
| Pt . | Age, sex at HIT . | Setting . | 4Ts . | HIT-associated thrombosis or other sequelae . | Serotonin release, % . | EIA-GAM (OD units) . | EIA-G (OD units) . | HIT . |
|---|---|---|---|---|---|---|---|---|
| 1 | 55, M | DVT treatment | 7 | DVT extends | 100 | ND | 2.65 | Definite |
| 2 | 65, F | General surgery | 7 | Limb artery | 100 | ND | 2.61 | Definite |
| 3 | 60, M | Cardiac surgery | 8 | Adrenal vein | 85 | 2.82 | 2.65 | Definite |
| 4 | 48, M | Vascular surgery | 6 | Nil | 84 | 1.50 | 2.56 | Definite |
| 5 | 60, F | Vascular surgery | 8 | Limb ischemia | ND | Pos | ND | Probable |
| 6 | 65, M | Cardiac surgery | 7 | Anaphylactoid | 60 | 1.66 | 2.04 | Definite |
| 7 | 75, F | Medical prophylaxis | 8 | DVT, PE | 93 | ND | ND | Definite |
| 8 | 51, M | Vascular surgery | 6 | Limb ischemia | 98 | ND | 3.10 | Definite |
| 9 | 77, F | Medical prophylaxis | 7 | MI | 99 | ND | 2.24 | Definite |
| 10 | 73, M | Vascular surgery | 5 | MI | 100 | ND | 2.56 | Definite |
| 11 | 72, M | Cardiac surgery | 7 | Anaphylactoid | 100 | ND | 2.31 | Definite |
| 12 | 74, F | Vascular surgery | 6 | Nil | 100 | 2.91 | 2.51 | Definite |
| 13 | 48, F | PE treatment | 4 | Nil | ND | Pos | ND | Probable |
| 14 | 66, M | Thoracic surgery | 7 | PE | ND | Pos | ND | Probable |
| 15 | 57, M | Medical prophylaxis | 6 | Mesenteric vein | 85 | ND | 2.54 | Definite |
| 16 | 61, M | DVT treatment | 4 | MI, DVT | 93 | ND | 1.85 | Definite |
| 17 | 68, M | Cardiac surgery | 8 | DVT, PE | ND | >2.00 | ND | Definite |
| 18 | 48, M | Vascular surgery | 7 | MI, CVA, PE | ND | Pos | ND | Probable |
| 19 | 62, M | Hemodialysis | 4 | Nil | 82 | ND | ND | Definite |
| 20 | 54, M | Medical (SBE treatment) | 5 | Nil | 100 | ND | 2.45 | Definite |
| Pt . | Age, sex at HIT . | Setting . | 4Ts . | HIT-associated thrombosis or other sequelae . | Serotonin release, % . | EIA-GAM (OD units) . | EIA-G (OD units) . | HIT . |
|---|---|---|---|---|---|---|---|---|
| 1 | 55, M | DVT treatment | 7 | DVT extends | 100 | ND | 2.65 | Definite |
| 2 | 65, F | General surgery | 7 | Limb artery | 100 | ND | 2.61 | Definite |
| 3 | 60, M | Cardiac surgery | 8 | Adrenal vein | 85 | 2.82 | 2.65 | Definite |
| 4 | 48, M | Vascular surgery | 6 | Nil | 84 | 1.50 | 2.56 | Definite |
| 5 | 60, F | Vascular surgery | 8 | Limb ischemia | ND | Pos | ND | Probable |
| 6 | 65, M | Cardiac surgery | 7 | Anaphylactoid | 60 | 1.66 | 2.04 | Definite |
| 7 | 75, F | Medical prophylaxis | 8 | DVT, PE | 93 | ND | ND | Definite |
| 8 | 51, M | Vascular surgery | 6 | Limb ischemia | 98 | ND | 3.10 | Definite |
| 9 | 77, F | Medical prophylaxis | 7 | MI | 99 | ND | 2.24 | Definite |
| 10 | 73, M | Vascular surgery | 5 | MI | 100 | ND | 2.56 | Definite |
| 11 | 72, M | Cardiac surgery | 7 | Anaphylactoid | 100 | ND | 2.31 | Definite |
| 12 | 74, F | Vascular surgery | 6 | Nil | 100 | 2.91 | 2.51 | Definite |
| 13 | 48, F | PE treatment | 4 | Nil | ND | Pos | ND | Probable |
| 14 | 66, M | Thoracic surgery | 7 | PE | ND | Pos | ND | Probable |
| 15 | 57, M | Medical prophylaxis | 6 | Mesenteric vein | 85 | ND | 2.54 | Definite |
| 16 | 61, M | DVT treatment | 4 | MI, DVT | 93 | ND | 1.85 | Definite |
| 17 | 68, M | Cardiac surgery | 8 | DVT, PE | ND | >2.00 | ND | Definite |
| 18 | 48, M | Vascular surgery | 7 | MI, CVA, PE | ND | Pos | ND | Probable |
| 19 | 62, M | Hemodialysis | 4 | Nil | 82 | ND | ND | Definite |
| 20 | 54, M | Medical (SBE treatment) | 5 | Nil | 100 | ND | 2.45 | Definite |
For 5 of the 20 patients (patients 5, 13, 14, 17, and 18), an SRA was not performed because the previous episode of HIT occurred at a non-Hamilton hospital.
CVA, cerebrovascular accident (thrombotic); EIA-G, EIA that detects antibodies against IgG only; GAM, EIA that detects antibodies against IgG, IgA, and IgM antibodies; F, female; M, male; MI, myocardial infarction; ND, not done; Pos, positive; Pt, patient; SBE, subacute bacterial endocarditis.
Recurrent HIT
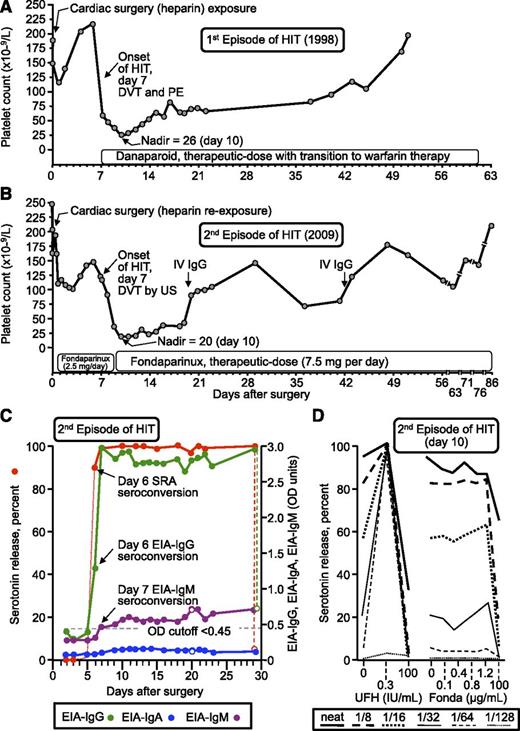
One of the 20 (5%) patients (patient 17) developed recurrent HIT after reexposure to UFH for cardiac surgery, as shown by seroconversion from a negative baseline to a strong positive SRA (serotonin release at 0.1 and 0.3 IU/mL UFH, 100%; reference range, <20%) and a strong positive EIA-IgG (2.74 OD units; reference range <0.45), and that was accompanied by an otherwise unexplained 86% decline in the platelet count that began on postoperative day 7 and while receiving postoperative thromboprophylaxis with fondaparinux (2.5 mg/day). Figure 1A shows the patient’s initial episode of HIT that occurred 11 years earlier (1998), whereas Figure 1B summarizes the second episode of HIT (2009). Remarkably, the clinical features of the second episode of HIT (onset day 7, platelet count nadir = 20 [day 10], platelet count recovery by ∼80 days, deep vein thrombosis (DVT), generalized maculopapular rash, full recovery with a therapeutic dose of fondaparinux 7.5 mg/day) virtually recapitulated his prior 1998 HIT episode (also postcardiac surgery, onset day 7, platelet nadir = 26 [day 10], recovery by ∼50 days, DVT/pulmonary embolism, generalized maculopapular rash, full recovery with a therapeutic dose of danaparoid).
Patient with recurrent HIT after heparin reexposure (patient 17). (A) First episode of HIT (1998). (B) Second episode of HIT after intraoperative heparin rechallenge (2009). The patient’s platelet count rose transiently on 2 occasions after administration of high-dose intravenous IgG. (C) Timing of anti-PF4/heparin seroconversion after heparin rechallenge. Both the EIA-IgG and SRA became positive on day 6; IgM seroconversion occurred on day 7. Reactivity in the EIA in the presence of high heparin (100 IU/mL) is shown by the open circles; thus, the increase in IgM levels was not inhibited by high heparin, whereas the increase in IgG levels and reactivity in the SRA were inhibited by high heparin. (D) Assessment of heparin- and fondaparinux-dependent platelet activation in the presence of patient serum. Strong serum-induced platelet activation (≥80% serotonin release) was observed in the absence of heparin (0 IU/mL) using neat and 1/8 diluted serum; strong heparin-dependent platelet activation was shown by the increase in serotonin release at 0.3 IU/mL UFH, compared with 0 IU/mL UFH, at higher dilutions of patient serum (1/16, 1/32, 1/64, 1/128). The absence of fondaparinux-dependent platelet activation argues against fondaparinux cross-reactivity as an explanation for the patient’s persisting thrombocytopenia. Fonda, fondaparinux; IV, intravenous; PE, pulmonary embolism; US, ultrasound.
Patient with recurrent HIT after heparin reexposure (patient 17). (A) First episode of HIT (1998). (B) Second episode of HIT after intraoperative heparin rechallenge (2009). The patient’s platelet count rose transiently on 2 occasions after administration of high-dose intravenous IgG. (C) Timing of anti-PF4/heparin seroconversion after heparin rechallenge. Both the EIA-IgG and SRA became positive on day 6; IgM seroconversion occurred on day 7. Reactivity in the EIA in the presence of high heparin (100 IU/mL) is shown by the open circles; thus, the increase in IgM levels was not inhibited by high heparin, whereas the increase in IgG levels and reactivity in the SRA were inhibited by high heparin. (D) Assessment of heparin- and fondaparinux-dependent platelet activation in the presence of patient serum. Strong serum-induced platelet activation (≥80% serotonin release) was observed in the absence of heparin (0 IU/mL) using neat and 1/8 diluted serum; strong heparin-dependent platelet activation was shown by the increase in serotonin release at 0.3 IU/mL UFH, compared with 0 IU/mL UFH, at higher dilutions of patient serum (1/16, 1/32, 1/64, 1/128). The absence of fondaparinux-dependent platelet activation argues against fondaparinux cross-reactivity as an explanation for the patient’s persisting thrombocytopenia. Fonda, fondaparinux; IV, intravenous; PE, pulmonary embolism; US, ultrasound.
Figure 1C shows the seroconversion profile for the second episode of HIT. This demonstrated that seroconversion to a positive SRA and EIA-IgG were both evident on postoperative day 6, thus preceding the onset of HIT (day 7, as judged by the beginning of the platelet count decline) by 1 day. Figure 1D shows that the patient’s serum contained antibodies that had strong heparin-independent platelet-activating properties, based upon the strong platelet activation (>80% serotonin release at neat and 1/8 dilutions) that occurred in the absence of heparin. In addition, the antibodies had heparin-dependent properties characteristic of HIT, as the serum-induced reactivity was greatly enhanced (at 1/16 to 1/64 serum dilutions) in the presence of 0.3 IU/mL UFH. Further, serum-induced platelet activation was inhibited in the presence of very high heparin concentrations (100 IU/mL), and by Fc receptor–blocking monoclonal antibody (data not shown), features characteristic of HIT sera.5,19 Figure 1D further shows that there was no substantial increase in platelet activation in the presence of pharmacological concentrations of fondaparinux (0.1, 0.4, 0.8, and 1.2 μg/mL), indicating that fondaparinux cross-reactivity was unlikely to be the explanation for the persisting thrombocytopenia. Also, as expected for HIT antibodies,22 inhibition of serum-induced platelet activation occurred in the presence of suprapharmacologic concentrations of fondaparinux (100 μg/mL). The significance of the serological features shown in Figure 1D is that they help to explain how a patient could develop recurrent HIT 1 week after cardiac surgery even when no postoperative heparin was being given (ie, the antibodies are able to activate platelets strongly in the absence of pharmacological heparin).
Frequency of seroconversion with repeat heparin exposure
Table 2 summarizes qualitatively for each patient whether he or she developed SRA seroconversion or anti-PF4/heparin seroconversion (each immunoglobulin isotype is listed separately) or both. Eight of 17 (47%) patients who were reexposed to heparin because of cardiac or vascular surgery developed seroconversion to a positive SRA, even though none of these 17 patients continued to receive UFH or LMWH postoperatively (patient 18, who underwent UFH reexposure on 2 occasions, is only counted once); 7 of these 8 patients developed a strong positive SRA result (shown as +++ in the SRA column, ie, ≥80% release), with the remaining patient developing a moderate positive SRA (shown as ++ in the SRA column, ie, 50% to 79.9% serotonin release).
Anti-PF4/heparin antibody responses after heparin reexposure
| Pt . | Interval (weeks) . | Rechallenge (postoperative antithrombotic therapy) . | SRA pre/post . | IgG pre/post . | IgA pre/post . | IgM pre/post . | Seroconversion by . | |
|---|---|---|---|---|---|---|---|---|
| SRA . | EIA . | |||||||
| Reexposure in medical patients of a full anticoagulant course of treatment (n = 3) | ||||||||
| 1 | 703 | UFH × 11-day course | NA/− | NA/− | NA/− | NA/− | No | No |
| 2 | 477 | UFH × 17-day course | NA/− | NA/− | NA/− | NA/− | No | No |
| 7 | 408 | LMWH × 10-day course | −/− | +*/+* | −/− | −/− | No | No |
| Reexposure in surgical patients (post-cardiac/vascular surgery)—intraoperative UFH use only (n = 17) | ||||||||
| 3 | 132 | CPB UFH (danaparoid) | −/+++ | −/+++ | −/++ | −/+ | Yes | Yes |
| 4 | 8 | Vasc UFH (danaparoid) | −/− | −/− | −/− | −/− | No | No |
| 5 | 8 | CPB UFH (nil) | −/− | −/− | −/− | −/− | No | No |
| 6 | 307 | Vasc UFH (danaparoid) | −/− | −/− | −/− | −/− | No | No |
| 8 | 180 | CPB UFH (danaparoid) | −/+++ | −/+++ | −/++ | −/+ | Yes | Yes |
| 9 | 21 | Vasc UFH (clopid/ASA) | −/− | +/+ | −/− | −/− | No | No |
| 10 | 37 | CPB UFH (danaparoid) | −/++ | +/+++ | +/++ | −/− | Yes | Yes |
| 11 | 47 | Vasc UFH (clopid/ASA) | −/+++ | +*/+++* | −/− | −/− | Yes | Yes* |
| 12 | 62 | Vasc UFH (danaparoid) | −/− | −/− | −/− | −/− | No | No |
| 13 | 22 | CPB UFH (danaparoid) | −/− | ++/++ | −/+* | −/− | No | Yes* |
| 14 | 22 | Vasc UFH (nil) | −/+++ | +/+++ | −/+++ | −/− | Yes | Yes |
| 15 | 515 | CPB UFH (danaparoid) | −/+++ | ++/+++ | −/+ | +*/++* | Yes | Yes |
| 16 | 422 | Vasc UFH (danaparoid) | −/+++ | +/+++ | −/− | −/+ | Yes | Yes |
| 17 | 597 | CPB UFH (fondaparinux) | −/+++ | −/+++ | −/− | −/+* | Yes† | Yes |
| 18a | 414 | Vasc UFH (fondaparinux) | −/− | −/− | −/− | −/+ | No | Yes |
| 18b | +132 | Vasc UFH (fondaparinux) | −/− | −/− | −/− | −/+ | No | Yes |
| 19 | 166 | CPB UFH (warfarin) | −/− | ++/++ | −/− | −/− | No | No |
| 20 | 20 | CPB UFH (fondaparinux) | −/− | ++/+++ | −/+ | −/− | No | Yes |
| Pt . | Interval (weeks) . | Rechallenge (postoperative antithrombotic therapy) . | SRA pre/post . | IgG pre/post . | IgA pre/post . | IgM pre/post . | Seroconversion by . | |
|---|---|---|---|---|---|---|---|---|
| SRA . | EIA . | |||||||
| Reexposure in medical patients of a full anticoagulant course of treatment (n = 3) | ||||||||
| 1 | 703 | UFH × 11-day course | NA/− | NA/− | NA/− | NA/− | No | No |
| 2 | 477 | UFH × 17-day course | NA/− | NA/− | NA/− | NA/− | No | No |
| 7 | 408 | LMWH × 10-day course | −/− | +*/+* | −/− | −/− | No | No |
| Reexposure in surgical patients (post-cardiac/vascular surgery)—intraoperative UFH use only (n = 17) | ||||||||
| 3 | 132 | CPB UFH (danaparoid) | −/+++ | −/+++ | −/++ | −/+ | Yes | Yes |
| 4 | 8 | Vasc UFH (danaparoid) | −/− | −/− | −/− | −/− | No | No |
| 5 | 8 | CPB UFH (nil) | −/− | −/− | −/− | −/− | No | No |
| 6 | 307 | Vasc UFH (danaparoid) | −/− | −/− | −/− | −/− | No | No |
| 8 | 180 | CPB UFH (danaparoid) | −/+++ | −/+++ | −/++ | −/+ | Yes | Yes |
| 9 | 21 | Vasc UFH (clopid/ASA) | −/− | +/+ | −/− | −/− | No | No |
| 10 | 37 | CPB UFH (danaparoid) | −/++ | +/+++ | +/++ | −/− | Yes | Yes |
| 11 | 47 | Vasc UFH (clopid/ASA) | −/+++ | +*/+++* | −/− | −/− | Yes | Yes* |
| 12 | 62 | Vasc UFH (danaparoid) | −/− | −/− | −/− | −/− | No | No |
| 13 | 22 | CPB UFH (danaparoid) | −/− | ++/++ | −/+* | −/− | No | Yes* |
| 14 | 22 | Vasc UFH (nil) | −/+++ | +/+++ | −/+++ | −/− | Yes | Yes |
| 15 | 515 | CPB UFH (danaparoid) | −/+++ | ++/+++ | −/+ | +*/++* | Yes | Yes |
| 16 | 422 | Vasc UFH (danaparoid) | −/+++ | +/+++ | −/− | −/+ | Yes | Yes |
| 17 | 597 | CPB UFH (fondaparinux) | −/+++ | −/+++ | −/− | −/+* | Yes† | Yes |
| 18a | 414 | Vasc UFH (fondaparinux) | −/− | −/− | −/− | −/+ | No | Yes |
| 18b | +132 | Vasc UFH (fondaparinux) | −/− | −/− | −/− | −/+ | No | Yes |
| 19 | 166 | CPB UFH (warfarin) | −/− | ++/++ | −/− | −/− | No | No |
| 20 | 20 | CPB UFH (fondaparinux) | −/− | ++/+++ | −/+ | −/− | No | Yes |
Positive anti-PF4/heparin EIA not inhibited by >30% in the presence of high heparin.
