Abstract
Published data demonstrating the efficacy of complement inhibition therapy in patients with atypical hemolytic uremic syndrome (aHUS) are remarkable in contrast to the historically poor long-term prognosis for aHUS patients treated with plasma-based therapy. Although both aHUS and acquired thrombotic thrombocytopenic purpura (TTP) remain clinical diagnoses, an increased understanding of both conditions has improved our ability to differentiate aHUS from acquired TTP. These same data have also demonstrated the importance of a more rapid identification and diagnosis of aHUS as the recovery of end-organ injury present appears to be related to the time to initiate therapy with eculizumab. The diagnosis of acquired TTP can be confirmed by the finding of severely deficient ADAMTS13 activity (<10%) with evidence of an ADAMTS13 antibody inhibitor whereas merely deficient ADAMTS13 activity in the absence of an ADAMTS13 autoantibody is more consistent with congenital TTP. In the absence of an objective diagnostic test, clinicians must rely collectively on platelet count, serum creatinine, and ADAMTS13 activity in the context of the response to plasma exchange therapy to identify patients whose diagnosis is most consistent with aHUS, and thus be more likely to benefit from therapy with eculizumab.
Case presentation
A 25-year-old man was admitted with complaints of diarrhea and severe abdominal pain. Initial studies included stool cultures, which failed to demonstrate an infectious cause for the diarrhea, and an enzyme-linked immunosorbent assay for Shiga toxin in the stool that was negative. He had significant hypertension on presentation (185/110 mmHg) and a leukocytosis of 29 × 109/L with a left-shifted differential, but the platelet count and the remainder of the complete blood count were otherwise normal. Three days later, he developed an altered sensorium and became anuric with a rising serum creatinine that required the initiation of hemodialysis. At the same time, he developed a significant drop in his platelet count to 35 × 109/L with a significant number of schistocytes in the peripheral blood smear. His deteriorating mental status and inability to protect his airway required that he be placed on mechanical ventilation. Imaging studies that included a computed tomography scan and magnetic resonance imaging of the brain showed no significant abnormalities to explain his altered mental status. Given the thrombotic microangiopathic (TMA) findings, daily plasma exchange (PEX), using 1 plasma volume and fresh frozen plasma as the replacement fluid, was initiated. After 4 daily PEX procedures, there was no significant hematologic or renal recovery with continued deterioration of his mental status. The results of the pretreatment ADAMTS13 activity returned on day 4 and demonstrated his ADAMTS13 activity to be 75%, without evidence for an antibody inhibitor of ADAMTS13. Complement protein mutation studies and complement factor H (CFH) autoantibody studies were done at presentation but the results were not yet available.
Introduction
The case summary presented illustrates well the difficulties clinicians face when presented with a patient with an acute TMA and the need to clinically differentiate atypical hemolytic uremic syndrome (aHUS) from thrombotic thrombocytopenic purpura (TTP). Recent advances in the treatment of aHUS with eculizumab (Soliris), an antibody inhibitor of terminal complement activation, have placed a greater importance on the timely and accurate differentiation of aHUS from acquired TTP. Dramatic hematologic responses and recovery of renal injury (4 of 5 patients becoming independent of dialysis) in patients refractory to PEX after treatment with eculizumab1 are striking in contrast to the historical data that showed that up to two-thirds of patients die or go on to end-stage renal disease in the first year after their initial presentation2 (Table 1). There are also published data that suggest the improvement in end-organ injury after therapy with eculizumab is related to the time to initiate therapy,1,3 with a shorter time to initiate therapy with eculizumab being associated with greater rates of recovery of renal function. These data have collectively “raised the bar” for physicians who are charged with accurately, and now more rapidly, clinically differentiating acquired TTP from aHUS.
Eculizumab in aHUS not responding to PEX (n = 17)
| . | Demographic and laboratory data . | Week 26 . | Week 64 . |
|---|---|---|---|
| Data at presentation | |||
| Median age, y | 28 (17-68) | ||
| Median platelets, ×109/L | 118 (62-161) | ||
| Median creatinine, mg/dL | 2.9 (1.4-8.9) | ||
| LDH (>ULN) | 10/17 (59) | ||
| Response criteria | |||
| Normalized platelet count | 13/15 (87)* | 13/15 (87)* | |
| Decreased creatinine by 25% | 11/17 (65)† | 13/17 (76)† | |
| Increased est. GFR ≥ 15 mL/min/1.73m2 | 8/17 (47) | 9/17 (53) |
| . | Demographic and laboratory data . | Week 26 . | Week 64 . |
|---|---|---|---|
| Data at presentation | |||
| Median age, y | 28 (17-68) | ||
| Median platelets, ×109/L | 118 (62-161) | ||
| Median creatinine, mg/dL | 2.9 (1.4-8.9) | ||
| LDH (>ULN) | 10/17 (59) | ||
| Response criteria | |||
| Normalized platelet count | 13/15 (87)* | 13/15 (87)* | |
| Decreased creatinine by 25% | 11/17 (65)† | 13/17 (76)† | |
| Increased est. GFR ≥ 15 mL/min/1.73m2 | 8/17 (47) | 9/17 (53) |
The clinical data both at presentation and after therapy with eculizumab are presented for the 17 patients enrolled in the prospective study of eculizumab in patients with a progressive TMA after PEX therapy. Fifteen patients completed 26 weeks of therapy, with 13 patients continuing therapy beyond 26 weeks, and were available for follow-up after a median of 64 weeks of therapy.
GFR, glomerular filtration rate; ULN, upper limit of normal.
Two patients with normal platelet count at start of therapy not evaluable.
Four of 5 patients able to become independent of the need for dialysis after therapy.
Clinical definitions
The terminology used to define TMAs has evolved as we have gained a better understanding of the pathophysiology of the specific conditions associated with the development of a TMA. The term TMA defines the clinical syndrome that includes: a non-immune-mediated hemolytic anemia, fragmented cells in the peripheral blood, and thrombocytopenia. After excluding secondary TMAs due to underlying diseases present, TMAs can be divided into 2 broad categories: TTP and hemolytic uremic syndrome (HUS). The diagnosis of acquired TTP can be confirmed by the finding of severely deficient pretreatment ADAMTS13 activity (<10%) mediated by an antibody inhibitor of ADAMTS13, with severely deficient ADAMTS13 activity in the absence of an antibody inhibitor of ADAMTS13 consistent with congenital TTP. The term HUS has been used to define a TMA with renal impairment, but can be further divided in typical and atypical forms of the disease.4 Typical HUS is a TMA secondary to Shiga toxin–producing organisms (most commonly Escherichia coli and Shigella dysenteriae) that begins with bloody diarrhea in the majority of cases and is more common in children.5 The term aHUS is best reserved for the TMA that is associated with the dysregulation of the alternative pathway of complement secondary to complement gene mutations or CFH autoantibodies. There are also conditions that have been associated with the development of TMA and are best labeled as secondary TMAs. These conditions include: malignant hypertension, septicemia, autoimmune disorders, Streptococcus pneumoniae infections in children, cobalamin C deficiency, glomerulopathies (membranoproliferative glomerulonephritis, systemic lupus erythematosis]), viral infections (HIV, influenza), and malignancies.4,6 These entities should be considered part of the disease that triggered the TMA and labeled with an etiology-based name (ie, malignant hypertension-associated TMA) rather than the term aHUS. Additionally, there are diagnoses that may present with TMA findings that historically have been labeled related to their clinical presentation, but have recently been demonstrated to be mediated at least in part by dysregulated complement activity. Examples of such diagnoses include: preeclampsia, hematopoietic stem cell transplantation–associated TMA, solid organ transplant–associated TMA, and antiphospholipid antibody syndrome.7-12
Pathophysiology of acquired TTP and aHUS
In acquired TTP, the microvascular injury is mediated by the development of an autoantibody inhibitor of the ADAMTS13 protease, leading to circulating ultra-large von Willebrand factor multimers that may spontaneously aggregate platelets under conditions of higher shear forces.13-16 In contrast, the microvascular injury present in aHUS is mediated by uncontrolled complement activation, resulting in microvascular injury, activation of platelets, coagulation, and leukocytes, and the development of a systemic TMA.2,6,17,18 The lack of objective diagnostic tests to confirm the clinical diagnoses of aHUS and TTP had been a major stumbling block to differentiate these disorders. Although a severe deficiency of the ADAMTS13 protease (<10%) and documented mutations of complement proteins are not required for the diagnosis of acquired TTP and aHUS, respectively, both findings in the appropriate clinical context serve to confirm the clinical diagnosis.
Clinical symptoms, acquired TTP, and aHUS
Several key features in the case presented accurately highlight the reasons that clinical symptoms alone cannot be used to differentiate aHUS and TTP. The presence of “bloody diarrhea” has historically been associated with the diagnosis of typical HUS, a TMA caused by a Shiga toxin–producing bacteria. The terms diarrhea “positive” or “negative” HUS have also been used historically to differentiate typical HUS and aHUS.19 However, given that gastrointestinal symptoms can commonly be seen in patients with aHUS and acquired TTP, the clinical utility of gastrointestinal symptoms to differentiate these conditions is limited.19,20 Similarly, neurologic symptoms have been used previously as a criterion to differentiate TTP from other TMAs. Although neurologic injury has been reported in 25% to 79% of patients with ADAMTS13-deficient TTP,21-25 severe and devastating neurologic injury similar to the case presentation has also been described in aHUS patients where the diagnosis was confirmed by the presence of complement protein mutations.20,26-28
Although acute renal failure requiring kidney dialysis at presentation is a prominent clinical feature in patients with aHUS,20 patients with ADAMTS13-deficient TTP may also rarely present with acute renal failure. Despite renal injury in ADAMTS13-deficient TTP being common, renal failure requiring dialysis is a relatively uncommon event, occurring in <10% of patients.21-23,29 In these studies that looked at renal insufficiency in ADAMTS13-deficient TTP patients, complement mutation studies were not reported for any patients.
ADAMTS13 activity and the differentiation of aHUS and TTP
Following the discovery of the ADAMTS13 protease and its role in the pathophysiology of acquired TTP,13,14 the measurement of ADAMTS13 activity has been increasingly used as an objective diagnostic test to differentiate acquired TTP from other TMAs. Published studies reported varying rates of severely deficient ADAMTS13 activity led to significant debates on this issue.14,30-32 Differing rates of severely deficient ADAMTS13 activity reported for patients clinically characterized as aHUS or TTP, however, were likely the result of differing (and unreliable) clinical criteria used to define each condition for study.
Differing thresholds for determining severely deficient ADAMTS13 activity (5%-15%) have been used based more on expert opinion rather than data.21,22,29,33-35 To clarify this important diagnostic issue, we analyzed the data from patients we treated in our medical center who presented with acute TMA episodes as defined by thrombocytopenia (<100 × 109/L), hemolytic anemia, and the presence of fragmented cells in the peripheral blood. Fifty-seven patients were clinically diagnosed with acquired TTP based on their clinical presentations, treatment response, and clinical evaluation during longitudinal follow-up. In this cohort of acquired TTP, all patients demonstrated presence of either an ADAMTS13 inhibitor or ADAMTS13 immunoglobulin G (IgG) autoantibody. The control TMA patient group included 19 patients with clinically diagnosed aHUS and 38 randomly selected patients with diagnosis of TMA due to underlying disease (disseminated intravascular coagulation, solid organ transplantation, HIV infection, antiphospholipid syndrome, preeclampsia/hemolysis, elevated liver function tests, low platelets syndrome, and malignancy). ADAMTS13 activity levels ranged from <1% to 9% (median <1%) in the acquired TTP cohort. The TMA control group had ADAMST13 activity levels ranging from 14% to >100% (median 61%). Receiver operating characteristic (ROC) analysis was performed and showed that using a threshold for determining ADAMTS13 deficiency of 10% yielded a sensitivity and specificity of 100% in differentiating acquired TTP from other types of TMAs, but with a threshold of 5% the specificity remained 100%, but the test sensitivity decreased to 95%.
Platelet count, serum creatinine, and the ADAMTS13 activity
Historic reports have associated higher platelet counts and more significant renal injury (serum creatinine) at the time of presentation with nondeficient ADAMTS13 activity21,22,29,33-36 (Table 2). A more recent statistical analysis by the French Reference Center for Thrombotic Microangiopathies evaluated the ability of the pretreatment platelet count, serum creatinine, and antinuclear antibodies (ANAs) to predict severely deficient ADAMTS13 activity (<5%). They reported similar findings as previous investigators, but characterized the association of the platelet count (<30 × 109/L) as most strongly associated with severely deficient ADAMTS13 activity, followed by the serum creatinine (≤2.25 mg/dL). Although not meant to replace the measurement of pretreatment ADAMTS13 activity, these data may be useful as a confirmatory tool for reported ADAMTS13 activity and for allowing clinicians to anticipate the result of the ADAMTS13 activity while evaluating a patient’s response to PEX therapy.
ADAMTS13 activity
| . | ADAMTS13 threshold, % . | ADAMTS13 activity . | |||
|---|---|---|---|---|---|
| Severely deficient . | Nondeficient . | ||||
| Platelets, ×109/L . | Creatinine, mg/dL . | Platelets, ×109/L . | Creatinine, mg/dL . | ||
| Raife et al35 | 15 | 13 | 1.2 | 44 | 2.7 |
| Coppo et al21,34 | 5 | 17 | 1.3 | 67 | 5.1 |
| Kremer Hovinga et al22 | 10 | 11 | 1.6 | 22 | 4.6 |
| Cataland et al29 | 10 | 12 | 1.7 | 66 | 6.7 |
| Bentley et al36 | 15 | 16 | 1.1 | 64 | 3.5 |
| . | ADAMTS13 threshold, % . | ADAMTS13 activity . | |||
|---|---|---|---|---|---|
| Severely deficient . | Nondeficient . | ||||
| Platelets, ×109/L . | Creatinine, mg/dL . | Platelets, ×109/L . | Creatinine, mg/dL . | ||
| Raife et al35 | 15 | 13 | 1.2 | 44 | 2.7 |
| Coppo et al21,34 | 5 | 17 | 1.3 | 67 | 5.1 |
| Kremer Hovinga et al22 | 10 | 11 | 1.6 | 22 | 4.6 |
| Cataland et al29 | 10 | 12 | 1.7 | 66 | 6.7 |
| Bentley et al36 | 15 | 16 | 1.1 | 64 | 3.5 |
Reported platelet count and serum creatinine at presentation from studies of TMA patients comparing the clinical features of patients with severely deficient and nondeficient ADAMTS13 activity.
C3, C4, complement mutation studies, and the differentiation of aHUS and TTP
Complement activation mediated by the alternative pathway in aHUS would be predicted to result in low levels of C3 and normal C4 levels. Although this has been reported in aHUS patients,18,37,38 additional studies suggest that no more than half of patients diagnosed with aHUS confirmed by mutation studies will demonstrate the expected low C3 and normal C4 levels, limiting their utility in the diagnosis of aHUS.18,39 More importantly, recent studies have demonstrated complement activation to be present in patients with ADAMTS13-deficient TTP, further limiting the potential utility of C3 and C4 to differentiate aHUS from acquired TTP.40-42
Documented mutations of 1 or multiple complement proteins could confirm the clinical diagnosis of aHUS, but the results of such studies are not available in real time to confirm the clinical diagnosis of aHUS. Despite the delay in receiving results of the mutation analysis, we still routinely perform these studies during follow-up to help in the categorization of their TMA. Whereas only half of patients will be found to have a mutation of CFH, CFI, C3, CFB, THBD, or MCP, when present, a mutation could confirm that the diagnosis of aHUS provided the rationale for long-term therapy with eculizumab.2,3,20 The percentage of patients with a documented mutation is expected to increase given recent reports of novel mutations, including those involving the coagulation system, associated with the clinical diagnosis of aHUS.43 Although these data may not be useful in the upfront diagnosis of aHUS, their importance may ultimately lie in their prognostic value as studies associate specific mutations with clinical outcomes and response to therapy.2,20
aHUS and response to plasma-based therapy
Plasma-based therapy (PEX or infusion) historically has been the first line of therapy for aHUS, based more upon expert opinion than prospective clinical trials.6,44-47 Drawing conclusions regarding the effectiveness of PEX from retrospective case series is problematic given the differing timing and application of PEX and plasma infusion (PI). Plasma-based therapies theoretically could be effective not only via the replacement of normal levels of CFH, CFI, factor B, and C3, but also via the removal of mutated complement factors6,47 and CFH autoantibodies via PEX. Responses to PEX/PI have been defined as complete (hematologic and renal remission) and partial (hematologic remission with renal sequelae).18,47 In 1 large retrospective study reported by Noris et al, plasma therapy induced a complete or partial remission in 63%, 25%, 57%, 88%, 75%, and 69% of episodes in patients with mutations of CFH, CFI, C3, thrombomodulin (THBD), anti-CFH autoantibodies, and no mutations, respectively.18 In patients with membrane cofactor protein (MCP) mutations, 97% of plasma-treated episodes and all 14 non-plasma-treated episodes achieved remission, consistent with the good prognosis associated with aHUS and mutations of MCP.2,48 With the exception of patients with mutations of THBD and MCP, complete response rates with PEX were much lower ranging from 5% to 43% in patients with mutations of CFH, CFI, C3, anti-CFH autoantibodies and patients with no mutations.18 More problematic, though, was the end point of end stage renal disease (ESRD) and death associated with the acute episode that ranged from 13% to 75% after excluding patients with MCP mutations. Long-term follow-up for all patients in this retrospective series demonstrated a poor prognosis in all patients except those with MCP mutations. After 3 years of follow-up, the combined end point of ESRD or death ranged from 50% to 77%, demonstrating the poor long-term prognosis with or without plasma-based therapy.18
In patients responding to plasma therapy, the therapy should be tapered with the long-term treatment plan individualized. The demonstration of an MCP mutation should allow the withdrawal of plasma therapy, while the finding of a mutation of CFH would indicate the need for long-term plasma therapy.6 In other patients, plasma therapy can be tapered and discontinued, assuming there is no evidence of active hemolysis and other manifestations of aHUS.6 The practical limitations (vascular access, risk for thrombosis, infection) of PEX in addition to the potential for adverse reactions to plasma are problematic for patients who will require long-term plasma therapy.4,6 More importantly, the prospective data published by Legendre et al in patients previously being maintained on plasma therapy demonstrated a time-dependent increase in the glomerular filtration rate after discontinuing plasma therapy and initiating therapy with eculizumab, suggesting that therapy with eculizumab more effectively controlled the disease manifestations of aHUS than plasma therapy.1
CFH autoantibodies and immune-suppressive therapy
There are published data supporting the use of the combined modalities of PEX and immune-suppressive therapy in patients with aHUS mediated by anti-CFH antibodies.6,49 Sinha et al recently demonstrated that the prompt use of PEX and immune-suppressive therapy can improve outcomes in patients with anti-CFH antibody-mediated aHUS.49 Risk factors for adverse outcomes in this study included a high antibody titer and a delay in PEX therapy. Immune-suppressive therapy reported to be effective in patients included: steroids, azathioprine, mycophenolate mofetil, cyclophosphamide, and rituximab.49-53 Relapse after achieving remission appeared to be related to anti-CFH antibody titers,6,49 with relapse-free survival better in patients receiving maintenance immunosuppressive therapy.49 Decisions regarding the length of PEX therapy as well as the choice and duration of immune-suppressive therapy therefore should be guided by the anti-CFH antibody titer.
Initial therapy of patients with suspected TTP and aHUS
Except when the diagnosis of aHUS is known, PEX therapy should be started as soon as possible in all patients presenting with a suspected diagnosis of aHUS or TTP.3,4,6 Even in cases with more pronounced renal failure where the suspicion of aHUS is higher, PEX should be initiated as soon as possible until additional data are available not only to confirm the diagnosis, but also to assess their initial response to PEX therapy. It is also important to remember to obtain a pretreatment measurement of the ADAMTS13 activity and CFH autoantibodies. Although it may take several days to obtain the results, the initial management will not be affected given the recommendation to rapidly initiate PEX in all patients. Although results of complement mutation studies may take several months, more rapid turnaround is available for CFH autoantibody testing and should be a part of the initial evaluation of a patient with suspected aHUS. While awaiting the results of the initial diagnostic testing, it is the consensus of expert opinions to assess the response to PEX over 3 to 5 days.3,4,6
Response to PEX and ADAMTS13 activity
In patients responding to daily PEX, our practice is to continue therapy until both the platelet count and lactate dehydrogenase (LDH) have normalized. It is also important to assess the recovery of renal injury present. Although some time may be required for complete recovery, at least stabilization, if not improvement, in the serum creatinine should be seen over the first 3 to 5 days when present.3,4 In patients responding to PEX therapy (normalization of platelet count, LDH, and stable or improved renal function), the finding of severely deficient pretreatment ADAMTS13 activity (<10%) would confirm the diagnosis of TTP. The finding of nondeficient ADAMTS13 activity (>10%) in a patient responding to PEX would not be typical for the diagnosis of TTP, and would support a diagnosis of aHUS that was responsive to PEX therapy. In these cases, complement mutation studies should be obtained which could confirm the diagnosis of aHUS.
Patients with nondeficient ADAMTS13 activity that initially respond to PEX, but are unable to discontinue PEX without a recurrence of hemolysis and end-organ injury, would also support the diagnosis of aHUS, and suggest the dependence on long-term plasma therapy. Patients with nondeficient, pretreatment ADAMTS13 activity and a hematologic response over the initial 3- to 5-day course of PEX, but with progressive renal injury, would also be more consistent with a diagnosis of aHUS, and a patient that is not completely responding to PEX therapy. In both cases, PEX therapy should be discontinued and therapy with eculizumab initiated.3,4,6 Similar to the patients with nondeficient, pretreatment ADAMTS13 activity that responded to PEX therapy, complement mutation studies should be obtained to attempt to confirm the diagnosis of aHUS.
Poor response to PEX and ADAMTS13 activity
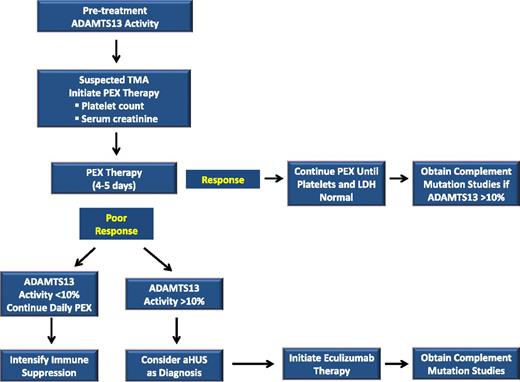
In patients unable to achieve a hematologic response or renal improvement with PEX over the first 3 to 5 days, the pretreatment ADAMTS13 activity provides important information for determining the next most appropriate course of therapy (Figure 1). Severely deficient, pretreatment ADAMTS13 activity would confirm the diagnosis of acquired TTP, and would be an indication for the addition or intensification of immune-suppressive therapy. In contrast, the finding of pretreatment ADAMTS13 activity that was not severely deficient, in a patient not responding to PEX, would be more consistent with a diagnosis of aHUS. In the absence of an alternative clinical explanation for their TMA (sepsis, malignancy, autoimmune disorder), PEX should be discontinued and therapy with eculizumab should be considered.
Conceptual algorithm. The algorithm depicted conceptually describes our approach to the treatment of patients presenting with an acute TMA. PEX is started initially in all patients, with the response to PEX serving as an important diagnostic criterion. Collectively, the pretreatment ADAMTS13, platelet count, serum creatinine, and the response to PEX are used to clinically differentiate acquired TTP from aHUS. After recovery, complement mutation studies may be useful to confirm the clinical diagnosis in cases where a complement protein mutation (CFH, CFI, C3, CFB, THBD, or MCP) is present.
Conceptual algorithm. The algorithm depicted conceptually describes our approach to the treatment of patients presenting with an acute TMA. PEX is started initially in all patients, with the response to PEX serving as an important diagnostic criterion. Collectively, the pretreatment ADAMTS13, platelet count, serum creatinine, and the response to PEX are used to clinically differentiate acquired TTP from aHUS. After recovery, complement mutation studies may be useful to confirm the clinical diagnosis in cases where a complement protein mutation (CFH, CFI, C3, CFB, THBD, or MCP) is present.
Initiating therapy with eculizumab
Although the difficulty in making the clinical diagnosis of aHUS is readily apparent, the urgency to recognize aHUS as an alternative diagnosis is not always appreciated. In a patient responding poorly to PEX therapy, there are also significant risks associated with PEX therapy that must be considered, especially in the absence of clinical benefit from therapy.54 Despite the data suggesting that the more rapid initiation of therapy with eculizumab is associated with greater improvements in renal function, there are several factors that collectively prevent the initiation of therapy prior to a window of 3 to 5 days. In cases where the diagnosis of aHUS is known at the time of the acute presentation (previous mutation studies) or suspected in children where TTP is less common, first-line therapy with eculizumab should be considered in lieu of PEX.3 In nearly all other cases presenting in adults, it is not possible to differentiate aHUS from TTP with certainty. For this reason, PEX must be started in all patients presenting with an acute TMA. The pretreatment ADAMTS13 activity is also an important tool as discussed previously to differentiate aHUS from TTP, but the result for nearly all physicians will not be available for at least 3 to 5 days given the reliance on reference laboratories for this testing for most physicians. Although it is important to initiate eculizumab as soon as possible, these practical concerns regarding the accurate diagnosis of aHUS, and the assessment of the initial response to PEX, necessitate this short delay potentially in starting therapy with eculizumab.
Prior to therapy, all patients should receive a meningococcal vaccine because of the increased risk for meningococcal infections in patients treated with eculizumab.55 Recommendations for vaccination vary in different countries. In the United States, a quadrivalent meningococcal vaccine (Menactra or Menevo) should be administered,56 but it should be emphasized that these vaccines do not cover group B meningococcus, 1 of the common disease-causing serotypes in the United States.57,58 A vaccine against group B serotype meningococcus is available in Europe, but not presently in the United States. Ideally, patients should wait 2 weeks prior to starting therapy to allow for the full effectiveness of the vaccine, but in nearly all cases, therapy cannot be delayed. In these cases, prophylactic antibiotics should be initiated simultaneously with the initiation of therapy with eculizumab and continued for 2 weeks. Therapy with eculizumab is initiated IV at a dose of 900 mg weekly for the first 4 weeks, and then beginning on week 5 maintenance therapy every other week at a dose of 1200 mg IV is administered. Dose adjustments should be considered for patients with a body weight <40 kg.
Time to respond to therapy with eculizumab
Though the clinical response data with eculizumab in aHUS are impressive,1 rapid improvements may not always be immediately apparent. When addressing the question of the expected time to respond to therapy with eculizumab, it is important to keep in mind the mechanism of action. Eculizumab blocks terminal complement activation via its binding to C5, preventing the activation of C5 that can lead to the formation of the membrane attack complex that mediates the end-organ injury in aHUS. With the initiation of therapy, further complement-mediated end-organ injury should be prevented, but recovery (and the time to recovery) is a function of the extent and duration of injury present. Whereas hematologic responses occur more quickly, recovery of renal function and other end-organ injury present may take longer. In the prospective study of eculizumab for the treatment of aHUS patients refractory to PEX, improvement of the platelet count was used as a surrogate for activity of the complement-mediated TMA.1 In this study, all 13 patients with a low platelet count at the start of therapy normalized their platelet counts by week 26, but only half of these patients had a normal platelet count by day 7. In this same study of PEX refractory patients, 65% of patients decreased their serum creatinine by 25% at week 26, with this number increasing to 76% by week 64, providing evidence for the time-dependent nature of the recovery in eculizumab-treated patients (Table 1). In terms of the recovery of neurologic injury, the only guidance available comes from published case reports. In 1 patient treated at our institution that presented with a devastating neurologic injury, clinically apparent improvements in her neurologic state did not begin until 8 weeks after initiating therapy with eculizumab.59 Whereas improvement in the platelet count and the LDH seen early in the course of therapy may provide initial signs of response to therapy, therapy with eculizumab may need to be continued for several weeks to accurately assess the recovery of end-organ injury.
Duration of therapy with eculizumab
As patients recover from their acute presentation and hopefully return to their previous baseline of functioning, the question regarding the duration of therapy will be raised by both physicians and patients. In the absence of data, current recommendations are based on expert opinion3 and our understanding of aHUS as an inherited disorder of complement dysregulation. After recovery from their acute illness, given the presumption of their inability to regulate complement activity, patients could suffer another acute episode after any clinical event (infection, surgery, pregnancy) that could activate complement. There are reports of recurrent acute TMA episodes and complications in patients who have either discontinued therapy or missed or delayed doses of eculizumab.1,3,59 Ultimately, data from the ongoing international aHUS Registry and prospective studies will be required to determine which patients can safely discontinue eculizumab.
Nonresponders to eculizumab
Although improvement in renal function after therapy with eculizumab may not be seen due to the presence of irreversible kidney injury present prior to the initiation of therapy with eculizumab, hematologic responses to therapy should still be appreciated in these patients. In patients failing at least to have a hematologic response after 6 to 8 weeks of therapy, the clinical diagnosis of aHUS should be questioned. Given that roughly half of patients with aHUS will not have an identifiable complement mutation, these studies may not be helpful in all nonresponding patients. In these cases, potential alternative diagnoses and etiologies of their aHUS need to be considered. Included in this category should be the recent finding of mutations of diacylglycerol kinase ε (DGKE) in patients with suspected aHUS.60 Mutations of DGKE (an intracellular enzyme) have been associated with an aHUS-like condition (microangiopathy, endothelial injury, and proteinuria) that is not related to complement dysregulation, and therefore would not be expected to respond to complement inhibition therapy.12
Future directions: complement activation biomarker studies
Despite the remarkable advances in treatment of patients with aHUS, there remain many unmet needs including the lack of an objective, diagnostic test to confirm the clinical diagnosis of aHUS. Our group and others have reported that complement activation can also be seen in ADAMTS13-deficient TTP patients.40-42,61 While the presence of complement activation may not differentiate aHUS and acquired TTP, the magnitude and/or mechanism of complement activation along with the ADAMTS13 may be informative. In studies on pretreatment plasma samples by our group characterizing the complement activation “footprint” of 19 patients clinically diagnosed with aHUS, more pronounced activation of the alternative pathway (Factor Bb) was seen compared with the classic pathway (C4d) as might be expected in patients with aHUS (S.R.C., V. M. Holers, S. Yang, and H.M.W., manuscript in preparation). Comparing these 19 patients to previously published data from our group that evaluated pretreatment samples from 38 patients with acquired TTP, levels of C5a (generalized complement activation) and C5b-9 (membrane attack complex) were significantly higher in the 19 patients classified clinically as aHUS. Although these data are preliminary, they hold out hope for the development of more objective diagnostic testing that could be used to confirm the diagnosis of aHUS. These candidate biomarkers might also be useful to monitor an individual’s response to complement inhibition therapy, serving as a more rapid and objective surrogate marker of disease response.
The remarkable advances in the development of complement inhibition therapy for the treatment of aHUS have forced the issue regarding the accurate differentiation of aHUS from TTP, leading to improvements not only in our ability to clinically differentiate aHUS from acquired TTP, but also in the development of objective diagnostic tests to confirm the clinical diagnosis of aHUS. Future advances in our ability to objectively define and differentiate TMA patients will be essential as we move toward more objective definitions of each TMA and away from the reliance on the clinical classification of these disorders.
Authorship
Contribution: S.R.C. and H.M.W. wrote and edited the manuscript.
Conflict-of-interest disclosure: S.R.C. and H.M.W. have both received research funding and both consulting and speaking income from Alexion.
Correspondence: Spero R. Cataland, Department of Hematology, The Ohio State University, A361 Starling Loving Hall, 320 W 10th Ave, Columbus, OH 43210; e-mail: spero.cataland@osumc.edu.