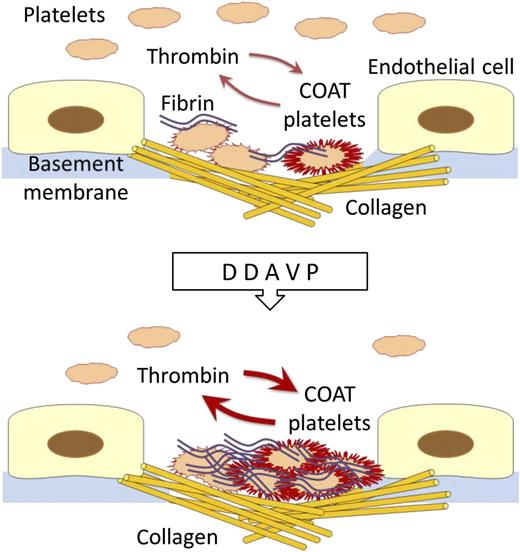
In this issue of Blood, Colucci et al provide experimental data supporting the hypothesis that desmopressin (DDAVP) favors the hemostatic process not only by inducing the release of von Willebrand factor (VWF) from endothelial cells, but also by enhancing the procoagulant activity of platelets.1
When platelets encounter a damaged vessel wall, they immediately recognize and adhere to exposed collagen and other subendothelium proteins. The adherent platelets become activated and recruit other platelets to the site of vascular injury. Meanwhile, the initiation of the coagulation cascade generates thrombin, which further activates platelets. By the combined action of thrombin and collagen, platelets express on their surface a number of specific molecules that promote both thrombin formation and cell-cell interactions (COAT platelets). It has been suggested that desmopressin infusion enhances formation of COAT platelets and thus improves the hemostatic activity of platelets.
When platelets encounter a damaged vessel wall, they immediately recognize and adhere to exposed collagen and other subendothelium proteins. The adherent platelets become activated and recruit other platelets to the site of vascular injury. Meanwhile, the initiation of the coagulation cascade generates thrombin, which further activates platelets. By the combined action of thrombin and collagen, platelets express on their surface a number of specific molecules that promote both thrombin formation and cell-cell interactions (COAT platelets). It has been suggested that desmopressin infusion enhances formation of COAT platelets and thus improves the hemostatic activity of platelets.
DDAVP is an analog of the antidiuretic hormone that interacts with type 2 vasopressin receptors of endothelial cells and induces secretion of ultralarge VWF multimers, resulting in sustained rise in plasma levels of VWF and associated factor VIII (FVIII). After demonstration in 1977 that the release of VWF occurs and is hemostatically effective in subjects with mild hemophilia and von Willebrand type 1 diseases,2 DDAVP has become a mainstay of treatment of these conditions. The excitement about this important achievement encouraged studies to test this drug, even in hemorrhagic conditions that, based on their pathogenesis, were not expected to benefit from increased levels of VWF or FVIII. The results dashed hopes that DDAVP could be a sort of panacea—good for all purposes—since negative or conflicting results have been obtained in most cases.3 However, some findings supported the efficacy of DDAVP in platelet disorders. In particular, clinical studies with surrogate end points showed that this drug was effective in improving hemostasis in subjects with thrombocytopenia associated with bone marrow failure,4 in platelet dysfunction due to antiaggregant agents,5 and in some inherited thrombocytopenias and inherited defects of platelet function.6,7 Moreover, a few case reports suggested that DDAVP was effective in halting or preventing bleeding in specific forms of inherited platelet disorders.8 Despite these appealing premises, the interest of researchers in this topic progressively diminished over time, partly because of the lack of plausible mechanisms explaining the possible efficacy of DDAVP in platelet disorders. Because of the shortage of sound clinical evidence on relevant end points, recent guidelines on management of patients with platelet defects recommended using this drug only as a last resort to stop bleeding after all other treatments failed.8
The proposal in this issue of Blood of a novel mechanism by which DDAVP improves hemostasis by a platelet-mediated effect is therefore important news that could bring the topic back to the attention of researchers.
When platelets are costimulated in vitro with collagen and thrombin, a portion of them expose on their surfaces negatively charged aminophospholipids together with α-granule proteins, including fibrinogen, VWF, thrombospondin, fibronectin, alpha2-antiplasmin, and factor V (collagen- and thrombin-activated [COAT] platelets).9 It is expected that COAT platelets are formed in vivo under circumstances of extreme hemostatic need, as immobilization of platelets on the collagen surface of a damaged vessel in the presence of thrombin generation. COAT platelets therefore represent a unique component of hemostasis, since their coating with adhesive and procoagulant proteins is potentially able to boost the hemostatic process at the sites of vascular injury.
Colucci et al administered DDAVP intravenously to 78 patients with mild primary platelet secretion disorders and investigated the percentage of COAT platelets generated in their blood samples by the combined action of thrombin and convulxin, a rattlesnake venom that activates platelets by mimicking the action of collagen. They found that generation of COAT platelets in blood samples taken after DDAVP was significantly increased with respect to baseline. Moreover, they also observed that DDAVP enhanced platelet-dependent thrombin generation.
The main hemostatic role of platelets is to localize the coagulation cascade at the site of a vascular injury (see figure). Circulating platelets immediately recognize exposed subendothelium and interact with specific molecules that include collagen. Then, platelets undergo a series of changes that support blood clotting and result in a mesh-like fibrin deposition that stops bleeding. COAT platelets are expected to do this job better because of their increased adhesiveness and enhanced procoagulant activity. It therefore conceivable that the improved hemostatic activity of platelets induced by DDAVP can compensate for quantitative or qualitative defects of these cells.
Colucci et al investigated patients with mild defects of platelet secretion who usually do not present with spontaneous bleeding. It would be interesting to know whether DDAVP also promotes COAT platelet formation in patients with Bernard-Soulier syndrome or Glanzmann thrombasthenia, who have a much more severe tendency toward bleeding and could benefit most from an enhanced platelet function. Obtaining this information is important because it has also been suggested that DDAVP has little effect on hemostasis in Glanzmann thrombasthenia while it is effective in Bernard-Soulier syndrome.7 The demonstration that DDAVP-induced formation of COAT platelets differs between these two conditions would be an indirect confirmation of the hemostatic role of this platelet subpopulation.
Acquiring the ability to improve the hemostatic activity of platelets in patients with functional platelet defects is clinically relevant, especially in Bernard-Soulier syndrome and Glanzmann thrombasthenia. In fact, patients with these conditions are particularly prone to developing refractoriness to platelet transfusions, and treatment of bleeding in this situation represents a major clinical problem. The data presented by Colucci et al provide a rationale for hoping that DDAVP can be effective in these emergencies as well as in preparation of patients for surgery or invasive procedures.
Much work is needed before recommending DDAVP in platelet disorders. From a pharmacologic point of view, it would be desirable to have more direct evidence that this drug improves in vivo hemostatic effectiveness of platelets. As pointed out by Colucci et al,1 the mechanisms of this effect of DDAVP remain undetermined. Most importantly, clinical trials are essential not only to measure the clinical effectiveness of DDAVP administration, but also to evaluate its risk-benefit ratio since the use of any molecule that potentiates hemostasis cannot be without the risk of thrombosis. DDAVP does not seem to represent an exception to the rule, since cases of myocardial infarction have been reported in patients receiving this drug.10
It may be that the story of DDAVP in platelet disorders is starting again.
Conflict-of-interest disclosure: The authors declare no competing financial interests.