In this issue of Blood, Weckbach et al demonstrate that midkine (MK), a described regulator of inflammation, supports neutrophil recruitment by promoting the high-affinity conformation of the β2 integrin lymphocyte function-associated antigen 1 (LFA-1), a step required for neutrophil arrest on the activated endothelium.1
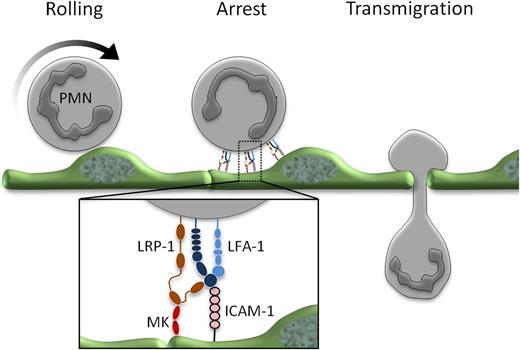
Midkine (MK) in leukocyte recruitment. Following rolling, neutrophils arrest on the inflamed endothelium and then transmigrate. The conversion from rolling to arrest is regulated by MK binding to LRP-1 and the subsequent conformational change of LFA-1 to its high-affinity state, a step required to allow firm integrin binding to ICAM-1 on the activated endothelium.
Midkine (MK) in leukocyte recruitment. Following rolling, neutrophils arrest on the inflamed endothelium and then transmigrate. The conversion from rolling to arrest is regulated by MK binding to LRP-1 and the subsequent conformational change of LFA-1 to its high-affinity state, a step required to allow firm integrin binding to ICAM-1 on the activated endothelium.
The authors report that the heparin-binding growth factor MK (also referred to as neurite growth-promoting factor 2) is essential for neutrophil arrest at the site of inflammation.1 Mice deficient for MK had markedly reduced numbers of adherent neutrophils in response to tumor necrosis factor (TNF), as assessed by intravital microscopy. This correlated with an impressive decrease in extravasated neutrophils and amelioration of tissue damage in a model of limb ischemia.1 These findings are in line with previous studies showing that MK-deficient mice are protected from organ damage in a variety of sterile inflammatory models, including rheumatoid arthritis, renal ischemia/reperfusion injury, and inflammatory bowel disease.2 Furthermore, others have used anti-sense oligoDNA and an RNA aptamer to inhibit MK functions and have shown a reduction in leukocyte infiltration in models of nephritis and autoimmune encephalitis (for an overview, see Muramatsu3 ). Thus, several lines of evidence suggest that MK plays a critical role in leukocyte recruitment.
Weckbach et al now identify a molecular mechanism that can explain the observed MK-dependent leukocyte accumulation. The authors show that MK is required specifically for the induction of the high-affinity conformation of the LFA-1 adhesion molecule on neutrophils.1 This β2 integrin possesses at least 3 distinct molecular configurations: low affinity (inactive); intermediate affinity, as induced by selectins during slow leukocyte rolling; and high affinity, as triggered by chemokine activation. Neutrophil arrest critically depends on the activation of LFA-1 to a high-affinity conformation.4 Interestingly, the authors found that only the induction of the high-affinity conformation required MK, as assessed by reporter antibodies, while MK was dispensable for the intermediate-affinity state, as implied by the lack of effect of MK deficiency on leukocyte slow rolling (see figure). Adhesion strengthening, a process involving clustering of high-affinity LFA-1 at the neutrophil-endothelial interface and cytoskeletal rearrangements following chemokine activation4 was independent of MK even though MK was necessary for inducing the integrin conformation required for this step. It is worth mentioning that β2 integrins are also required for leukocyte recruitment steps after arrest, such as intravascular crawling, transmigration, and detachment.4 Although the authors’ results can be explained by MK’s requirement for the adhesion step alone, it would be interesting to explore whether consecutive steps are affected in a similar way in neutrophils as well as mononuclear populations. The latter could be particularly interesting because monocytes and T cells rely on both β2 integrins and the β1 integrin VLA-4 for arrest4 and might therefore not have an absolute requirement for MK for this step. However, all leukocyte populations seem to require LFA-1 for transmigration,4 which may predict that transmigration of these cells is equally affected by MK independently of the requirement for MK in the arrest step. Although differences in the recruitment of other cells to the TNF-inflamed cremaster muscle was not observed, this model is not optimal for evaluating mononuclear cell accumulation.
Several interesting characteristics of MK were revealed in this study. It does not behave like a canonical cytokine, since it had no effect on neutrophil activation per se and did not induce endothelial cell activation. Reminiscent of chemokines such as stromal cell-derived factor 1α (SDF-1α), immobilization of MK was required to induce polymorphonuclear neutrophil (PMN) adhesion and transition of the β2 integrin to the high-affinity state. Thus, in vivo, the observed rescue of adhesion in MK−/− mice by delivery of soluble MK may reflect the activity of MK bound to proteoglycans presented on the endothelium together with intercellular adhesion molecule 1 (ICAM-1). In line with this, the sulfated glycosaminoglycan Heparin, which can potentially interfere with this binding has been shown to inhibit many MK functions.3
Multiple receptors for MK have been identified, including the anaplastic lymphoma kinase receptor (CD246) and receptor-like protein tyrosine phosphatase ζ.3 Relevant to the current study, low-density lipoprotein receptor-related protein 1 (LRP-1) has previously been described as being associated with the I-domain of the α-chain of the β2 integrin macrophage antigen-1 (Mac-1)5 and being required for integrin clustering and adhesion of the monocytic cell line U937.6 Using pharmacologic inhibition, the authors provide evidence that LRP-1 is the receptor for MK on neutrophils because blockade of this receptor inhibited the MK-mediated LFA-1 high-affinity conformation and neutrophil adhesion in vitro.
MK is produced by immune cells and also by endothelial cells.3 In vitro, PMN-derived MK does not appear to be required for adhesion, suggesting that in vivo, MK produced by the endothelium possibly serves as a local regulator, with concentrations of this molecule potentially playing a key role in modulating leukocyte recruitment. In line with this concept, hypoxia, activation of the nuclear factor κB (NF-κB) pathway as well as cytokines like TNF-α and interleukin-1β (IL-1β) upregulate MK expression.7 Moreover, MK levels may be downregulated by its internalization by the endocytic receptor LRP-1.8
This study raises several questions for fruitful future investigation. For example, given the significant effects on transmigration, does MK influence ICAM-1 signaling, which affects junctional reorganization associated with transmigration? How does LRP-1 mechanistically regulate β2 integrin activation? Is MK association with LRP-1 and subsequent modulation of LFA-1 modulated by the priming of neutrophils?
In summary, Weckbach et al have provided a molecular mechanism for the observed requirement for MK in leukocyte extravasation in many inflammation models. The specific function of MK for a discrete process in leukocyte recruitment and its extracellular accessibility make it an ideal target for pharmacologic intervention.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal