Key Points
Eltrombopag promotes hematopoiesis in patients with severe aplastic anemia by stimulating stem and progenitor cells.
Eltrombopag can be discontinued safely in robust responders with maintenance of hematopoiesis.
Abstract
About a quarter of patients with severe aplastic anemia remain pancytopenic despite immunosuppressive therapy. We have previously demonstrated that eltrombopag has efficacy in this setting with 44% (11/25) of patients having clinically significant hematologic responses. We now report safety and efficacy data on a further 18 patients and long-term follow-up on the entire cohort of 43 patients. The overall response rate was 17 of 43 patients (40%) at 3 to 4 months, including tri- and bilineage responses. The majority of patients who remained on eltrombopag in an extension study (14/17) continued to show improvement, and 7 eventually had significant increases in neutrophil, red cell, and platelet lineages. Five patients with robust near-normalization of blood counts had drug discontinued at a median of 28.5 months after entry (range, 9-37 months), and all maintained stable counts a median of 13 months (range, 1-15 months) off eltrombopag. Eight patients, including 6 nonresponders and 2 responders, developed new cytogenetic abnormalities on eltrombopag, including 5 with chromosome 7 loss or partial deletion. None evolved to acute myeloid leukemia to date. Eltrombopag is efficacious in a subset of patients with aplastic anemia refractory to immunosuppressive therapy, with frequent multilineage responses and maintenance of normalized hematopoiesis off treatment. This study is registered at www.clinicaltrials.gov as #NCT00922883.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1973.
Disclosures
Bob Löwenberg, Editor, the authors, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the initial efficacy of eltrombopag in patients with refractory severe aplastic anemia, based on an open-label study.
Describe maintenance of response once eltrombopag is discontinued in patients with severe aplastic anemia.
Describe the safety of eltrombopag in patients with severe aplastic anemia.
Release date: March 20, 2014; Expiration date: March 20, 2015
Introduction
Aplastic anemia is a bone marrow failure syndrome characterized by marrow hypoplasia and hematopoietic stem cell (HSC) deficiency. An immune-mediated pathophysiology has been inferred from response to immunosuppressive therapy (IST), demonstration of immune activation, and animal models.1-3 Depletion of primitive hematopoietic stem and progenitor cells (HSPCs) is profound and may persist even in patients responding to IST.4 While outcomes for patients have markedly improved with the advent of allogeneic stem cell transplantation and IST,5 about 30% of patients with severe acquired aplastic anemia (SAA) remain pancytopenic after treatment with horse antithymocyte globulin and cyclosporine.6,7 A fraction of patients who fail to improve with 1 course of IST will respond to a second round. In the remaining two-thirds, nontransplantation options are limited and include growth factors, transfusional support, and androgen therapy.7 Management of these patients is challenging. Allogeneic stem cell transplantation is an option for patients who have suitable donors, but infectious complications, graft-versus-host disease, and graft failure often intervene, especially in older patients and in those without a well-matched family or unrelated donor.5
Thrombopoietin (TPO) is known to be a critical regulator of hematopoiesis.8-10 The TPO receptor c-mpl is expressed on HSCs,11 and knockout mice that lack c-mpl are deficient in HSCs.12,13 Eltrombopag is an oral TPO receptor agonist and was originally developed to stimulate thrombopoiesis in patients with immune thrombocytopenias.14 However, animal models15 and insights from congenital marrow failure syndromes indicate involvement of TPO signaling in HSC homeostasis as well as in thrombopoiesis.10,16,17
We treated an initial cohort of 25 patients with SAA refractory to IST on a pilot dose-escalation study, administering eltrombopag for 3 to 4 months. Hematologic response was observed in 44%.18 Blood count improvement was often robust and durable. Study design allowed responding patients to continue on eltrombopag in an extension arm, and we noted continued count improvement in multiple lineages over time. Two nonresponding patients evolved to monosomy 7 at the 3- to 4-month response assessment.
To confirm and expand these initial observations, we added a second cohort. Here, we report on a larger cohort with longer follow-up, with focus on duration and quality of responses, the impact of eltrombopag discontinuation in robust responders, and the incidence of clonal progression.
Methods
Study participants and oversight
Consecutive patients with refractory SAA who fulfilled eligibility criteria were enrolled between July 2009 and February 2013. This pilot, investigator-initiated, nonrandomized trial was registered at www.clinicaltrials.gov as #NCT00922883; was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute; and was monitored by a data safety and monitoring board. Drug was provided by GlaxoSmithKline (Collegeville, PA), but the company provided no financial support and did not play a role in data collection or analysis. All authors had full and independent access to data.
Patients eligible for inclusion were 12 years or older and previously diagnosed with SAA as defined by standard criteria,19 refractory to at least 1 course of antithymocyte-globulin–based IST initiated at least 6 months previously, and with platelet counts <30 × 103/µL. Fanconi anemia was excluded by diepoxybutane testing in patients under 40 years, and no patients with familial marrow failure or clinical characteristics of dyskeratosis congenita were included. Primary refractory patients had never responded to IST, and relapsed refractory patients had responded to at least 1 prior cycle of IST but were refractory to the most recent course of IST. Bone marrow aspiration and biopsy were performed prior to enrollment to exclude myelodysplastic syndrome (MDS) by morphology and cytogenetic analysis. Paroxysmal nocturnal hemoglobinuria (PNH) was assessed by flow cytometry, measuring the frequency of red cells and neutrophils lacking glycosylphosphatidylinositols (GPI)-anchored proteins, and a PNH clone was considered present if red cells or neutrophils deficient in GPI-anchored proteins were >1%. The characteristics of the first 25 patients have been described previously,18 and entry criteria for the additional 18 patients enrolled in the expanded trial were identical, with the exception of decreasing the lower age limit to 12 years from the prior 18 years, based on the availability of more pharmacologic information in pediatric patients treated with eltrombopag in trials for immune thrombocytopenic purpura (ITP).20 Informed consent was obtained in accordance with the Declaration of Helsinki.
Study design
The initial treatment plan was unchanged from the original protocol.18 Subjects commenced eltrombopag at a dose of 50 mg, which was increased by 25 mg every 2 weeks if the platelet count had not increased by 20 × 103/µL, to a maximum dose of 150 mg. Patients continued supportive care with platelet and red blood cell transfusions as required. Continuation of cyclosporine was permitted.
Blood counts and chemistries were monitored weekly through the primary end point and response assessment was at 3 to 4 months. Liver enzyme elevation greater than 6 times normal required suspension of eltrombopag, with reinstitution at the next-lowest dose level when transaminases had returned to <5 times normal. Adverse events were monitored and graded according to the Common Terminology Criteria for Adverse Events version 3.0. Bone marrow aspirate and biopsy with cytogenetic analysis was done at study entry and repeated at the 3- to 4-month response assessment and then every 6 months in responding patients remaining on drug. Reticulin staining was graded according to standard guidelines.21
Those patients who responded were offered the option of continuing on eltrombopag in an extension study. In August 2012, a modification to the extension study was approved that included a taper of eltrombopag over 16 weeks in those who achieved a robust response, defined as platelets >50 × 103/µL, hemoglobin >10 g/dL, and neutrophils >1 × 103/µL for longer than 8 weeks without transfusion support.
Study end points
The primary end point was hematologic response at 3 to 4 months and defined as uni- or multilineage recovery by 1 or more of the following criteria: (1) platelet response (increase to 20 × 103/μL above baseline or stable platelet counts with transfusion independence for a minimum of 8 weeks in those who were transfusion dependent on entry into the protocol); (2) erythroid response (when pretreatment hemoglobin was <9 g/dL, defined as an increase in hemoglobin by 1.5 g/dL or, in transfused patients, a reduction in the units of packed red blood cell transfusions by an absolute number of at least 4 transfusions for 8 consecutive weeks, compared with the pretreatment transfusion number in the previous 8 weeks); and (3) neutrophil response (when pretreatment absolute neutrophil count [ANC] of <0.5 × 103/μL as at least a 100% increase in ANC, or an ANC increase >0.5 × 103/μL, and the toxicity profile as measured using Common Terminology Criteria for Adverse Events).
Secondary end points were change in blood counts as a continuous variable, incidence of bleeding, change in serum TPO, health-related quality of life (as measured by the Medical Outcomes Study 36-Item Short Form General Health Survey, version 2; Quality-Metric), and clonal evolution to monosomy 7 or complex cytogenetics. Telomere lengths in peripheral blood cells were measured as previously described and analyzed as a predictor of response or clonal progression.22 DNA was extracted from bone marrow mononuclear cells for comparative genomic hybridization (CGH) using the single-nucleotide polymorphism (SNP)–based CytoScan high-density microarrays from Affymetrix.
Statistical analysis
We used the 2-stage “minimax” design23 with a response probability of ≤10% as the null hypothesis and a response probability of ≥30% as the alternative hypothesis in the initial patient cohort. We added a second cohort of 18 patients to explore the rate of clonal evolution for this patient population and to better define the response rate, adding as an exploratory secondary end point clonal evolution to monosomy 7 or complex cytogenetics. Univariate and multivariate logistic regression models were used to evaluate the effects of risk factors on the probabilities of response and clonal evolution. The R and S-plus software packages were used compute the numerical results.
Results
Patient characteristics
A total of 44 patients were enrolled on study, with 43 patients receiving eltrombopag. Initial results on the first 25 patients have been previously reported.18 One patient’s diagnosis was reclassified as hypoplastic MDS after study entry but prior to eltrombopag initiation. The characteristics of the entire cohort are shown in Table 1. Six patients met criteria for very severe aplastic anemia at study entry, defined as SAA and neutrophil count <200/µL. Three patients were on stable doses of cyclosporine throughout the study. One patient received granulocyte colony-stimulating factor (G-CSF) for a short period, and this patient was not assessed for a neutrophil response.
Patient characteristics at baseline
| Baseline characteristic . | Value . |
|---|---|
| Age, y | |
| Median | 44 |
| Range | 17-77 |
| Sex, n (%) | |
| Male | 24 (56) |
| Female | 19 (44) |
| Ethnic group, n (%) | |
| White | 20 (47) |
| Black | 13 (30) |
| Hispanic | 9 (21) |
| Asian | 1 (2) |
| Time since last IST, mo | |
| Median | 9 |
| Range | 6-117 |
| Prior number of courses of IST | |
| Median | 2 |
| Range | 1-4 |
| Response to prior IST, n (%) | |
| Primary refractory | 33 (77) |
| Relapsed refractory | 10 (23) |
| Transfusion dependency, n (%) | |
| Red cells | 40 (93) |
| Platelets | 42 (98) |
| PNH clones, n (%) | |
| Yes | 26 (60) |
| No | 17 (40) |
| Laboratory parameters | |
| Platelets (×10−3/mm3) | |
| Median | 8 |
| Range | 2-22 |
| Hemoglobin (g/dL) | |
| Median | 7.9 |
| Range | 6-13.3 |
| Neutrophils (×10−3/mm3) | |
| Median | 0.57 |
| Range | 0.07-2.8 |
| Baseline characteristic . | Value . |
|---|---|
| Age, y | |
| Median | 44 |
| Range | 17-77 |
| Sex, n (%) | |
| Male | 24 (56) |
| Female | 19 (44) |
| Ethnic group, n (%) | |
| White | 20 (47) |
| Black | 13 (30) |
| Hispanic | 9 (21) |
| Asian | 1 (2) |
| Time since last IST, mo | |
| Median | 9 |
| Range | 6-117 |
| Prior number of courses of IST | |
| Median | 2 |
| Range | 1-4 |
| Response to prior IST, n (%) | |
| Primary refractory | 33 (77) |
| Relapsed refractory | 10 (23) |
| Transfusion dependency, n (%) | |
| Red cells | 40 (93) |
| Platelets | 42 (98) |
| PNH clones, n (%) | |
| Yes | 26 (60) |
| No | 17 (40) |
| Laboratory parameters | |
| Platelets (×10−3/mm3) | |
| Median | 8 |
| Range | 2-22 |
| Hemoglobin (g/dL) | |
| Median | 7.9 |
| Range | 6-13.3 |
| Neutrophils (×10−3/mm3) | |
| Median | 0.57 |
| Range | 0.07-2.8 |
Hematologic responses
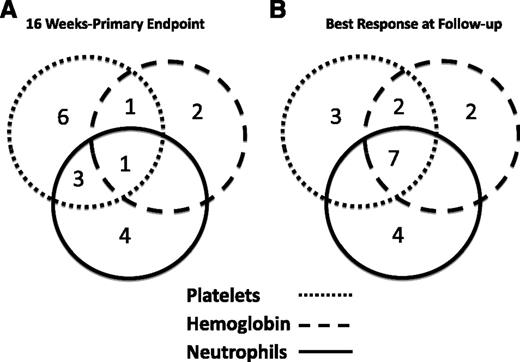
We previously reported that 11 of the initial 25 patients (44%) had a hematologic response to eltrombopag at 3 to 4 months.18 In the second cohort, we enrolled an additional 18 patients. Of these, 6 of 18 responded in at least 1 lineage at response assessment for an overall response rate of 17 of 43 (40%). Figure 1A summarizes the response characteristics of the 17 responders at the initial response assessment. One patient achieved a trilineage response, and there were 4 bilineage responses. A total of 9 of 15 patients who were receiving platelet transfusions became transfusion independent. Of the 8 neutrophil responders, 4 initially had severe neutropenia (<500/µL). Median time to initial response was 12 weeks (range, 8-14 weeks). Nine responders met standard response criteria, defined as no longer meeting criteria for SAA, after a median time on drug of 6 months (range, 3-21 months). There was no significant difference in the number of bleeding episodes and infections between responders and nonresponders.
Responses to eltrombopag by lineage. These Venn diagrams show the numbers of patients with uni- and multilineage responses at response assessment (A) and best response at follow-up (B). All biological best responses are included in panel B, including a >1.5 g increase in hemoglobin, even if it began at >9 g/dL. The lineage affected is indicated by the shade of the circle.
Responses to eltrombopag by lineage. These Venn diagrams show the numbers of patients with uni- and multilineage responses at response assessment (A) and best response at follow-up (B). All biological best responses are included in panel B, including a >1.5 g increase in hemoglobin, even if it began at >9 g/dL. The lineage affected is indicated by the shade of the circle.
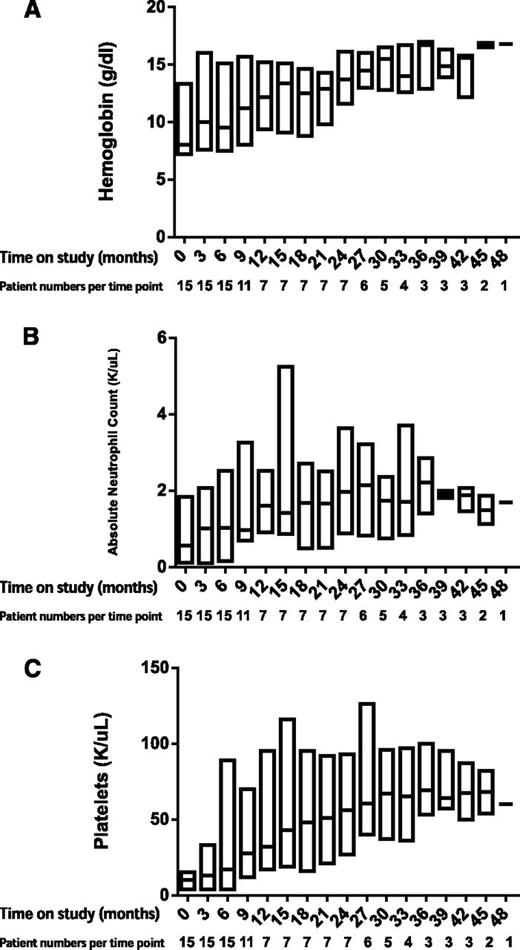
A total of 14 of 17 responders continued on eltrombopag in the extension arm, with a median time on drug of 12 months (range, 6-37 months). The majority of patients who remained on eltrombopag continued to show hematologic improvement, and 7 eventually achieved trilineage responses. Longitudinal responses are shown in Figure 2. Figure 1B summarizes best hematologic responses achieved during follow-up. One nonresponder reached response criteria 4 weeks after discontinuing eltrombopag, without institution of any additional therapies remaining so for more than 4 months to date, with platelet counts of 15 to 20 × 103/μL. A second patient who was red cell–transfusion dependent for a year prior to protocol entry did not meet response criteria at 16 weeks, and eltrombopag was discontinued. Her reticulocyte count continued to increase, and she has not required red cell transfusion for over 2 years, with hemoglobin stable at 10 g/dL (as of this writing). Thus, taking into account these 2 delayed responses, 19 of 43 patients (44%) showed significant clinical improvement after protocol entry.
Hematologic responses over time by lineage. Boxplots show erythroid response (A), neutrophil response (B), and platelet response (C).
Hematologic responses over time by lineage. Boxplots show erythroid response (A), neutrophil response (B), and platelet response (C).
Eltrombopag discontinuation
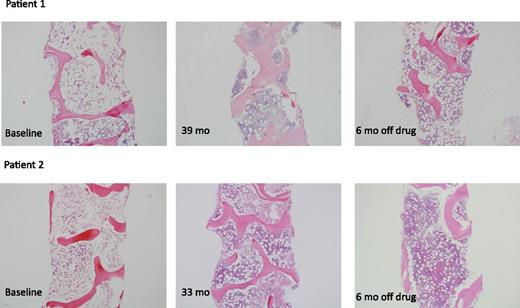
As reported previously,18 1 patient who discontinued drug at 10 weeks because of a cataract misdiagnosis had a trilineage response and continues to be transfusion independent nearly 3.5 years following protocol entry. We modified the protocol in August 2012 to include tapering of eltrombopag and discontinuation in patients with platelets >50 × 103/μL, hemoglobin >10 g/dL, and neutrophils >1 × 103/μL for more than 8 weeks, without transfusions. Five patients fulfilled these criteria, and eltrombopag was tapered and then discontinued after a median of 28.5 months (range, 9-37 months). All 5 patients have maintained stable counts with a median follow-up off drug of 13 months (range, 1-15 months) (supplemental Figures 2A-C, available on the Blood Web site). Their bone marrows have remained normocellular (Figure 3).
Bone marrow cellularity in patients 1 and 2. The left panels (for both Patients 1 and 2) show cellularity in these robust responders at baseline. The middle panels show cellularity just prior to discontinuing eltrombopag. The right panels demonstrate that, for Patients 1 and 2, the marrows remain cellular. The images were taken on an Olympus BX41 microscope with an Olympus DP72 camera, using a 4× UPlanFL N Olympus objective (original magnification ×40).
Bone marrow cellularity in patients 1 and 2. The left panels (for both Patients 1 and 2) show cellularity in these robust responders at baseline. The middle panels show cellularity just prior to discontinuing eltrombopag. The right panels demonstrate that, for Patients 1 and 2, the marrows remain cellular. The images were taken on an Olympus BX41 microscope with an Olympus DP72 camera, using a 4× UPlanFL N Olympus objective (original magnification ×40).
Three patients lost their responses while on the extension arm. All were on the full dose of 150 mg at the time of relapse. Two had achieved only erythroid responses, defined by a reduction in red cell–transfusion requirements, and after 6 months on drug returned to their previous transfusion requirements. One patient had a haplocord transplant, and the other continued supportive care with transfusions. The third relapse occurred when a neutrophil response was lost after 6 months, and the patient succumbed to an infectious episode.
Response predictors
We confirmed that absolute reticulocyte count was the only pretreatment predictor for response (responders vs nonresponders, 41.8 vs 24.2; P = .023). Age, neutrophil count, age-adjusted telomere length, presence or absence of more than 1% GPI-deficient neutrophils, number of prior cycles of immunosuppression, time since the last cycle of immunosuppression, duration of aplastic anemia diagnosis, and primary refractory disease vs relapsed refractory disease were not predictive of response.
Quality of life
Quality-of-life scores were measured using the Medical Outcomes Study 36-Item Short Form General Health Survey. At study entry, physical health scores were significantly lower (P < .001) than the US general population mean specifically in the areas of physical function, role function (physical and emotional), social function, and general health (supplemental Figure 2); all differences were clinically relevant. Mental health scores did not differ (P = .12). A total of 27 patients had surveys completed at the 3- to 4-month primary response assessment time point, and at that time, there was no significant change in pre- vs posteltrombopag physical or mental scores.
Toxicity
Similar to our prior report on the initial cohort, we observed a favorable toxicity profile in this expanded cohort, with a longer follow-up period. There were no dose-limiting toxicities other than reversible transaminitis,18 similar to the much larger safety and efficacy studies in patients with ITP, despite a higher dose used in our study.24 Two patients had reversible transaminitis related to drug; both required dose interruption, and 1 is currently maintained on 75 mg with a sustained response. Liver function abnormalities in the other patient returned to baseline after stopping drug for 4 days, and he tolerated reinstitution at 150 mg.
Contrary to reports suggesting that TPO receptor agonist use can increase bone marrow reticulin,25 we found no significant increase in fibrosis in biopsy specimens throughout our entire study cohort after a median follow-up of 13 months (range, 3-51 months) with biopsies performed every 6 months. There were no thrombotic events while receiving eltrombopag, but 1 robust responding patient experienced a lower extremity deep venous thrombosis 14 months after eltrombopag had been discontinued, with a platelet count of 60 × 103/μL and normal hemoglobin and neutrophil levels.
Clonal evolution
Eight patients developed clonal cytogenetic abnormalities during eltrombopag administration (Table 2). Seven had a normal karyotype confirmed within 3 months of starting drug, assessed by conventional cytogenetics. One patient (#42) had insufficient metaphases on his sample prior to entering the study but a normal karyotype 9 months prior to entering the study. Only 2 of 8 patients had dysplastic changes when new cytogenetic abnormalities appeared, but some samples were severely hypocellular, making assessment of morphology difficult. None had increased myeloblasts. Clonal evolution events occurred in 6 of 8 nonresponding patients, and the new cytogenetic changes were detected on the marrow performed at response assessment. Two responding patients evolved. One responder (#26), whose counts had been gradually increasing while on the extension arm, was noted to have falling counts at 13 months, and the marrow showed mild dyserythropoeisis and 13q deletion. A second responder (#32) had stable blood counts and on routine evaluation marrow at 10 months had also developed del 13q, but no dysplastic features were seen. Chromosome 7 abnormalities developed in 5 of 8 evolvers. Because many of these evolvers proceeded immediately to transplant, sequential cytogenetics were available on only 4, who showed no significant change in clone size 1 to 9 months later.
Characteristics of patients who evolved while on eltrombopag
| Patient # . | Age (y) . | Response . | Baseline . | At evolution . | Time on eltrombopag (mo) . | Dysplasia . | Outcome . | Time posttransplant (mo) . |
|---|---|---|---|---|---|---|---|---|
| 7 | 60 | NR | 46XY[20] | −7[20] | 3 | N | Died of progressive cytopenias | N/A |
| 8 | 18 | NR | 46XX[6] | +8[9]/46XX[11] | 3 | N | Transplanted successfully | 31 |
| 19 | 20 | NR | 46XY[20] | −7[5]t(1;16) [3]/46XY[12] | 3 | N | Transplanted successfully | 18 |
| 26 | 67 | R | 46XY[20] | del(13)[19]/46XY[1] | 13 | Mild dyserythropoeisis | Transplanted | 8 |
| 31 | 41 | NR | 46XY[20] | +21[3]/46XY[17] −7[2]/46XY[19] | 3 6 | Mild dyserythropoeisis | Awaiting transplant | N/A |
| 32 | 66 | R | 46XY[20] | 46XYdel13q[2]/46XY[18] | 9 | N | Under observation | N/A |
| 36 | 23 | NR | 46XY[20] | −7[5],XY[15] | 3 | N | Transplanted successfully | 3 |
| 42 | 17 | NR | No metaphases | +1,der(1;7) [4]/46XY[16] | 3 | N | Transplanted successfully | 4 |
| Patient # . | Age (y) . | Response . | Baseline . | At evolution . | Time on eltrombopag (mo) . | Dysplasia . | Outcome . | Time posttransplant (mo) . |
|---|---|---|---|---|---|---|---|---|
| 7 | 60 | NR | 46XY[20] | −7[20] | 3 | N | Died of progressive cytopenias | N/A |
| 8 | 18 | NR | 46XX[6] | +8[9]/46XX[11] | 3 | N | Transplanted successfully | 31 |
| 19 | 20 | NR | 46XY[20] | −7[5]t(1;16) [3]/46XY[12] | 3 | N | Transplanted successfully | 18 |
| 26 | 67 | R | 46XY[20] | del(13)[19]/46XY[1] | 13 | Mild dyserythropoeisis | Transplanted | 8 |
| 31 | 41 | NR | 46XY[20] | +21[3]/46XY[17] −7[2]/46XY[19] | 3 6 | Mild dyserythropoeisis | Awaiting transplant | N/A |
| 32 | 66 | R | 46XY[20] | 46XYdel13q[2]/46XY[18] | 9 | N | Under observation | N/A |
| 36 | 23 | NR | 46XY[20] | −7[5],XY[15] | 3 | N | Transplanted successfully | 3 |
| 42 | 17 | NR | No metaphases | +1,der(1;7) [4]/46XY[16] | 3 | N | Transplanted successfully | 4 |
N/A, not applicable; NR, nonresponder; R, responder.
We retrospectively performed CGH using the SNP-based CytoScan high-density microarrays on DNA extracted from pretreatment and evolution bone marrow mononuclear cells from these patients, and we could not detect chromosome 7 abnormalities in the pretreatment samples. The combined SNP/CGH array technology is reportedly more sensitive to mosaicism than standard cytogenetics and can detect levels as low as 5%; however, the pretreatment samples were profoundly hypocellular and therefore much of the DNA assayed may have originated from nonhematopoietic cells such as fibroblasts or adipocytes.26,27
No predictive factors for evolution were identified (Table 3). In previous work, leukocyte telomere content at SAA diagnosis predicted risk of clonal evolution: patients with telomere length at diagnosis in the shortest age-adjusted quartile had the highest proportion of clonal evolution.22 In the current study, all 8 patients that clonally evolved were in the shortest quartile of telomere content at study entry. Of the 33 nonevolvers, 29 were also on the shortest quartile, and telomere content in the lowest quartile did not predict clonal evolution in the current study (P = .262 by Fisher’s exact test), likely due to the limited numbers and overall very short telomeres possibly related to a longer history of disease and prior therapies. Patients with relapsed refractory disease showed a trend of being more likely to evolve than those with primary refractory disease (4/33 vs 4/10; P = .052).
Univariate and multivariate logistic regression analysis for clonal evolution
| Factor . | Univariate logistic regression analysis: evolution . | Multivariate logistic regression analysis: evolution . | ||||
|---|---|---|---|---|---|---|
| Coefficient . | SD . | P value . | Coefficient . | SD . | P value . | |
| Age | −0.0225 | 0.0211 | .2873 | −0.0122 | 0.0279 | .6603 |
| Male gender | 2.0031 | 1.1212 | .0740 | 2.3316 | 1.7582 | .1848 |
| ANC | −0.1307 | 0.7649 | .8643 | 0.7459 | 0.9201 | .4176 |
| ARC | −0.0188 | 0.0193 | .3301 | −0.0229 | 0.0256 | .3697 |
| Relapsed refractory | 1.5755 | 0.8373 | .0599 | 2.2455 | 1.9191 | .2420 |
| Prior IST treatments | ||||||
| 1 IST course | — | — | — | — | — | — |
| 2 IST courses | 15.0620 | 906.943 | .9868 | 13.0273 | 866.145 | .9880 |
| 3 IST courses | 15.5852 | 906.943 | .9863 | 14.3640 | 866.149 | .9868 |
| 4 IST courses | 15.8729 | 906.944 | .9860 | 14.7658 | 866.155 | .9864 |
| Years since last IST | −0.0078 | 0.0241 | .7463 | −0.3367 | 0.4787 | .4818 |
| Years since diagnosis | 0.0866 | 0.1059 | .4133 | −0.0337 | 0.3942 | .9318 |
| PNH clone | −0.8109 | 0.8851 | .3595 | −2.3858 | 1.9912 | .2308 |
| Factor . | Univariate logistic regression analysis: evolution . | Multivariate logistic regression analysis: evolution . | ||||
|---|---|---|---|---|---|---|
| Coefficient . | SD . | P value . | Coefficient . | SD . | P value . | |
| Age | −0.0225 | 0.0211 | .2873 | −0.0122 | 0.0279 | .6603 |
| Male gender | 2.0031 | 1.1212 | .0740 | 2.3316 | 1.7582 | .1848 |
| ANC | −0.1307 | 0.7649 | .8643 | 0.7459 | 0.9201 | .4176 |
| ARC | −0.0188 | 0.0193 | .3301 | −0.0229 | 0.0256 | .3697 |
| Relapsed refractory | 1.5755 | 0.8373 | .0599 | 2.2455 | 1.9191 | .2420 |
| Prior IST treatments | ||||||
| 1 IST course | — | — | — | — | — | — |
| 2 IST courses | 15.0620 | 906.943 | .9868 | 13.0273 | 866.145 | .9880 |
| 3 IST courses | 15.5852 | 906.943 | .9863 | 14.3640 | 866.149 | .9868 |
| 4 IST courses | 15.8729 | 906.944 | .9860 | 14.7658 | 866.155 | .9864 |
| Years since last IST | −0.0078 | 0.0241 | .7463 | −0.3367 | 0.4787 | .4818 |
| Years since diagnosis | 0.0866 | 0.1059 | .4133 | −0.0337 | 0.3942 | .9318 |
| PNH clone | −0.8109 | 0.8851 | .3595 | −2.3858 | 1.9912 | .2308 |
—, baseline value; ARC, absolute reticulocyte count; SD, standard deviation.
One patient died of progressive cytopenias, and 5 patients have undergone cord blood/haplo or matched unrelated donor HSC transplantation. All engrafted with no relapses.
Discussion
Patients with SAA refractory to IST have a poor prognosis and represent an unmet clinical need. Infectious complications are common and can be fatal.28 Intensive transfusion support is necessary for the majority of patients and can be complicated by hemosiderosis, alloimmunization, and transfusion transmitted infections. New therapies are needed for this challenging clinical situation. We confirm that eltrombopag therapy resulted in clinically significant increases in blood counts and/or decreased transfusion requirements in 40% of patients with refractory SAA. Some patients achieved multilineage responses by 3 months of dose-escalating therapy; however, further improvements in blood counts were observed in patients who remained on drug, and bi- and trilineage responses were common and more likely with greater duration of drug exposure. As previously reported in the large ITP cohorts, patients tolerated eltrombopag extremely well.
The responses seen in our clinical trial provide strong evidence for the stimulation of human HSCs by eltrombopag in vivo. In ITP, patients relapse when eltrombopag is stopped.24 In contrast, continual exposure to eltrombopag may not be necessary to sustain adequate hematopoiesis in patients with SAA. The clinically relevant mechanisms in the 2 diseases may be different, with direct stimulation of HSPCs in SAA to restore physiologic HSC numbers vs supraphysiologic stimulation of megakaryocytes by eltrombopag in ITP. The eltrombopag dosages resulting in response in the ITP trials were lower than in this study. No patients responded to doses less than 100 mg per day. It is possible that stimulation of HSPCs vs megakaryocytes requires higher eltrombopag concentrations and longer treatment time to push quiescent HSPCs into active hematopoiesis, but differentiating which factor is more important is difficult because of the dose-escalation design. In the current SAA study, 5 patients have remained transfusion-free with normal or near-normal blood counts and marrow cellularity after discontinuation of eltrombopag (4 followed off therapy for over 12 months). Three of these patients were particularly heavily pretreated, failing 3 previous rounds of IST, suggesting that abrogation of immune attack may be necessary for a sustained response to eltrombopag in patients with SAA.
Stem cell depletion is a central pathological feature of SAA,4 and our results suggest that pharmacologic expansion of this compartment can be effective in addressing this pathophysiology. Baseline reticulocyte count was the only factor predicting response in this trial. The importance of higher reticulocyte numbers as predictors of response to IST have been noted previously, perhaps reflecting residual HSC numbers.29,30 Why endogenous pathways do not stimulate normalization of the HSPC compartment, if immune attack has abated, particularly given the very high endogenous circulating levels of TPO in patients with SAA, is unclear.31,32 It is likely that a critical mass of HSPCs is required for bone marrow recovery in patients, although HSPC numbers remain low even in those who normalize blood counts.33 In an ongoing trial, we have observed responses to eltrombopag in patients with moderate aplastic anemia (MAA) not previously treated with IST, who presumably have higher residual numbers of HSCs. These responses suggest that baseline HSPC numbers are a crucial determinant of response and that some patients with MAA may not have an underlying or active immune etiology for their marrow failure, consistent with data suggesting lower response rates to immunosuppression in patients with moderate as compared with severe aplastic anemia.34 There are ongoing efforts to elucidate any possible impact of eltrombopag on immune cell function; however, we did not find any changes in T-cell subsets, including regulatory T cells, comparing samples collected before and following 3 to 4 months of treatment from the first 25 patients.
The number of patients who clonally evolved on this study is concerning. To date, 8 of 43 patients (19%) have developed new cytogenetic abnormalities, 3 to 13 months after beginning eltrombopag. Only 2 patients had morphologic dysplasia, and none had increased myeloblasts. None progressed to AML, although 5 of 8 have undergone allogeneic stem cell transplantation. Patients with SAA are at risk of progression to clonal marrow dysfunction, with the largest experience reporting up to 15% of patients clonally progressing by 10 years from diagnosis.35 Refractoriness to IST has been shown to be a risk factor for evolution, but it is difficult to discern true rates for evolution in this population due to the problem of competing outcomes, because refractory patients are referred for transplant at different time points and therapies for refractory patients are varied and applied in only small single-arm trials.36 The emergence of clones with chromosome 7 abnormalities confers a poor prognosis in SAA19 and is most common in patients who have failed to respond to therapy.37 We have treated 81 patients with experimental immunosuppression for refractory disease at our institution, of which clonal evolution was observed in 9 subjects (an additional 2 evolutions were observed among cases receiving immunosuppression followed by eltrombopag therapy). Among these 9 subjects, only 2 developed frank leukemia, with the remaining having monosomy 7 (4 patients), trisomy 6 (2 patients), and 1 subject with t(6;14). Median time to evolution from first IST was approximately 2 years, and median time to evolution from second IST was approximately 1 year. Five patients in the current study developed chromosome 7 abnormalities with 3 monosomy 7, 1 del 7p, and 1 der(1;7) (Table 2). All were nonresponders, and just 1 patient had evidence of dysplasia.
Any hematopoietic cytokine, particularly one that directly affects HSPCs via the c-mpl receptor, could impact on proliferation or self-renewal and theoretically on the emergence of abnormal clones. G-CSF therapy has been associated with monosomy 7 in retrospective studies of patients with aplastic anemia.36 Although large prospective multi-institutional studies did not confirm an impact of G-CSF on clonal progression, follow-up may not have been sufficiently long to accurately assess risk.38,39 In studies of patients with MDS, cytokine therapy has not been associated with an increased risk of progression to AML.40,41 Eltrombopag increases megakaryocytic proliferation when added to mononuclear cells from patients with AML and MDS but in the majority of samples inhibited blast cell proliferation.42 Romiplostim, a subcutaneous TPO receptor agonist, has shown promise in improving thrombocytopenia in low-risk MDS patients enrolled in a single-arm study.43 A subsequent phase 3 study treating patients with low- to intermediate-risk-1 MDS with romiplostim was terminated early due to more cases of progression to AML in the treatment arm. However, longer follow-up of this cohort, published as an abstract in 2012, found no difference in rates of progression between the placebo and romiplostim-treated groups.44 Eltrombopag is also being investigated for the treatment of cytopenias in MDS and AML, and 1 patient with primary refractory AML and monosomy 7 has been reported to have reached a clinical, morphologic, and cytogenetic response to eltrombopag, sustained for 6 months.45 Preliminary results from a randomized controlled trial of eltrombopag vs placebo in 98 patients with advanced MDS/AML have not shown any increased rate of disease progression.46
Two responders evolved, both acquiring deletion of 13q. Patients with both de novo MDS and MDS evolved from SAA with deletions of 13q are considered to have an excellent prognosis, generally without progression to frank MDS/AML.37,47 The first patient underwent transplant, and we are currently observing the second. His counts fell initially but have remained stable for 2 months, and he remains transfusion independent. Although transient cytogenetically abnormal clones have been reported in aplastic anemia,48 this phenomenon is infrequent.37 Because the majority of patients who evolved in this study underwent HSC transplantation, we cannot rule out transience of these clones or assess the rate of progression to frank MDS/AML.
Two hypotheses may be offered to explain clonal evolution. First, eltrombopag therapy may have stimulated the expansion of dormant clones. Perhaps these clones were initially below the limit of detection for metaphase cytogenetics or were quiescent and only became apparent when eltrombopag stimulation initiated cycling, providing metaphases for analysis. To investigate this possibility, we screened pretreatment marrow samples using combined CGH-SNP arrays. This technique was used to detect cryptic clonal genomic aberrations in aplastic anemia, undetectable by traditional metaphase cytogenetics.49 We did not find evidence for preexisting clones in our cohort, although this technique will not detect clones making up <5% of the DNA sample. Alternatively, chronic pharmacologic stimulation may have driven HSPC proliferation, and in the presence of shortened telomeres or other perturbations due to stem cell deficits or chronic immune attack, subsequent destabilization of the genome is accelerated and abnormal clones emerge. Evolution may have been inevitable in this group but was hastened by the use of eltrombopag. We note that prior to drug discontinuation in robust responders, 6 patients remained on 150 mg eltrombopag for a median of 20 months (range, 7-30 months), without clonal evolution, so if eltrombopag does hasten clonal evolution, only a subset of refractory patients appear to be susceptible.
Eltrombopag is efficacious in a substantial subset of patients with aplastic anemia refractory to IST. Multilineage responses are frequently seen, particularly with extended therapy. We now report sustained and durable hematopoiesis in patients who discontinued eltrombopag. Dosing schedules incorporating drug taper when robust responses are achieved may be optimal for safety. Clonal evolution rates are of concern, although a direct association with eltrombopag is unclear. Identification of patients with preexisting clonal hematopoiesis using newer, more sensitive techniques or delineation of other factors predisposing patients to clonal progression may prove helpful to guide physicians in referring patients for early allogeneic transplantation vs a trial of eltrombopag and should be the focus of future clinical trials. Given these concerns, eltrombopag should be used in patients with refractory aplastic anemia with careful attention to potential risks and benefits. Ongoing clinical research trials will help answer some of these questions. We are currently conducting clinical trials with this very promising drug in MAA and unilineage bone marrow failure syndromes and low- to intermediate-risk MDS and in upfront therapy in combination with IST. Our results open new strategies for treatment of acquired and potentially congenital bone marrow failure states and suggest that eltrombopag is a potent stimulator of in vivo HSPC function.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Katie L. Dawson and others at GlaxoSmithKline for their helpful contributions, Stephen D. Klagholz for his analysis of the quality-of-life data, the nurses at the Clinical Center and Hematology Branch of the National Heart, Lung, and Blood Institute for patient care, and the patients for their participation.
This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda.
Authorship
Contribution: R.D. was the principal investigator for this study and participated in amending the protocol, execution of the study; data collection, analysis, and interpretation; and drafting of the manuscript; C.E.D., N.S.Y, M.J.O., and P.S. participated in the primary conception of the study; K.B., M.B., and B.D. participated in collecting data; C.E.D., N.S.Y., M.J.O., and B.D. participated in analyzing the data; B.D. performed the CGH; K.B. cared for the patients; D.M.T., M.J.O., P.S., and A.R.P. participated in interim discussions and data interpretation and critically revised the manuscript; K.R.C. read the pathology specimens; and C.O.W. provided statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cynthia Dunbar, Molecular Hematopoiesis Section, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Building 10-CRC, Room 4E-5132, 10 Center Dr, Bethesda, MD 20892; e-mail: dunbarc@nhlbi.nih.gov.