Patient 17 developed HIT based upon both clinical picture and SRA+ status.
Yes* Any/all EIA seroconversion(s) for that patient were not inhibited >30% by high heparin.
Strength of assay results: EIA: –, negative; +, weak positive (0.40 [or 0.45] to 0.99 OD units), ++, moderate positive (1.00-1.99 OD units); +++, strong positive (≥2.00 OD units). SRA: –, negative (<20% serotonin release); +, weak positive (20.0-49.9% release); ++, moderate positive (50.0-79.9% release); +++, strong positive (≥80% release).
Clopid/ASA, clopidogrel/acetylsalicylic acid (aspirin); CPB, cardiopulmonary bypass; NA, not available; pre/post, test results of blood samples available shortly before heparin reexposure and after heparin reexposure, respectively; Pt, patient; Vasc, vascular surgery.
As shown in Table 2, none of the 3 patients who received a repeat course of UFH or LMWH for a medical indication developed recurrent antibodies, either by SRA or by EIA. In contrast, 9 of the 17 (53%) patients who received intraoperative UFH developed an anti-PF4/heparin immune response, with reactivity in at least 1 immunoglobulin isotype able to be inhibited by >30% in the high heparin inhibition step (indicated as “Yes” in the EIA seroconversion column; once again, patient 18 is only counted once). With the inclusion of 2 additional surgical patients (patients 11 and 13) who developed antibodies detectable in the anti-PF4/heparin EIA that were not inhibited by >30% with high heparin (indicated as “Yes*” in the EIA seroconversion column), there were 11/17 (64%) patients who exhibited some evidence of an immune response against PF4-dependent antigens (P = .0737 for Fisher’s exact test for the comparison between EIA seroconversion between surgical and medical patient groups).
Of these 11 immune responses, 9 represented IgG anti-PF4/heparin seroconversions that were classified as strong (+++) based upon a peak OD reading ≥2.00 OD units; 8 of these 9 patients with strong EIA-IgG seroconversion also developed a positive SRA. The most common antibody isotype was IgG (n = 9 patients), followed by IgA (n = 7) and IgM (n = 6; again, patient 18 is only counted once).
We observed 1 patient (patient 11) who developed seroconversion to a strong positive SRA (which was inhibited by high heparin) but in whom neither the preoperative sample (weakly positive EIA-IgG measuring 0.55 OD units) nor the postoperative sample with the highest EIA reactivity (2.14 OD units) exhibited >30% inhibition by high heparin. This finding corroborates previous observations23,24 that on some occasions platelet-activating antibodies can be present even when the high heparin maneuver fails to inhibit reactivity in the EIA.
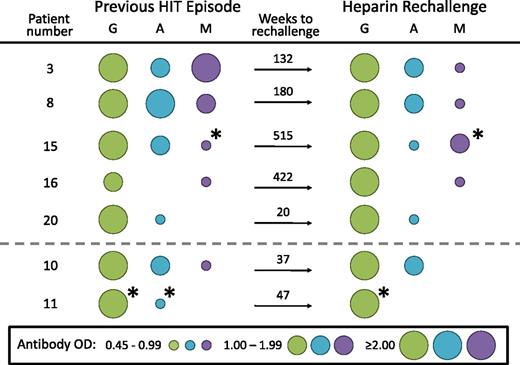
For 7 patients, we were able to compare the anti-PF4/heparin antibody isotype profile for the patients’ previous episodes of HIT with the isotype profile associated with anti-PF4/heparin antibody seroconversion after heparin reexposure. Figure 2 shows that for 5 of the patients (3, 8, 15, 16, and 20) the isotype profiles were identical, whereas for 2 of the patients (10 and 11), 1 of the 2 isotypes that was detectable at the time of their previous HIT episode was not regenerated at the time of subsequent heparin rechallenge–induced anti-PF4/heparin seroconversion.
Comparison of antibody isotype profiles between previous episode of HIT and antibodies generated after heparin reexposure. For each of 5 patients (3, 8, 15, 16, 20), the isotype profiles were identical for the previous HIT episode and the antibodies generated after UFH rechallenge, whereas for the 2 remaining patients (10, 11), 1 of the antibody isotypes present with the previous HIT episode was not regenerated upon UFH rechallenge. The size and color of the circles indicate the strength of EIA reactivity and the antibody isotype, respectively. *Indicates that reactivity in the EIA was not inhibited by >30% in the presence of high heparin.
Comparison of antibody isotype profiles between previous episode of HIT and antibodies generated after heparin reexposure. For each of 5 patients (3, 8, 15, 16, 20), the isotype profiles were identical for the previous HIT episode and the antibodies generated after UFH rechallenge, whereas for the 2 remaining patients (10, 11), 1 of the antibody isotypes present with the previous HIT episode was not regenerated upon UFH rechallenge. The size and color of the circles indicate the strength of EIA reactivity and the antibody isotype, respectively. *Indicates that reactivity in the EIA was not inhibited by >30% in the presence of high heparin.
SRA seroconversion without HIT
As described previously (see the “Recurrent HIT” section), only 1 of the 8 patients who developed SRA seroconversion after heparin rechallenge developed recurrent HIT (patient 17, Figure 1). The remaining 7 patients were judged not to have developed recurrent HIT because none developed either thrombosis or a recurrent platelet count fall at the time of SRA seroconversion. Interestingly, compared with SRA-seroconverting patient 17 (who developed recurrent HIT), none of the other 7 SRA-seroconverting patients (who did not develop HIT) exhibited strong (≥80% serotonin release) heparin-independent platelet activation: their mean serum-induced percent serotonin release (at buffer control) was 13% (range, 0% to 54%), compared with 95% serotonin release induced by serum from patient 17 in the absence of heparin.
Repeat heparin exposure in the presence of a positive baseline EIA
Nine of the 17 patients who underwent reexposure for cardiac or vascular surgery had IgG antibodies detected at preoperative baseline, although none had a positive baseline SRA. For these 9 patients, there was no significantly greater frequency of forming antibodies after reexposure compared with the 8 patients who did not have antibodies at baseline: 7/9 (78%) vs 4/8 (50%); P = .3348 (patient 18 who underwent 2 reexposures is only counted once in this analysis). Furthermore, presence of preoperative antibodies did not predict for SRA seroconversion after reexposure (5/9 [56%] vs 3/8 [38%]; P = .6372), and, most notably, the single patient who was judged to have developed recurrence of HIT (patient 17, Figure 1) after heparin reexposure tested negative for anti-PF4/heparin antibodies at preoperative baseline.
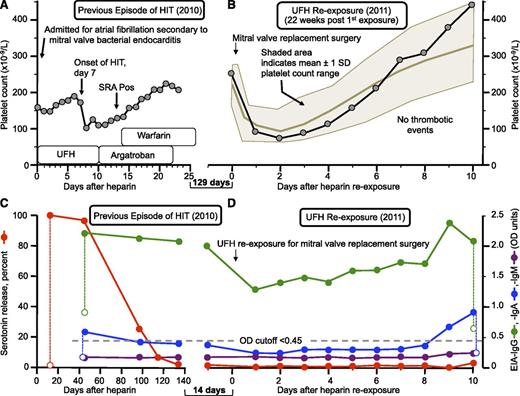
Figure 3 shows a representative case of a patient (patient 20) who underwent intraoperative UFH reexposure in 2011 despite having a positive EIA-IgG but with a negative SRA. Figure 3A illustrates the previous episode of HIT (2010), with the high-probability clinical picture corroborated by a strong positive (100% serotonin release) SRA. Figure 3B shows the platelet count sequence associated with the heparin reexposure (2011) given for mitral valve replacement surgery, illustrating that the platelet count profile was consistent with usual perioperative thrombocytopenia (see shaded area). This patient had an uneventful postoperative course, without thrombosis or other postoperative complications. Figure 3C shows the serial EIA and SRA results for the approximate 140-day period after the episode of HIT that preceded subsequent UFH reexposure at cardiac surgery, indicating that the EIA-IgG remained moderately positive (OD = 1.98 units) on the day before heparin reexposure at surgery). Figure 3D shows that after UFH reexposure at cardiac surgery, both the IgG and IgA levels, which declined modestly in the early postoperative period (presumably related to hemodilution) and then showed subsequent increases consistent with seroconversion. However, despite the post-reexposure IgG levels rising to ≥2.00 OD units by postoperative day 10, the SRA remained negative.
Repeat heparin exposure in the presence of a positive baseline EIA (patient 20). (A) Previous episode of HIT (2010). (B) Platelet counts after UFH rechallenge (2011) for urgent cardiac surgery (severe mitral regurgitation). (C) Anti-PF4/heparin antibodies and SRA results at the time of the previous episode of HIT and (D) at heparin rechallenge approximately 5 weeks after the first negative SRA. The data illustrate an uneventful UFH reexposure despite a moderate positive EIA-IgG (1.98 OD units)—with a negative SRA—at time of heparin rechallenge, which was accompanied by increase in IgG levels to almost 2.50 OD units as well as weak IgA seroconversion. The shaded area indicates a platelet count range expected for a postcardiac surgery patient population.2 Pos, positive; SD, standard deviation.
Repeat heparin exposure in the presence of a positive baseline EIA (patient 20). (A) Previous episode of HIT (2010). (B) Platelet counts after UFH rechallenge (2011) for urgent cardiac surgery (severe mitral regurgitation). (C) Anti-PF4/heparin antibodies and SRA results at the time of the previous episode of HIT and (D) at heparin rechallenge approximately 5 weeks after the first negative SRA. The data illustrate an uneventful UFH reexposure despite a moderate positive EIA-IgG (1.98 OD units)—with a negative SRA—at time of heparin rechallenge, which was accompanied by increase in IgG levels to almost 2.50 OD units as well as weak IgA seroconversion. The shaded area indicates a platelet count range expected for a postcardiac surgery patient population.2 Pos, positive; SD, standard deviation.
Timing of the recurrent anti-PF4/heparin immune response
For the 11 patients for whom evidence of a postoperative anti-PF4/heparin immune response was documented after heparin reexposure, the earliest day of detectability of antibodies was postoperative day 5 (patient 18), with a median (range) time to a positive test being SRA (day 8 [range, days 6 to 11], EIA-IgG (day 7 [range, days 6 to 9], EIA-IgA (day 9 [days 7 to 10], and EIA-IgM (day 8 [days 5 to 9]). This timing of onset of seroconversion is no sooner than would otherwise be expected in patients exhibiting anti-PF4/heparin seroconversion after heparin exposure, either with or without overt HIT.4-6
There were 5 patients (patients 3, 8, 14, 17, and 20) with previous typical-onset HIT for whom the precise day of onset of their previous HIT episode was known, and for whom recurrent antibodies, including recurrent platelet-activating antibodies (positive SRA) in 3 of the 5 patients, were regenerated after intraoperative reexposure to UFH. None of these 5 patients showed evidence for a speedier onset of seroconversion, in relation to the previous episode of HIT: median day of onset of previous HIT, day 7 [range, 7 to 10])—judged by onset of the HIT-associated platelet count fall—vs median day of onset of subsequent anti-PF4/heparin seroconversion, day 7 (range, 6 to 9). Given that the patients likely would have had detectable anti-PF4/heparin antibodies 1 or 2 days before the onset of platelet count fall for their previous episode of HIT, these intrapatient comparisons definitely argue against any tendency for a speedier antibody formation rate among patients with previous HIT who subsequently develop recurrent anti-PF4/heparin antibodies with subsequent heparin reexposure.
Discussion
Our study shows that approximately half of the patients with a previous history of HIT who undergo heparin rechallenge for cardiac/vascular surgery once again develop anti-PF4/heparin antibodies with platelet-activating properties. The high frequency of SRA seroconversion that we observed with intraoperative reexposure (8/17, or 47%) appears to be greater than the expected 3% to 20% (12%, median of 5 studies2,12-15 ) frequency of SRA seroconversion reported in the postcardiac surgery literature. This suggests that patients with a history of HIT could have an inherently greater capacity to form platelet-activating HIT antibodies compared with cardiac/vascular surgery patients who do not have a history of previous HIT. Nevertheless, because it takes at least 5 days after surgery to form platelet-activating antibodies, and because further heparin use can be avoided during the postsurgical period, it would appear that the frequency of recurrent HIT will likely be low. We believe that our findings therefore support consensus conference recommendations for intraoperative heparin use in patients with a previous history of HIT (provided that platelet-activating antibodies are no longer detectable), and to use an alternative (non-heparin) anticoagulant for postoperative anticoagulation.25-27 We recognize that few centers perform washed platelet activation assays, but in most cases, there is sufficient time to refer the patient serum to a center that is experienced in performing such assays, such as the SRA.
However, our study also points to the possibility of a patient developing recurrent HIT—even in the absence of continuing postoperative heparin—if the patient forms antibodies that are able to activate platelets strongly in vitro even in the absence of added pharmacologic heparin. One of our patients who received a repeat exposure to intraoperative UFH to permit urgent cardiac surgery—patient 17 (Figure 1)—developed a clinical picture strongly indicative of HIT, and one that essentially recapitulated his prior episode of HIT 11 years earlier. This patient’s serum strongly activated platelets even in the absence of heparin (heparin-independent platelet activation21 ), a serological feature consistent with the antibodies identified in patients with “delayed-onset HIT”28-31 as well as delayed recovery of thrombocytopenia after stopping heparin,32 and that plausibly explains why this patient developed HIT 1 week after cardiac surgery, even though no postoperative heparin was being given. A practical consequence of this clinical observation is that patients with previous HIT who undergo heparin rechallenge should undergo platelet count monitoring, perhaps for as long as 10 days, even if they do not receive any postoperative heparin.
Our study also provides a systematic assessment of the timing of repeat anti-PF4/heparin antibody responses in a patient population with well-characterized previous HIT. We found that antibody formation did not occur any more quickly upon heparin reexposure than had occurred with their previous HIT episode (when this information was available), or in relation to the known timing of typical-onset HIT, which is approximately day 6 to 7 (median).4 This median time to detectability of newly forming anti-PF4/heparin antibodies of approximately 1 week—irrespective of whether previous exposure to heparin has occurred or not5 or even whether a patient has had a previous history of HIT (present report)—provides support to the recent concept that HIT represents a misdirected immune response,33 in which all cases of HIT—even when patients are exposed to heparin for the first time—likely represent a type of “secondary” immune response, perhaps as a result of preceding “primary” immunization that may have occurred as a result of exposure to PF4-coated bacteria.33 Interestingly, in most instances, the isotype profile of the subsequent rechallenge-induced seroconversion response resembled that seen at the time of the previous episode of HIT (Figure 2), suggesting that the HIT immune response is indeed a secondary (anamnestic) response involving long-lived B-lymphocytes rather than a de novo immune reaction; the latter would likely result in a more heterogeneous pattern of isotype response.
We also observed that for 9 of our 17 surgical patients, anti-PF4/heparin IgG antibodies were present at the time of rechallenge, although none had platelet-activating antibodies (ie, all 9 patients had a negative SRA at the time of rechallenge). None of these patients appeared to develop recurrent HIT based upon their postoperative platelet count profiles and unremarkable clinical courses. This is consistent with a previous study by Selleng and colleagues34 who observed no evidence for acute HIT in patients who required urgent cardiac surgery soon after recovery from HIT but who had IgG antibodies without a positive platelet activation assay. Thus, for the common practice of checking by EIA for presence of anti-PF4/heparin antibodies before planning a recurrent heparin exposure in patients with a previous history of HIT, a negative EIA would indicate absence of platelet-activating antibodies35 (and no need to perform the SRA), but the presence of non–platelet-activating anti-PF4/heparin antibodies—as shown by a positive EIA but a negative SRA—before surgery should not deter from considering UFH reexposure for cardiac or vascular surgery.
Limitations of our study include the lack of SRA documentation of previous HIT in 5 of our 20 patients, the possibility that some of our patients may have developed antibodies after discharge from the hospital (in which case the frequency of anti-PF4/heparin and/or SRA seroconversion might have been even higher than we observed), and the single-center nature of our study (which limits generalizability). In addition, our reported frequency of recurrent HIT among all of our patients (both medical and surgical), which was 1 in 20 (5%), has a wide 95% confidence interval (0.13-24.9); thus, determining the true frequency of recurrent HIT among heparin-reexposed patients will require assessment in additional patients. Heparin reexposure in patients with well-documented previous HIT is relatively uncommon; we identified only approximately 1 patient per year (ie, 21 reexposures in 20 patients over an 18-year period). Nevertheless, our data suggest that deliberate intraoperative UFH reexposure for patients who require cardiac or vascular surgery is a reasonable treatment option, provided that platelet-activating antibodies are not detectable before surgery, especially given that most medical centers will not have experience with non-heparin anticoagulants for cardiac or vascular surgery.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The anti-PF4/heparin serology studies and the SRA were supported by the Heart and Stroke Foundation of Ontario (operating grant T6950).
Authorship
Contribution: T.E.W. identified eligible patients, designed and supervised the experiments, analyzed the data, interpreted the results, and was the primary author of the paper; J.-A.I.S. performed the experiments, analyzed the data, and interpreted the results. Both authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.E.W. has received lecture honoraria from Pfizer Canada and Instrumentation Laboratory, has provided consulting services to, and/or has received research funding from GlaxoSmithKline, W.L. Gore, Immucor GTI Diagnostics, and Paringenix, and has provided expert witness testimony relating to HIT. The remaining author declares no competing financial interests.
Correspondence: Theodore (Ted) E. Warkentin, Hamilton Regional Laboratory Medicine, Hamilton Health Sciences, Hamilton General Site, Rm 1-270B, 237 Barton St E, Hamilton, ON, L8L 2X2; e-mail: twarken@mcmaster.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal