Key Points
Somatic indels of CALR exon 9 are present in about 20% to 25% of sporadic patients with essential thrombocythemia or primary myelofibrosis.
These mutations are found also in familial cases of essential thrombocythemia or primary myelofibrosis as somatically acquired events.
Abstract
Somatic mutations in the calreticulin (CALR) gene were recently discovered in patients with sporadic essential thrombocythemia (ET) and primary myelofibrosis (PMF) lacking JAK2 and MPL mutations. We studied CALR mutation status in familial cases of myeloproliferative neoplasm. In a cohort of 127 patients, CALR indels were identified in 6 of 55 (11%) subjects with ET and in 6 of 20 (30%) with PMF, whereas 52 cases of polycythemia vera had nonmutated CALR. All CALR mutations were somatic, found in granulocytes but not in T lymphocytes. Patients with CALR-mutated ET showed a higher platelet count (P = .017) and a lower cumulative incidence of thrombosis (P = .036) and of disease progression (P = .047) compared with those with JAK2 (V617F). In conclusion, a significant proportion of familial ET and PMF nonmutated for JAK2 carry a somatic mutation of CALR.
Introduction
Myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), have in most instances sporadic occurrence.1 However, familial clustering of MPN has been reported by us and others.2,3 Common mutations involved in the pathogenesis of MPN such as JAK2, MPL, CBL, and TET2 are mainly somatically acquired also in familial cases,3-5 although rare cases of germline MPL, CBL, and TET2 mutations have been reported.6-8 The JAK2 GGCC haplotype confers susceptibility to MPN, but does not explain familial clustering.5
Two recent articles demonstrated that most of the patients with sporadic ET or PMF not associated with JAK2 or MPL alterations carry somatic mutations of calreticulin (CALR).9,10 The clinical course of sporadic CALR-mutated patients is more indolent than that of JAK2-mutated patients.9
The role of CALR mutations in familial MPN remains to be clarified. In this study, we aimed to investigate the frequency of CALR mutations in familial MPN, their germline or somatic occurrence, and their correlation with clinical phenotype.
Patients and methods
This study was approved by the Ethics Committee of Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia, Italy. The procedures followed were in accordance with the Helsinki Declaration, and samples were obtained with patients’ written informed consent.
We identified in our database a total of 154 consecutive patients with familial MPN diagnosed and followed from 1970 to 2013 at the Department of Hematology Oncology, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia. DNA was available in 127 cases belonging to 78 families, which were included into the study. Patients were defined as familial cases if 2 or more individuals within the same pedigree were affected.2 Diagnosis of MPN was made in accordance with the criteria in use at the time of the first observation, as previously described.11
JAK2 (V617F) mutation status was assessed in granulocyte DNA as previously described.12 JAK2 wild-type patients were further evaluated for JAK2 exon 12 mutations, MPL exon 10 mutations, and CALR exon 9 mutations.9,13-16 Patients with nonmutated JAK2, MPL exon 10, and CALR exon 9 were sequenced for the other 8 exons of CALR.9 Female patients belonging to pedigrees with all affected members negative for JAK2, MPL, and CALR were evaluated for clonality using X-chromosome–inactivation pattern (XCIP).12
Overall survival was estimated using the Kaplan-Meier product limit method, and survival curves were compared by the log-rank test. The cumulative incidence of disease transformation and that of thrombotic events were estimated with a competing risk approach, considering death from all causes as a competing event.17 The comparison of cumulative incidence curves in different groups of patients was carried out using the Pepe-Mori test.18 All P values were considered statistically significant when smaller than .05 (2-tailed). Statistical analyses were performed using Stata12.1 (StataCorp LP, College Station, TX) software.
Results and discussion
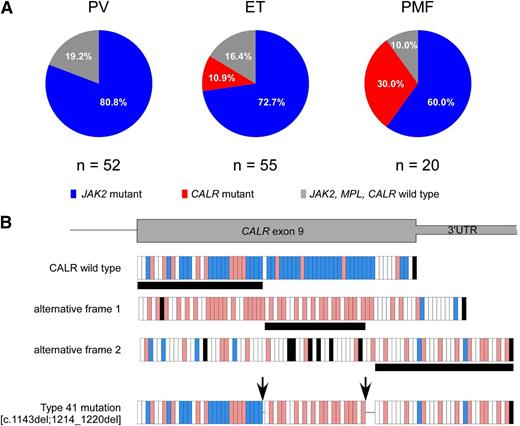
Of 127 patients, 94 carried JAK2 mutations (91 JAK2 V617F and 3 exon 12 mutations), 12 carried CALR mutations, and 21 did not carry any mutations. We did not find any MPL mutation. The distribution of mutations according to diagnosis was significantly different (P = .003), with CALR mutations being associated only with ET and PMF, as reported in Figure 1A.
Distribution of mutations according to diagnosis and description of the novel CALR mutation. (A) JAK2 mutations were present in 42 of 52 (80.8%) patients with PV, 40 of 55 (72.7%) patients with ET, and 12 of 20 (60%) patients with PMF. CALR mutations were identified in 6 of 55 (10.9%) patients with ET and 6 of 20 (30%) patients with PMF. The remaining 10 patients (19.2%) with PV, 9 patients (16.4%) with ET, and 2 patients (10%) with PMF did not carry any mutation. (B) The novel type 41 is a complex mutation, consisting of 2 separate deletion events of 1 bp and 7 bp. These 2 deletions were shown to be on the same allele by polymerase chain reaction product subcloning and sequencing, as described previously.9 The first deletion introduces a frameshift to alternative frame, which is common to all CALR exon 9 mutations, whereas the second deletion causes a frameshift to the third alternative frame at the end of exon 9. Because the next stop codon comes later in the third frame, this mutation creates the longest CALR protein described so far (20 amino acids longer than the wild type). Each vertical bar represents an amino acid: blue bars indicate negatively charged amino acids, red bars indicate positively charged amino acids, white bars indicate uncharged amino acids, and black bars indicate stop codons. Horizontal black bars below the frames denote the amino acid stretch encoded by the respective frame. The arrows indicate the location of the 2 deletions. 3′ UTR, 3′ untranslated region of the gene.
Distribution of mutations according to diagnosis and description of the novel CALR mutation. (A) JAK2 mutations were present in 42 of 52 (80.8%) patients with PV, 40 of 55 (72.7%) patients with ET, and 12 of 20 (60%) patients with PMF. CALR mutations were identified in 6 of 55 (10.9%) patients with ET and 6 of 20 (30%) patients with PMF. The remaining 10 patients (19.2%) with PV, 9 patients (16.4%) with ET, and 2 patients (10%) with PMF did not carry any mutation. (B) The novel type 41 is a complex mutation, consisting of 2 separate deletion events of 1 bp and 7 bp. These 2 deletions were shown to be on the same allele by polymerase chain reaction product subcloning and sequencing, as described previously.9 The first deletion introduces a frameshift to alternative frame, which is common to all CALR exon 9 mutations, whereas the second deletion causes a frameshift to the third alternative frame at the end of exon 9. Because the next stop codon comes later in the third frame, this mutation creates the longest CALR protein described so far (20 amino acids longer than the wild type). Each vertical bar represents an amino acid: blue bars indicate negatively charged amino acids, red bars indicate positively charged amino acids, white bars indicate uncharged amino acids, and black bars indicate stop codons. Horizontal black bars below the frames denote the amino acid stretch encoded by the respective frame. The arrows indicate the location of the 2 deletions. 3′ UTR, 3′ untranslated region of the gene.
All CALR mutations were demonstrated to be somatically acquired by genotyping T-cell DNA and showing the absence of CALR mutations in that control tissue. The CALR exon 9 mutations found in familial MPN patients are listed in Table 1. As reported previously for sporadic MPN,9 the type 1 (n = 4) and type 2 (n = 6) CALR mutations were predominant, with the remaining 2 patients carrying type 27 and a novel type (which we numbered continuously as type 41) CALR mutation. The novel type 41 is a complex mutation, consisting of 2 separate deletion events of 1 bp and 7 bp, as reported in Figure 1B; this mutation creates the longest mutant CALR protein described so far.
Somatic CALR mutations found in familial MPN patients
| Patient ID . | Sample ID . | Diagnosis . | CALR mutation type . | CALR mutation . | Mutation burden, % . |
|---|---|---|---|---|---|
| S47A9SM | MPC09_213 | PMF | 1 | c.1092_1143del | 27 |
| S48A9SM | MPC09_214 | PMF | 1 | c.1092_1143del | 40 |
| S12A12SM | MPC12_22 | PMF | 1 | c.1092_1143del | 52 |
| S29A12SM | MPC12_115 | ET | 1 | c.1092_1143del | 48 |
| S48A7SM | MPC07_162 | ET | 2 | c.1154_1155insTTGTC | 24 |
| S106A7SM | MPC07_395 | PMF | 2 | c.1154_1155insTTGTC | 44 |
| S20A10SM | MPC10_100 | ET | 2 | c.1154_1155insTTGTC | 27 |
| S113A10SM | MPC10_288 | ET | 2 | c.1154_1155insTTGTC | 40 |
| S157A10SM | MPC10_594 | PMF | 2 | c.1154_1155insTTGTC | 47 |
| S100A11SM | MPC11_627 | ET | 2 | c.1154_1155insTTGTC | 47 |
| S167A6SM | 906 | ET | 27 | c.1123_1125delinsTGTTT | 50 |
| S17A99SM | f5p3 | PMF | 41 | [c.1143del;1214_1220del] | 49 |
| Patient ID . | Sample ID . | Diagnosis . | CALR mutation type . | CALR mutation . | Mutation burden, % . |
|---|---|---|---|---|---|
| S47A9SM | MPC09_213 | PMF | 1 | c.1092_1143del | 27 |
| S48A9SM | MPC09_214 | PMF | 1 | c.1092_1143del | 40 |
| S12A12SM | MPC12_22 | PMF | 1 | c.1092_1143del | 52 |
| S29A12SM | MPC12_115 | ET | 1 | c.1092_1143del | 48 |
| S48A7SM | MPC07_162 | ET | 2 | c.1154_1155insTTGTC | 24 |
| S106A7SM | MPC07_395 | PMF | 2 | c.1154_1155insTTGTC | 44 |
| S20A10SM | MPC10_100 | ET | 2 | c.1154_1155insTTGTC | 27 |
| S113A10SM | MPC10_288 | ET | 2 | c.1154_1155insTTGTC | 40 |
| S157A10SM | MPC10_594 | PMF | 2 | c.1154_1155insTTGTC | 47 |
| S100A11SM | MPC11_627 | ET | 2 | c.1154_1155insTTGTC | 47 |
| S167A6SM | 906 | ET | 27 | c.1123_1125delinsTGTTT | 50 |
| S17A99SM | f5p3 | PMF | 41 | [c.1143del;1214_1220del] | 49 |
The clinical phenotype within the familial cluster was homogenous in 41 (53%) of 78 families (PV in 20 families, ET in 16 families, PMF in 5 families), whereas the remaining 37 families (47%) exhibited mixed phenotypes. In 45 of 78 families, DNA was available for all affected members; the somatic mutational and diagnosis pattern are reported in supplemental Table 1 on the Blood Web site. One family showed a homogeneous phenotype (ET) and coexistence of JAK2 and CALR mutations (supplemental Figure 1).
Interestingly, there were 3 families with all patients diagnosed with PV and nonmutated JAK2 and 3 families with all patients diagnosed with ET and nonmutated JAK2, MPL, and CALR (supplemental Table 2). Twelve of 14 samples from these 6 families were analyzed by Affymetrix SNP 6.0 arrays: 10 had normal karyotype and 2 had aberrations of the Y chromosome, which can be also related to aging. Five female patients were evaluated for XCIP: 2 samples were polyclonal, 2 were not evaluable, and 1 had ambiguous XCIP. Affected members of 1 of our families diagnosed with ET were recently found to carry a germline JAK2 mutation,16 as also shown by other investigators.19,20 Overall, absence of somatic mutations, normal karyotype, and polyclonal hematopoiesis in cases of familial ET or PV with nonmutated JAK2, MPL, and CALR suggest that these patients are very likely to be cases of hereditary thrombocytosis or erythrocytosis.21,22
We did not find significant differences in terms of clinical phenotype (age, sex, leukocyte count, hemoglobin, platelet count, erythropoietin, splenomegaly, thrombosis) at diagnosis according to genotype in ET and PMF patients, perhaps because of the low number of patients (supplemental Tables 3 and 4). The only significant difference (P = .017) was a higher platelet count in CALR-mutated ET patients (median 1222 × 109/L, range 563-1536) in comparison with JAK2-mutated ET patients (median 657 × 109/L, range 456-1500), confirming what was previously observed in ET sporadic cases.9,11,23
The whole cohort was observed for a median follow-up from diagnosis of 8.4 years (range, 0-32.8 years). Of 127 patients, 20 died; 17 (13.4%) patients had thrombotic events and 11 (8.7%) patients developed disease progression (7 secondary myelofibrosis and 4 leukemic evolutions). Cumulative incidences of thrombosis and disease progression at 10 years according to diagnosis and mutational status are reported in supplemental Table 5.
The molecular status (CALR-mutated vs JAK2-mutated vs nonmutated CALR/JAK2/MPL) did not affect overall survival in patients with ET and PV, whereas it did in patients with PMF (P = .001). In detail, in PMF, the median overall survival was 1.3 years in patients with nonmutated CALR/JAK2/MPL and not reached in CALR-mutated and JAK2-mutated patients, suggesting a worse prognosis in CALR/JAK2/MPL-unmutated PMF, as recently observed in sporadic cases.24 In ET, CALR-mutated patients showed a lower incidence of thrombosis (10-year confidence interval 0% vs 12.8%, P = .036) and a lower incidence of disease progression (10-year confidence interval 0% vs 6.6%, P = .047) in comparison with JAK2-mutated patients (supplemental Figure 2A-B), although the low number of patients does not allow any firm conclusion. The lower rate of thrombosis in CALR-mutated ET is consistent with what we previously observed in sporadic ET.9,11
In conclusion, in familial MPN, CALR mutations are somatically acquired and are associated with ET or PMF phenotype, as in sporadic MPN. In addition, in familial ET, CALR mutations are associated with a lower risk of thrombosis with respect to JAK2 (V617F), as observed in sporadic ET.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Riccardo Soldinger for editorial assistance.
Studies performed at the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, and Department of Molecular Medicine, University of Pavia, were supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), Fondo per gli investimenti della ricerca di base (FIRB, project no. RBAP11CZLK), Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR, PRIN 2010-2011) (M.C.), and from the Italian Ministry of Health (GR-2010-2312855) (E.R.). In particular, M.C. acknowledges funding from the AIRC Special Program “Molecular Clinical Oncology 5 per mille” AIRC Gruppo Italiano Malattie Mieloproliferative (project #1005). The studies performed in Vienna were supported by funding from the Austrian Science Fund (FWF23257-B12, FWF4702-B20) (R.K.).
Authorship
Contribution: E.R., A.S.H., R.K., and M.C. conceived this study, collected and analyzed data, and wrote the manuscript; I.C.C., M.B., C.C., E.S., and C.A. collected clinical data; A.S.H., D.P., J.D.M., N.C.C.T., C.M., T.B., and R.K. did molecular investigations; V.V.F. and C.P. did statistical analyses; and E.B. studied bone marrow biopsies.
Conflict-of-interest disclosure: R.K. reports having a pending patent application on the use of calreticulin gene mutations for the diagnosis and therapeutic targeting of MPNs. The remaining authors declare no competing financial interests.
Correspondence: Elisa Rumi, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: elisarumi@hotmail.com; and Mario Cazzola, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.
References
Author notes
E.R. and A.S.H. contributed equally to this paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal