Key Points
Human fetal and adult classical monocytes have distinct baseline transcriptional and signaling programs.
Transcriptional and signaling differences in fetal monocytes underlie stronger responses to cytokine stimulation.
Abstract
Preterm birth affects 1 out of 9 infants in the United States and is the leading cause of long-term neurologic handicap and infant mortality, accounting for 35% of all infant deaths in 2008. Although cytokines including interferon-γ (IFN-γ), interleukin-10 (IL-10), IL-6, and IL-1 are produced in response to in utero infection and are strongly associated with preterm labor, little is known about how human fetal immune cells respond to these cytokines. We demonstrate that fetal and adult CD14+CD16− classical monocytes are distinct in terms of basal transcriptional profiles and in phosphorylation of signal transducers and activators of transcription (STATs) in response to cytokines. Fetal monocytes phosphorylate canonical and noncanonical STATs and respond more strongly to IFN-γ, IL-6, and IL-4 than adult monocytes. We demonstrate a higher ratio of SOCS3 to IL-6 receptor in adult monocytes than in fetal monocytes, potentially explaining differences in STAT phosphorylation. Additionally, IFN-γ signaling results in upregulation of antigen presentation and costimulatory machinery in adult, but not fetal, monocytes. These findings represent the first evidence that primary human fetal and adult monocytes are functionally distinct, potentially explaining how these cells respond differentially to cytokines implicated in development, in utero infections, and the pathogenesis of preterm labor.
Introduction
Human gestation poses a unique and poorly understood challenge to normal mechanisms of immune recognition and rejection. The mother and her fetus are exposed to genetically distinct cells that bidirectionally cross the placenta,1,2 and each must learn to tolerate the other while simultaneously remaining responsive to pathogens. Previous studies have shown that the mother employs multiple strategies to maintain immune tolerance of her fetus.3 The fetal immune system also appears to play an active role in maintaining pregnancy.4 In contrast, production of inflammatory cytokines (eg, interferon-γ [IFN-γ], interleukin-6 [IL-6], tumor necrosis factor α, and IL-1) in response to bacterial infection characterizes the fetal inflammatory response syndrome (FIRS), which is associated with spontaneous abortion or preterm labor.5-10
The fetal immune system actively contributes to tolerance of maternal antigens.1,11 Upon stimulation with noninherited maternal alloantigens, fetal naïve CD4+ T cells preferentially differentiate into CD4+CD25+FoxP3+ regulatory T cells that specifically mediate tolerance of those antigens1 and that may also prevent harmful inflammatory responses against the mother. It is well recognized that the mouse has a developmentally limited fetal hematopoietic stem cell population that gives rise to unique hematopoietic lineages,12,13 including B-1 B cells, which generate “natural” antibodies.14-16 Human fetal T cells11 and erythrocytes17 also appear to arise from distinct fetal progenitors. Because hematopoietic stem cells have multilineage potential, we hypothesized that human fetal myeloid cells (specifically, fetal monocytes) arising from these cells would also have distinct functional characteristics compared with their adult counterparts.
Materials and methods
Isolation of bone marrow monocytes
Fetal bone marrow was obtained from 18- to 22-gestational-week specimens obtained under the auspices of Committee on Human Research–approved protocols from the Department of Obstetrics, Gynecology and Reproductive Science, San Francisco General Hospital. Fetal samples were obtained after elective termination of pregnancy. Samples were excluded in the case of (1) known maternal infection, (2) intrauterine fetal demise, and/or (3) known or suspected chromosomal abnormality. Fetal monocytes were isolated from femurs by bisection and mechanical dispersion of marrow in RPMI 1640 (Sigma Aldrich). Adult bone marrow samples were obtained from healthy donors (AllCells and Lonza Group). Both adult and fetal mononuclear cells were isolated by density centrifugation of a Ficoll-Hypaque gradient (Amersham Biosciences). All samples, both fetal and adult, were viably cryopreserved prior to use.
Flow cytometry
Mononuclear cell preparations were incubated in fluorescence-activated cell sorting (FACS) staining buffer (phosphate-buffered saline with 2% fetal bovine serum and 2 mM EDTA) with fluorochrome-conjugated, antihuman surface antibodies. Antibodies used for phenotyping included CD3 APC (BW264/56; Miltenyi), CD20 ECD (B9E9; Beckman Coulter), CD14 qDot605 (Tuk4; Invitrogen), CD16 Pacific Blue (3G8; BD Biosciences), HLA-DR PE-Cy7 (G46-6; BD Biosciences), CX3CR1 APC (2A9-1; Biolegend), CCR2 PerCP-Cy5.5 (K036C2; Biolegend), CD11c Alexa700 (Bly6; BD Biosciences), and CD11b APC-Cy7 (ICRF44; BD Biosciences). All cells were stained with a live/dead marker (Amine-Aqua/AmCyan; Invitrogen) to exclude dead cells from the analysis. For intracellular signal transducer and activator of transcription (STAT) staining, cells were first stained with CD14 and CD16 prior to fixation/permeabilization and subsequent STAT staining according to the manufacturer’s protocol (Cytofix Buffer and Permeabilization Buffer III; BD Biosciences). Intracellular antibodies used included STAT1 (pY701) Alexa488 (4a; BD Biosciences), STAT3 (pY705) PE-CF594 (4/P-STAT3; BD Biosciences), STAT5 (pY694) Alexa Fluor 647 (47/STAT5[pY694]; BD Biosciences), and STAT6 (pY641) PerCP-Cy5.5 (18/p-Stat6; BD Biosciences).
For IL-6 receptor (IL-6R) and SOCS3 staining, cells were first stained with a live/dead marker, HLA-DR, CD14, CD16, and IL-6R APC (UV4; Biolegend) prior to fixation/permeabilization according to the manufacturer’s protocol (Cytofix/Cytoperm Buffer; BD Biosciences). Cells were then stained with a rabbit polyclonal anti-SOCS3 (Abcam, ab16030) and then a donkey antirabbit Al488 secondary (Abcam, ab150069).
All data were acquired with an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo (TreeStar) software.
FACS
For basal gene expression microarrays, mononuclear cells were stained with the appropriate antibodies and filtered through 70-µM mesh filters (Falcon). The stained cells were subsequently sorted by FACS (FACS Aria; BD Biosciences) directly into RLT buffer (QIAGEN). Purity was assessed by reanalyzing a small fraction of sorted cells. For IFN-γ stimulation experiments, cells were incubated for 4 hours in sterile serum-free cell culture media (SF X-VIVO 20; Lonza Group) and appropriate amounts of IFN-γ. The cells were subsequently stained with the appropriate surface markers, filtered, and sorted by FACS into sterile phosphate-buffered saline. These cells were then resorted to a purity of greater than 99% directly into RNAqueous Micro lysis buffer (Ambion; Life Technologies).
RNA preparation for microarray analysis: basal gene expression microarray
RNA was isolated according to the manufacturer’s protocol (RNeasy Mini Kit; QIAGEN), and yield was determined on a Nanodrop spectrophotometer (Thermo Scientific). Sample preparation, labeling, and array hybridizations were performed according to standard protocols from the University of California, San Francisco Shared Microarray Core Facilities (http://www.arrays.ucsf.edu) and Agilent Technologies (http://www.agilent.com). Total RNA quality was assessed using a Pico Chip on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA was amplified using the Sigma whole-transcriptome amplification kits following the manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO), and subsequent Cy3-CTP labeling was performed using NimbleGen 1-color labeling kits (Roche-NimbleGen, Madison, WI). Labeled Cy3-cDNA was assessed using the Nandrop ND-8000 (Nanodrop Technologies, Wilmington, DE), and equal amounts of Cy3 labeled target were hybridized to Agilent whole-human-genome 4×44K arrays. Hybridizations were performed for 17 hours, according to the manufacturer’s protocol. Arrays were scanned using the Agilent microarray scanner, and raw signal intensities were extracted with Feature Extraction v10.6 software.
RNA preparation for microarray analysis: IFN-γ–stimulated microarrays
RNA was isolated from FACS-sorted samples using the RNAqueous-Micro kit (Life Technologies) and subjected to 2 rounds of linear amplification using the Aminoallyl MessageAmp II kit (Life Technologies). Cy3-coupled armored RNA was fragmented and hybridized overnight to a SurePrint G3 Human Gene Expression 8×60K microarray, which was washed and scanned per the manufacturer’s instructions (Agilent Technologies).
Statistical analysis of microarrays
For basal gene expression microarrays, the data set was normalized using the quantile normalization method proposed by Bolstad et al.18 No background subtraction was performed, and the median feature pixel intensity was used as the raw signal before normalization. A one-way analysis of variance linear model was fit to the comparison with estimate the mean M values and calculated moderated t statistic, B statistic, false discovery rate (FDR), and P value for each gene for the comparison of interest. Adjusted P values were produced by the method proposed by Holm (1979).19 All procedures were carried out using functions in the R package Limma in Bioconductor.20,21
For the IFN-γ–stimulated arrays, raw intensities were extracted using feature extraction software (Agilent) and quantile normalized using Limma.20 Differentially expressed genes were identified using Significance Analysis for Microarrays22 and data visualized as heatmaps using custom Perl scripts. Genes subsets demarcated as different between fetus and adult were determined by stratifying significantly differentially expressed genes such that they were not differentially expressed at baseline (based on relative expression and FDR ≤ 1%), were differentially expressed after stimulation with IFN-γ (based on relative expression and FDR ≤ 1%), and were differentially expressed between adult bone marrow (ABM) and fetal bone marrow (FBM) IFN-γ–treated samples (based on relative expression and FDR ≤ 5%) (supplemental Table 3, available on the Blood Web site). All data are available in the National Center for Biotechnology Information Gene Expression Omnibus under accession number GSE54818.
Quantitative PCR validation of microarray targets
Classical monocytes were isolated from ABM and FBM by FACS directly into RLT buffer (QIAGEN). RNA was isolated using the RNeasy Mini Kit (QIAGEN). A total of 275 ng of RNA was used according to the manufacturer’s protocol with Janus kinase (JAK)/STAT quantitative polymerase chain reaction (PCR) arrays (SABiosciences). Genes of interest were normalized to glyceraldehyde-3-phosphate dehydrogenase.
Cytokine stimulation and STAT staining
Approximately 1 × 106 mononuclear cells from FBM or ABM were stained with CD14 Qdot605 and CD16 Pacific Blue for 30 minutes prior to incubation in polystyrene tissue culture–treated 96-well plates with appropriate amounts of cytokine in sterile serum-free cell culture media (SF X-VIVO 20; Lonza Group) at 37°C for 5, 15, or 30 minutes (100 µL total volume). The cells were then fixed with Cytofix Buffer (BD Biosciences), permeabilized with Permeabilization Buffer III (BD Biosciences), and stained with HLA-DR, STAT1, STAT3, STAT5, and STAT6 according to the manufacturer’s instructions. Cytokines used were recombinant human IFN-γ, IL-4, IL-6 (all from Biolegend), and IL-10 (BD Biosciences).
Bioinformatic promoter analysis
Putative STAT1 and STAT5 promoter sites in genes enriched after IFN-γ stimulation in ABM or FBM were determined using the SABiosciences DECODE database. Genes identified with a STAT1 binding site were identified using STAT1 as the transcription factor. Genes identified with a STAT5 binding site were identified using STAT5a and STAT5b. A gene was considered to have a STAT5 binding site if it had either STAT5a or STAT5b binding sites in the promoter.
Statistics
All error bars represented are standard error of the mean. P values displayed in phenotypic characterizations were determined by nonparametric Student t test (Mann-Whitney U test). Sample size for statistical calculations was determined by use of the maximum number of unique biological replicates available. All experiments were performed two or more times with distinct biological samples (total of 8-12). Each individual experiment contained a minimum of 4 ABM and 4 FBM samples. No samples were excluded. See previous sections for microarray-specific statistics.
Results
To determine whether human fetal monocytes are functionally distinct from those of the adult, we isolated mononuclear cells from human FBM (18-22 gestational weeks) and ABM. Because the bone marrow becomes the primary site of myelopoiesis during the late second trimester of fetal development,23,24 we reasoned that differences that might exist between fetal and adult monocytes could be explored by directly comparing mononuclear cells obtained from this organ with cells from its adult counterpart. To determine whether human fetal monocytes could be readily identified using known myeloid and monocyte surface markers, bone marrow mononuclear cells were isolated from 18- to 22-week human FBM and ABM specimens for phenotypic characterization. Because classical monocytes (HLA-DR+CD14+CD16−) are thought to differentiate into nonclassical monocytes (HLA-DR+CD14+CD16+) upon stimulation and activation,25 we chose to examine the classical population as the earliest member of the mature monocyte lineage. Common monocyte markers (Figure 1A-C and supplemental Figures 1 and 2) were present on classical monocytes and absent on nonmonocyte antigen-presenting cells (HLA-DR+CD14−CD16−). The classical monocyte population identified as HLA-DR+CD14+CD16− is a relatively pure monocyte population and is not contaminated by CD3+ T cells, natural killer T cells, or CD20+ B cells (supplemental Figure 2). Compared with the nonmonocyte antigen-presenting cells, both the fetal and adult CD14+CD16− populations had uniformly higher expression of the myeloid and monocyte surface markers CD11b, CD11c, CCR2, and CX3CR1. Adult CD14+CD16− cells, on the other hand, had higher expression of the integrins CD11c and CD11b than fetal CD14+CD16− cells. CX3CR1 and CCR2 are commonly used to define classical and nonclassical monocyte populations and govern properties of cell migration and function.26 CX3CR1 was expressed only on the nonclassical population of adult monocytes but was expressed by both classical and nonclassical populations of fetal monocytes (Figure 1C, upper panel), suggesting that each of these cell populations might have an enhanced ability to enter and to establish resident macrophage and/or dendritic cell populations in tissues from which they would otherwise be restricted, such as the brain or skin.27,28 Conversely, both fetal and adult classical monocytes had similarly high expression of CCR2, whereas fetal nonclassical monocytes expressed half as much CCR2 as their adult counterpart (Figure 1C, lower panel). These observations of CCR2 expression on both monocyte subsets are contrary to what is seen in peripheral blood nonclassical monocytes that express little CCR2.29 Although not demonstrated in humans, CCR2 in mice is required for egress from the bone marrow30 ; we speculate that its expression on human adult nonclassical monocytes may reflect a similar role in bone marrow egress.
Phenotypic characterization of human bone marrow monocytes. (A) Representative plot of bone marrow monocytes in ABM and FBM. CD14+CD16− classical monocytes are gated as shown. (B) Mean fluorescence intensity (MFI) of CD11c, CD11b, CX3CR1, and CCR2 as expressed on classical monocytes or on the CD14− nonmonocyte population in FBM and ABM. (C) MFI of CX3CR1 and CCR2 in classical (CD14+CD16−) and nonclassical (CD14+CD16+) monocyte populations in ABM and FBM. Representative of 3 or more experiments; n = 12 (total from all experiments).
Phenotypic characterization of human bone marrow monocytes. (A) Representative plot of bone marrow monocytes in ABM and FBM. CD14+CD16− classical monocytes are gated as shown. (B) Mean fluorescence intensity (MFI) of CD11c, CD11b, CX3CR1, and CCR2 as expressed on classical monocytes or on the CD14− nonmonocyte population in FBM and ABM. (C) MFI of CX3CR1 and CCR2 in classical (CD14+CD16−) and nonclassical (CD14+CD16+) monocyte populations in ABM and FBM. Representative of 3 or more experiments; n = 12 (total from all experiments).
Given the observed differences in surface markers associated with migration and inflammation (eg, CX3CR1, CD11b, and CD11c), we wondered whether basal gene expression programs were different in fetal and adult monocytes. HLA-DR+CD14+CD16− classical monocytes were isolated from FBM and ABM (n = 4 each) by FACS and subjected to whole-genome gene expression analysis (Figure 2). Fetal and adult classical monocytes were found to have 2069 significantly differentially expressed genes (FDR ≤ 0.05, fold change ≥2), many of which are known to contribute to key immunologic pathways associated with monocyte function, including pathogen recognition and sensitivity to cytokines. These genes include IL1R1, IL6R, IL10RA, and IL12RB1, as well as TLR7, TLR8, NOD1, and NLRC4 (Figure 2B-C and supplemental Table 1).
Transcriptional characterization of human bone marrow monocytes. (A) Unbiased cluster analysis of gene expression of ABM and FBM classical monocytes. (B) Scatterplot of pairwise global gene expression comparing ABM and FBM classical monocytes (log2 signal intensity). Genes that were differentially expressed between groups are indicated in red and blue. Genes of interest in green are also significantly differentially expressed (FDR ≤ 0.05, fold change ≥2). (C) log2 fold change of genes in green (fetal vs adult).
Transcriptional characterization of human bone marrow monocytes. (A) Unbiased cluster analysis of gene expression of ABM and FBM classical monocytes. (B) Scatterplot of pairwise global gene expression comparing ABM and FBM classical monocytes (log2 signal intensity). Genes that were differentially expressed between groups are indicated in red and blue. Genes of interest in green are also significantly differentially expressed (FDR ≤ 0.05, fold change ≥2). (C) log2 fold change of genes in green (fetal vs adult).
Spontaneous abortion and the onset of preterm labor are strongly associated with FIRS, which is characterized by robust fetal production of the proinflammatory cytokine IL-6.6,31 Given the observation that the IL-6 receptor (IL6R) is expressed at higher levels in fetal as compared with adult monocytes, we tested the possibility that the fetal monocyte response to IL-6 is distinct from that found in the adult. Phosphorylation of canonical (pSTAT3)32 and noncanonical (pSTAT1 and pSTAT5) STATs was assessed in ABM and FBM mononuclear cells after stimulation with IL-6 for varying amounts of time (Figure 3 and supplemental Figure 3) and at varying concentrations (supplemental Figure 4). IL-6 induced phosphorylation of STAT3 in most fetal and adult monocytes, but a higher frequency of fetal cells phosphorylated STAT3 and did so more rapidly than adult cells (Figure 3B). Interestingly, the noncanonical mediators, STAT1 and STAT5, were phosphorylated in fetal monocytes, but not in adult monocytes, suggesting a tendency of the fetus to activate signaling pathways that are not normally activated in the adult (Figure 3A,C). These differences were observed even at very low IL-6 concentrations (supplemental Figure 4), demonstrating an exquisite sensitivity of fetal monocytes to IL-6. These findings show that fetal monocytes are highly attuned to the presence of IL-6 and are able to mount a strong canonical pSTAT3 response. We also found that even very low concentrations of IL-6 enhanced noncanonical STAT1 and STAT5 phosphorylation at low concentrations (supplemental Figure 4a,c), which has the potential to trigger unique gene expression pathways and monocyte maturation.33,34 The consequences of activating these pathways in fetal monocytes are unknown, but because JAK/STAT pathways have been characterized as potent mediators of cytokine signaling transduction, it may be that these variant pathways alter the functional response of fetal monocytes to IL-6.
STAT phosphorylation in response to IL-6 stimulation. (A) Percentage of classical monocytes in FBM and ABM expressing pSTAT1 after IL-6 stimulation. (B) Percentage of pSTAT3+ classical monocytes in FBM and ABM. (C) Percentage of pSTAT5+ in classical monocytes in FBM and ABM. Cells were stimulated with 70 ng/mL IL-6. All data are from 2 or more experiments; n = 8 (total from all experiments).
STAT phosphorylation in response to IL-6 stimulation. (A) Percentage of classical monocytes in FBM and ABM expressing pSTAT1 after IL-6 stimulation. (B) Percentage of pSTAT3+ classical monocytes in FBM and ABM. (C) Percentage of pSTAT5+ in classical monocytes in FBM and ABM. Cells were stimulated with 70 ng/mL IL-6. All data are from 2 or more experiments; n = 8 (total from all experiments).
JAK/STAT signaling mediates various aspects of monocyte migration, cytokine responses, and differentiation.5,35-37 Given the above observation of unique IL-6–mediated pSTAT responses in fetal monocytes, we wondered whether these cells might also respond to other physiologically relevant immunologic stimuli via noncanonical JAK/STAT phosphorylation. Of particular interest were the responses to IFN-γ, IL-4, and IL-10 due to their involvement in Th1/Th2 polarization and FIRS.6,31,38-40 Because the response of the fetus to these cytokines is unknown, we stimulated ABM and FBM mononuclear cells with IFN-γ, IL-10, or IL-4 for varying periods of time (Figure 4 and supplemental Figure 3) or varying concentrations (supplemental Figure 5), after which monocyte phosphorylation of STAT1, STAT3, STAT5, and STAT6 was assessed. Elevated circulating levels of IFN-γ in the fetus have been associated with complications of preterm birth, including injury to brain white matter.7 Just as both fetal and adult monocytes phosphorylate the canonical signaling intermediate STAT3 upon IL-6 stimulation, IFN-γ stimulation results in robust phosphorylation of the canonical intermediate STAT1 in both cell types (Figure 4A). Fetal monocytes were, however, even more sensitive to IFN-γ, as demonstrated by higher STAT1 phosphorylation at lower cytokine concentrations (supplemental Figure 5a). As we hypothesized, phosphorylation of a noncanonical intermediate (STAT5) was detected in fetal, but not adult, monocytes (Figure 4B).
STAT phosphorylation in response to IL-10, IFN-γ, and IL-4. (A) Percentage of pSTAT1+ classical monocytes in FBM and ABM after IFN-γ stimulation. (B) Percentage of pSTAT5+ classical monocytes in FBM and ABM after IFN-γ stimulation. Cells were stimulated with 40 ng/mL IFN-γ. (C) Percentage of pSTAT3+ classical monocytes in FBM and ABM after IL-10 stimulation. Cells were stimulated with 70 ng/mL IL-10. (D) Percentage of pSTAT6+ classical monocytes in FBM and ABM after IL-4 stimulation. (E) Percentage of pSTAT5 in classical monocytes in FBM and ABM after IL-4 stimulation. Cells were stimulated with 30 ng/mL IL-4. All data are from 2 or more experiments; n = 8 (total from all experiments).
STAT phosphorylation in response to IL-10, IFN-γ, and IL-4. (A) Percentage of pSTAT1+ classical monocytes in FBM and ABM after IFN-γ stimulation. (B) Percentage of pSTAT5+ classical monocytes in FBM and ABM after IFN-γ stimulation. Cells were stimulated with 40 ng/mL IFN-γ. (C) Percentage of pSTAT3+ classical monocytes in FBM and ABM after IL-10 stimulation. Cells were stimulated with 70 ng/mL IL-10. (D) Percentage of pSTAT6+ classical monocytes in FBM and ABM after IL-4 stimulation. (E) Percentage of pSTAT5 in classical monocytes in FBM and ABM after IL-4 stimulation. Cells were stimulated with 30 ng/mL IL-4. All data are from 2 or more experiments; n = 8 (total from all experiments).
Fetal and neonatal immune responses have been previously described to preferentially activate Th2 responses rather than Th1, though most of the evidence for this skew has been shown mice.41,42 Given that unexpected STAT phosphorylation responses were seen in response to a classical Th1 cytokine (IFN-γ), we sought to determine whether fetal monocyte responses to a keystone Th2 cytokine (IL-4) were also different from responses described in adults. As in the case of IFN-γ, stimulation with IL-4 prompted both fetal and adult monocytes to phosphorylate the canonical intermediate STAT6. The fetal STAT6 response was amplified compared with that of the adult (Figure 4D) and was triggered at a much lower concentration (supplemental Figure 5d). IL-4 also triggered a strong and unexpected pSTAT5 response in fetal classical monocytes, similar to that which was observed after stimulation with IL-6 and IFN-γ (Figure 4E and supplemental Figure 5e), implicating STAT5 as a common mediator of fetal cytokine responses. Finally, IL-10 is critical for maintenance of pregnancy in experimental models of spontaneous abortion.43 After IL-10 stimulation, both the fetal and adult monocytes displayed an equivalent phosphorylation of the canonical signaling mediator, STAT3 (Figure 4C), but no phosphorylation of any other STATs.
Taken together, these data indicate that fetal monocytes not only are more sensitive to activation of canonical signaling pathways in response to IL-6, IFN-γ, and IL-4 but also trigger unexpected noncanonical signaling pathways that are distinct from those observed in adult monocytes. In contrast, the fetal and adult responses to the anti-inflammatory cytokine, IL-10, are virtually identical. A unifying and distinctive feature of fetal monocytes is their robust phosphorylation of STAT5 upon exposure to cytokines that have diverse effects in adults (eg, IL-6, IFN-γ, and IL-4). STAT5 has been implicated as a mediator of monocyte maturation,33 suggesting that fetal monocytes may be poised to respond to cytokines through rapid and sensitive induction of canonical STAT pathways and through accelerated maturation via activation of noncanonical STAT5-mediated signaling pathways.
These observations led us to 2 questions: (1) how does differential STAT phosphorylation affect downstream functional programs, and (2) which factors regulate differential STAT phosphorylation after stimulation with IL-6, IFN-γ, and IL-4, but not IL-10? To assess the potential impact of differential STAT activation on functional programs, we investigated the response to IFN-γ stimulation in more depth. Having observed differential activation of STAT1 and STAT5 in fetal and adult monocytes after IFN-γ stimulation (Figure 4A-B), we sought to determine whether inflammatory cytokine stimulation might lead to activation of different gene-expression programs in fetal and adult monocytes. To address this question, FBM and ABM cells were stimulated with IFN-γ for 4 hours and subjected to whole-genome gene expression analysis. We identified genes that were not differentially expressed in fetal and adult monocytes at baseline but that did become differentially expressed in these 2 populations after IFN-γ stimulation (Figure 5, supplemental Figure 6, and supplemental Tables 2 and 3). Notably, many of the genes upregulated by adult monocytes after IFN-γ stimulation are associated with antigen presentation and costimulation, including CIITA (transports major histocompatibility complex II to the surface of antigen-presenting cells), CD40 (a costimulatory molecule that activates T cells by binding to CD40L on T cells), CD74 (major histocompatibility complex II invariant chain), and CD80 (B7-1, a costimulatory molecule that activates T cells by binding to CD28). In contrast, many genes that were upregulated by fetal monocytes after IFN-γ stimulation can trigger and mediate innate pathogen responses, including TBX21 (T-bet; expression in dendritic cells instructs them to promote Th1 differentiation in T cells),44,45 C1QB (a critical component of the first step in the complement deposition pathway), TLR7 (crucial for recognition of intracellular pathogens), and DEFB1 (a directly toxic antimicrobial peptide). Many genes that were expressed equivalently in IFN-γ–stimulated fetal and adult monocytes have been previously defined as canonical interferon-stimulated genes such as CXCL10, STAT1, TAP2, and FCGR1B (Figure 5C), consistent with our finding that canonical JAK/STAT signaling pathways that lead to induction of these genes are activated in both fetal and adult cells after cytokine stimulation (Figure 4A-B). Analysis of putative STAT1 and STAT5 binding sites in differentially expressed genes (Figure 5D) revealed an enrichment in STAT1 and STAT5 binding sites in those genes that are more highly expressed in fetal cells, supporting the hypothesis that differential STAT phosphorylation is responsible for activation of distinct gene programs.
IFN-γ–stimulated genes in FBM and ABM classical bone marrow monocytes. (A) Cells were stimulated with 40 ng/mL IFN-γ for 4 hours and the sorted to >99% purity for whole-genome gene microarray analysis. Panels A to C depict a representative subset of the differentially expressed genes. (A) A subset of genes is not differentially expressed at baseline between ABM and FBM but is significantly more highly expressed in FBM than ABM after stimulation with IFN-γ (based on relative expression and an FDR ≤ 5%). Relative expression is the log2 representation of gene expression compared with median normalized array values. All genes marked with an asterisk (*) have bioinformatically identified putative STAT1 binding sites in their promoters; all those marked with a dagger (†) have bioinformatically identified putative STAT5 binding site in their promoter. (B) A subset of genes is not differentially expressed at baseline between ABM and FBM but is significantly more highly expressed in ABM than FBM after stimulation with IFN-γ (based on relative expression and FDR ≤ 5%). (C) A subset of genes that is not differentially expressed at baseline between ABM and FBM and is not significantly differentially expressed between ABM and FBM and are induced in both subsets after stimulation with IFN-γ (based on relative expression and an FDR ≤ 5%). (D) Pie chart of putative STAT1 and STAT5 binding sites in the promoters of significantly differentially expressed genes. NA indicates that there is no bioinformatic information (on any transcription factor) available on the gene of interest.
IFN-γ–stimulated genes in FBM and ABM classical bone marrow monocytes. (A) Cells were stimulated with 40 ng/mL IFN-γ for 4 hours and the sorted to >99% purity for whole-genome gene microarray analysis. Panels A to C depict a representative subset of the differentially expressed genes. (A) A subset of genes is not differentially expressed at baseline between ABM and FBM but is significantly more highly expressed in FBM than ABM after stimulation with IFN-γ (based on relative expression and an FDR ≤ 5%). Relative expression is the log2 representation of gene expression compared with median normalized array values. All genes marked with an asterisk (*) have bioinformatically identified putative STAT1 binding sites in their promoters; all those marked with a dagger (†) have bioinformatically identified putative STAT5 binding site in their promoter. (B) A subset of genes is not differentially expressed at baseline between ABM and FBM but is significantly more highly expressed in ABM than FBM after stimulation with IFN-γ (based on relative expression and FDR ≤ 5%). (C) A subset of genes that is not differentially expressed at baseline between ABM and FBM and is not significantly differentially expressed between ABM and FBM and are induced in both subsets after stimulation with IFN-γ (based on relative expression and an FDR ≤ 5%). (D) Pie chart of putative STAT1 and STAT5 binding sites in the promoters of significantly differentially expressed genes. NA indicates that there is no bioinformatic information (on any transcription factor) available on the gene of interest.
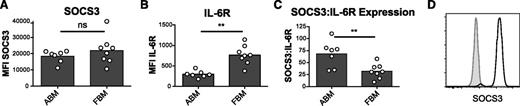
These data suggest distinct functional outcomes can arise after STAT phosphorylation in fetal vs adult monocytes. To delineate mechanisms that might control the differential phosphorylation of STATs, we focused on potential inhibitors of STAT phosphorylation that were identified by microarray analysis and validated by quantitative PCR (supplemental Tables 1 and 4). Of the common inhibitors, the most strikingly differentially expressed was SOCS3 (23-fold higher in the adult; Figure 2C, supplemental Figure 7, and supplemental Table 4), a member of the suppressor of cytokine signaling family that can directly inhibit JAK-mediated STAT phosphorylation via a KIR domain.46 SOCS3 was of particular interest because it selectively inhibits STAT1 and STAT3 phosphorylation downstream of the IL-6, but not the IL-10, receptor.46-48 Because SOCS3 messenger RNA is more abundant in adult monocytes compared with fetal monocytes, we wondered if SOCS3 could be responsible for the relatively attenuated pSTAT3 and absent pSTAT1 responses observed in adult monocytes. To address this question, relative protein expression levels of IL-6R and SOCS3 were assayed by flow cytometry. Surprisingly, SOCS3 protein was not differentially expressed between fetal and adult cells (Figure 6A). Because intracellular signaling from the IL-6R is directly inhibited by SOCS3, we wondered if IL-6 signaling in fetal cells might be enhanced compared with adult cells due to the relative abundance of IL-6R and SOCS3. As hypothesized, the IL-6R was more highly expressed in the fetal monocytes (Figure 6B) and the ratio of SOCS3/IL-6R was lower in fetal monocytes compared with adult monocytes (Figure 6C). This suggests that there are more repressive SOCS3 molecules available per IL-6R in adult monocytes. These data support a putative mechanism in which a higher proportion of SOCS3 to IL-6R in the adult monocytes results in attenuated STAT1 and STAT3 phosphorylation, consistent with our findings above (Figure 3A-B and supplemental Figure 8).
SOCS3 and IL-6R expression in FBM and ABM monocytes. (A) Mean fluorescence intensity (MFI) of SOCS3 in classical ABM and FBM monocytes. (B) MFI of IL-6R in classical ABM and FBM monocytes. (C) The relative ratio of SOCS3 to IL-6R expression based on MFI. All SOCS3/IL-6R characterizations represent 2 or more independent experiments with ABM (n = 7) and FBM (n = 8). (D) Representative histogram of SOCS3 staining in FBM classical monocytes. The gray histogram is stained with secondary alone and the white with anti-SOCS3 primary antibody plus secondary antibody.
SOCS3 and IL-6R expression in FBM and ABM monocytes. (A) Mean fluorescence intensity (MFI) of SOCS3 in classical ABM and FBM monocytes. (B) MFI of IL-6R in classical ABM and FBM monocytes. (C) The relative ratio of SOCS3 to IL-6R expression based on MFI. All SOCS3/IL-6R characterizations represent 2 or more independent experiments with ABM (n = 7) and FBM (n = 8). (D) Representative histogram of SOCS3 staining in FBM classical monocytes. The gray histogram is stained with secondary alone and the white with anti-SOCS3 primary antibody plus secondary antibody.
Discussion
In aggregate, our findings indicate that fetal and adult monocytes are phenotypically and transcriptionally different from one another at baseline. In particular, we also demonstrate that fetal monocytes generate distinct JAK/STAT signaling responses after stimulation with IFN-γ, IL-6, or IL-4. In response to the key proinflammatory cytokine, IL-6, fetal monocytes phosphorylate noncanonical STATs (eg, STAT1 and STAT5), suggesting that IL-6–induced STAT5-mediated signaling may lead to maturation of monocytes into functional phagocytes, whereas concurrent phosphorylation of STAT1 may lead to activation of classical proinflammatory genes. Together, these signaling responses that are associated with maturation and immune activation may then also optimize the fetus and newborn’s chance of successfully combating a microbial invader.
Our data suggest a mechanism wherein high levels of canonical (STAT3) and noncanonical (STAT1) STAT phosphorylation are found in fetal monocytes due to their lower ratio of SOCS3 to IL-6R and, hence, lower levels of STAT inhibition (supplemental Figure 8). We show that fetal monocytes have stronger JAK/STAT responses to stimulation by multiple cytokines as compared with adult monocytes. It may be that these differential responses are also due to relatively decreased influence of signaling inhibitors. If so, fetal innate immune responses could potentially be modulated using small-molecule JAK/STAT inhibitors.
Differences in oxygen tension and overall cellular composition in FBM vs ABM may contribute to the observed differences in STAT signaling. In addition, and as an unavoidable condition of these experiments, observations made on cells in vitro are not necessarily reflective of those that occur in vivo (even though cells from all tissues were cryopreserved immediately after isolation).
Overall STAT levels may also contribute to the differences observed in STAT phosphorylation. Results of transcript analysis by quantitative reverse-transcription PCR at baseline and protein analysis in unstimulated phosphoflow samples suggest that there are no major differences in basal STAT levels; however, we also acknowledge that, due to cell number constraints, we are unable to determine the absolute protein levels of the various STATs by immunoblotting.
Upon stimulation with IFN-γ, we show that fetal monocytes fail to upregulate costimulatory and antigen-presentation genes but instead upregulate genes involved in primitive antimicrobial responses in response to IFN-γ. We propose that failure to generate antigen-presentation and costimulation responses in fetal monocytes is a strategy to prevent activation of adaptive (ie, T-cell–mediated) immune responses (supplemental Figure 9). Although such adaptive responses are crucial for clearance of pathogens in the postnatal period, they may trigger potentially harmful responses in the fetus (such as antiself or antimaternal rejection), resulting in preterm labor and delivery. Thus, rather than promoting a strong adaptive and potentially inflammatory response that could trigger labor and expulsion of the fetus, fetal monocytes mount a more primitive, but potentially protective, innate antimicrobial response. Our findings provide a foundation for understanding the myeloid immune response to inflammatory cytokines implicated in the pathogenesis of FIRS, spontaneous abortion, and preterm labor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank N. Bhakta helpful discussions regarding bioinformatic analyses and manuscript preparation, the Sandler Asthma Basic Research Center Functional Genomics Core facility (R. Barbeau, A. Barczak, and D. Erle) for assistance with microarray processing and analysis, and the patients, staff, and providers of the Women’s Options Center at San Francisco General Hospital.
This study was supported by grants R21 AI09009 and R01 100082 from the National Institute of Allergy and Infectious Diseases (J.M.M.) and K08 HD067295 from the National Institute of Child Health and Human Development (T.B.). Support was also provided by the Harvey V. Berneking Living Trust. J.M.M. is a recipient of the National Institutes of Health Director’s Pioneer Award Program, part of the National Institutes of Health Roadmap for Medical Research, through grant number DPI 0D00329 from the Office of the Director.
Authorship
Contribution: E.K.-L. designed and performed research, collected and analyzed data, and wrote the manuscript; C.C.K. contributed vital analytical tools, analyzed data, performed statistical analysis, and wrote the manuscript; T.D.B. helped design experiments and interpret data and wrote the manuscript; and J.M.M. helped design experiments and interpret data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph M. McCune, 1001 Potrero Ave, Building 3, Room 601, UCSF Box 1234, San Francisco, CA, 94143-1234; e-mail: mike.mccune@ucsf.edu.