In this issue of Blood, Passamonti et al provide evidence that therapy with ruxolitinib, a Janus activated kinase (JAK) 1/JAK2 inhibitor, modifies the natural history of primary myelofibrosis (MF).1
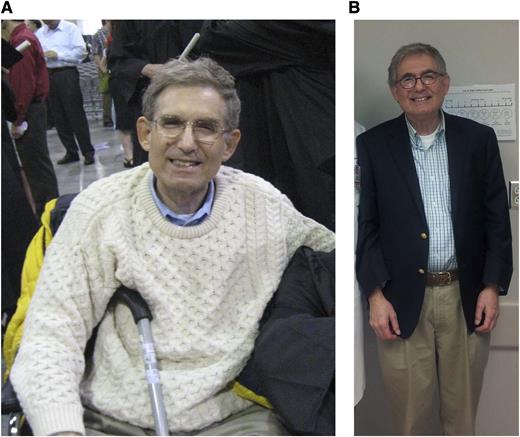
Patient with advanced MF at the start of ruxolitinib therapy (A) April 2008; and currently on ruxolitib therapy (B) January 2014.
Patient with advanced MF at the start of ruxolitinib therapy (A) April 2008; and currently on ruxolitib therapy (B) January 2014.
Typical clinical manifestations of MF, the most aggressive of the classic myeloproliferative neoplasms, are extramedullary hematopoiesis with enlargement of the spleen and liver, progressive bone marrow fibrosis with worsening blood cell count, and debilitating MF-related constitutional symptoms, including fatigue, night sweats, low-grade fever, pruritus, and bone pain, all leading to decreased performance status, cachexia, and premature death.2 Whereas 20% to 30% of patients may die of transformation to acute myeloid leukemia, most succumb to MF-related complications, commonly referred to as “disease progression.”3 Median survival from diagnosis ranges from 2 to 11 years, depending on the presence of several risk factors included in the International Prognostic Scoring System (IPSS).3 Apart from allogeneic stem cell transplant, to which <10% of patients with MF submit, no treatment so far has been shown to change outcome in MF.2 Passamonti and colleagues1 report that patients with advanced MF (intermediate-2 or high risk by IPSS; expected median survivals of 4 and 2 years, respectively) treated with ruxolitinib had longer survival than those who received conventional therapy. Ruxolitinib therapy in MF significantly reduces splenomegaly and improves MF-related constitutional symptoms and quality of life in most patients.2 However, it does not eliminate the disease. How, then, would ruxolitinib prolong survival?
Hyperactive JAK-signal transducers and activators of transcription (STAT) intracellular signaling is a feature of MF, resulting in the proliferation of hematopoietic progenitor cells and a proinflammatory state.2 The JAK2V617F mutation discovered in 2005, present in 50% to 60% of patients with MF, results in constitutive JAK-STAT activation, similar to the recently identified calreticulin mutation present in most of JAK2V617F mutation-negative patients with MF.2,4 Other rare mutations (eg, LNK, MPL, SOCS, and CBL) may explain a hyperactive JAK-STAT pathway in the remaining patients with MF.2 Ruxolitinib, a JAK inhibitor, inhibits JAK-STAT pathway and may clinically benefit any patient with MF, regardless of their mutational status, due to its antiproliferative and antiinflammatory properties.2 Looking for signs of anti-MF activity, we have traditionally looked at the effect of a therapy on the malignant clone or on bone marrow (BM) fibrosis as biological markers. Indeed, long-term therapy with ruxolitinib gradually reduces JAK2V617F allelic burden over the course of therapy.5 Recent analysis of BM biopsies in patients on long-term (5 years) ruxolitinib therapy revealed an improvement in BM fibrosis in a subgroup of patients.6 Yet neither a decrease in JAK2V617F mutation load nor a change in BM fibrosis grade has been correlated with clinical response or long-term outcome of patients on ruxolitinib therapy. However, the levels of inflammatory cytokines do correlate with clinical response. Levels of inflammatory cytokines are known to be extremely high in patients with MF and have been correlated with constitutional symptoms, transfusion need, leukocytosis, thrombocytopenia, splenomegaly, and overall survival.7 The levels of inflammatory cytokines significantly decrease in patients on ruxolitinib therapy and have been correlated with an improvement in constitutional symptoms.8 In a study with fedratinib, an investigational JAK inhibitor, a decrease in inflammatory cytokines was correlated with improvement in splenomegaly.9 Therefore, an important question is whether inflammatory cytokines are a biological marker that may partly explain the life-prolonging benefit of ruxolitinib in MF. By inhibiting inflammatory cytokines and controlling the signs and symptoms of MF, the patient’s body condition improves as the disease is kept under good control for a prolonged period of time, preventing “disease progression” (see figure).10
There are several prerequisites related to the optimal management of patients on ruxolitinib therapy in order to provide long-term benefit and potentially extend life expectancy.2,10 Guidelines for the starting dose of ruxolitinib are well established and should be followed closely: most dose adjustments happen within the first 3 months of therapy. This is a period where most benefits are also observed. Due to its short half-life, ruxolitinib should be used in a twice daily (BID) schedule; daily dosing was reported overall to be ineffective. Proactive dose adjustments are recommended to maintain patients on therapy with an effective dose and without interruptions. The higher the dose of ruxolitinib, the better is the spleen response. This appears to be important for survival benefit: 2 studies so far reported a correlation between the degree of spleen reduction and survival. However, 10 mg of ruxolitinib BID is equally as effective in controlling constitutional symptoms as higher doses (maximum dose is 25 mg BID). If starting with a low dose (eg, 5 mg BID in patients with low platelets), dose increases should be made monthly, if safe; later increases provide less benefit. Anemia has been identified as the most common side effect of ruxolitinib. The development of significant anemia on ruxolitinib therapy does not diminish its benefits: patients with or without ruxolitinib-related anemia experienced the same level of improvements in spleen and quality of life. In addition, with proper dose adjustments, there is usually a rebound in hemoglobin to near baseline levels in patients on therapy. In general, interruption of ruxolitinib therapy leads to the return of constitutional symptoms to baseline within 7 to 10 days, while regrowth of spleen usually happens at a slower rate. In a case of significant myelosuppression, 5 mg ruxolitinib BID can be used, but doses of 10 mg BID or higher have been shown to be good maintenance therapy.
How to further optimize therapy with ruxolitinib is a goal of many ongoing clinical studies, where new investigational agents are being combined with ruxolitinib to further increase its benefits, decrease its side effects (eg, improve platelets or red blood cell count), or bring additional benefits (eg, antifibrotic agents). These efforts may make MF even more indolent and prolong life further.
Conflict-of-interest disclosure: S.V. receives research funding for the conduct of clinical trials from Incyte Corporation, Astrazeneca, Lilly Oncology, Geron, NS Pharma, Bristol Myers Squibb, Novartis, Celgene, YM Biosciences, Gilead, Seattle Genetics, Promedior, and Cell Therapeutics, Inc.