In this issue of Blood, Wong et al emphasize the implication of cellular and X-linked inhibitors of apoptosis (cIAP1, cIAP2, and XIAP) in the regulation of myeloid cell homeostasis through the inhibition of receptor-interacting protein kinase (RIPK1 and RIPK3)–dependent cytokine production and inflammation.1
Loss of cIAP and XIAP expression induces RIPK1-dependent TNF mRNA expression and RIPK3-dependent caspase activation and apoptosis in macrophages. The systemic inflammatory disease developed by cIAP and XIAP TKO mice, which is significantly but not fully rescued in a TNF-deficient background, is most likely driven by other inflammatory cytokines and/or DAMPs.
Loss of cIAP and XIAP expression induces RIPK1-dependent TNF mRNA expression and RIPK3-dependent caspase activation and apoptosis in macrophages. The systemic inflammatory disease developed by cIAP and XIAP TKO mice, which is significantly but not fully rescued in a TNF-deficient background, is most likely driven by other inflammatory cytokines and/or DAMPs.
Inhibitors of apoptosis (IAPs) define a family of proteins that inhibit cell death either by inactivation of caspases or by promoting prosurvival signaling from the tumor necrosis factor receptor (TNFR) complex I and/or limiting formation of the TNFR complex II. It has been previously reported that IAPs are proto-oncogenes rearranged and/or overexpressed in a wide range of human tumors, and there is also evidence that the expression of IAP proteins correlates with a poorer prognosis in patients suffering from certain types of cancer.2 In line with these findings, Smac mimetics that inhibit IAPs have been developed as antitumor agents taking advantage of their ability to efficiently kill tumor cells by triggering cIAP degradation, ultimately resulting in TNF/TNFR1-mediated cell death.3,4 Death domain–containing members of the TNFR family can induce caspase-dependent apoptosis and, under certain circumstances, necrosis, provided that the function of caspase 8 is inhibited.5 It is also well documented that RIPK3 is required for the induction of necroptosis.5,6 Of note, deletion or pharmacologic depletion of IAPs favors the formation of the Ripoptosome that triggers necroptotic death via RIPK1.
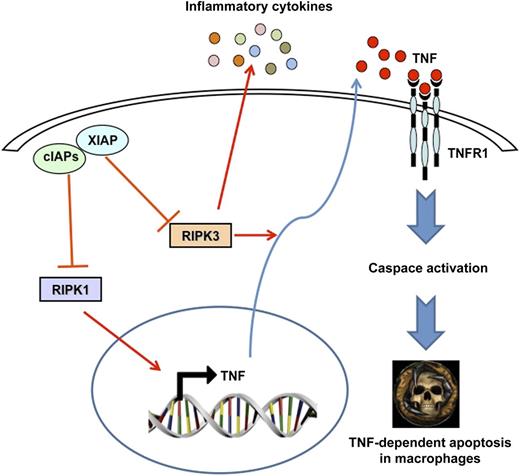
In the present study, Wong et al took advantage of an elegant genetic mouse model combined with pharmacologic approaches using Smac mimetics in order to highlight the role of these 3 members of the IAP family on systemic inflammation and cytokine production in myeloid cells. Of note, they show that triple knockout (TKO) mice specifically deleted in the myeloid lineage suffer from an inflammatory disease characterized by increased cytokine production, granulocytosis, and splenomegaly. This complex phenotype can be reproduced partially ex vivo using IAPs antagonists. The authors conclude that cIAP1 and cIAP2 together with XIAP suppress the production of multiple cytokines and chemokines, including TNF, thereby regulating the homeostasis and function of mature myeloid cells.1 They also established that the combined deletion of cIAPs and XIAP is required for TNF induction and TNF/TNFR1-mediated killing of macrophages (see figure). Finally, they demonstrated, that in this particular context, TNFα messenger RNA (mRNA) production and TNF secretion are dependent on RIPK1 and RIPK3 activation, respectively. This suggests that RIPK1 and RIPK3 regulate cytokine production directly and that this function resides upstream of and likely distinct from the implication of both kinases in the induction of necroptosis. In line with the present manuscript, the same authors recently reported that IAPs limit the activation of RIPKs by TNFR1 during development. Indeed, the death of cIAP1−/− cIAP2−/− transgenic mice was rescued in a TNFR1-deficient background. Moreover, deletion of RIPK3 or hemizygosity for RIPK1 also allowed double mutant mice to survive past birth. cIAPs appear, therefore to limit activity of RIP kinases in the TNFR1 signaling pathway.7
The main strength of the manuscript by Wong et al resides in the development of this complex but interesting TKO mice model. However, one important point that is not fully explored in the present study is the mechanism that drives the inflammatory syndrome and granulocytosis in TKO mice. The authors point to the crucial role of TNF in transgenic mice and also in macrophages challenged ex vivo with this cytokine, and accordingly, the phenotype can be significantly but not fully rescued in a TNF-deficient background. Finally, they also bring evidence that injection of anti-TNF in TKO mice can reduce cytokine production and granulocytosis, highlighting further the role of TNF in driving systemic inflammatory disease in vivo. Therefore, it seems that other cytokines or danger-associated molecular pattern (DAMP) molecules are likely at play in the overall phenotype.
IAP proteins have emerged recently as promising new targets for molecular therapy of cancer, and agents antagonizing IAP proteins (Smac mimetics) have been shown to trigger apoptotic and nonapoptotic cell death in cancer cells. For instance, the prognostic significance of XIAP has been evaluated in acute myeloid leukemia, and increased XIAP expression was found to be associated with patients’ higher risk groups. In addition, IAP antagonists in combination with TNF synergistically trigger cell death in leukemia cells making this combination of drugs highly attractive for cancer therapy.4
It is well established that RIPK1 and RIPK3 are critically required to induce cell death in a FADD-deficient or caspase 8–deficient context.8 As cancer cells are often characterized by defects in caspase activation and apoptosis, one can assume that triggering necroptosis using IAP antagonists might favor the elimination of cancer cells. Importantly, recent findings in the literature suggest that there is a window of opportunity for IAP antagonists in combination with TNF in myeloid malignancies.9 The work by Wong et al that highlights the implication of IAP proteins to limit RIPK activation in order to sustain myeloid cell homeostasis supports the notion that IAP antagonists combined with TNF could be beneficial in the clinic for treatment of myeloid malignancies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal