Key Points
A chimeric molecule that does not bind TF is as effective as FVIIa in preventing bleeding.
An in vivo model of TF dependence of FVIIa supports the phospholipid-binding model.
Activated factor VII is approved for treating hemophilia patients with autoantibodies to their factor IX or FVIII; however, its mechanism of action remains controversial. Some studies suggest that FVIIa requires tissue factor (TF) for function and that the reason for the high dose requirement is that it must compete with endogenous FVII for tissue factor. Others suggest that FVIIa binds platelets where it activates FX directly; the high concentration required would result from FVIIa’s weak affinity for phospholipids. We address this question by infusing a chimera of mouse FIX (Gla and EGF1) with FVIIa (EGF2 and catalytic domain) into hemophilia B mice. This mutant has no TF-dependent activity because it cannot functionally bind TF at physiologically relevant concentrations. In vivo, this mutant is as effective as mouse FVIIa in controlling bleeding in hemophilia B mice. Our results suggest that the hemostatic effect of pharmacologic doses of FVIIa is TF independent.
Introduction
Human coagulation factor VIIa is approved to treat bleeding in hemophilia patients with inhibitors. Two theories attempt to explain why high doses of FVIIa are required for hemostasis. The tissue factor (TF)-dependent mechanism suggests that FVIIa’s hemostatic effect requires its binding to TF, which is expressed on cell surfaces at the site of injury.1,2 In this scenario, the high concentration of FVIIa must compete with endogenous FVII for TF. The alternative phospholipid-dependent mechanism postulates that FVIIa binds phospholipids on the platelet surface and activates factor X. Because FVIIa has low affinity for phospholipid, a high concentration of FVIIa is required to achieve hemostasis.3,4 Related to the platelet-binding mechanism, the possibility that platelet GP1bα binds FVIIa and contributes to its TF-independent activity has also been proposed.5
Recently, Shibeko et al6 used in vitro data and mathematical modeling and concluded that both mechanisms contribute to enhanced thrombin generation but that the TF-dependent mechanism dominates.
To test whether the TF pathway is dominant, we used a chimeric FVIIa molecule, mFIXgla-egf1FVIIa, with minimal affinity for TF. FVIIa is a multidomain enzyme containing an amino-terminal γ-carboxyglutamic acid–rich (Gla) domain, 2 epidermal growth factor (EGF)-like domains, and a trypsin-like serine protease domain. Previous studies indicate that the Gla domain, the first EGF domain (EGF1), and the protease domain all contribute to TF binding.7,8 Furthermore, our unpublished observations indicate that the affinity of human FIXgla-egf1FVIIa for TF is reduced about 600 000-fold compared with wtFVIIa. Our chimeric mFVIIa molecule, mFIXgla-egf1FVIIa, has had its Gla and EGF1 domains replaced by those of mouse FIX (which has a similar domain structure); its functional affinity for TF is reduced to unmeasurable levels, yet its ability to catalyze the conversion of FX to FXa in the presence of Ca++ and phospholipid is similar to that of FVIIa. If FVIIa, as used pharmacologically, is active only when in a functional complex with TF, then mFIXgla-egf1FVIIa, with its much-reduced TF-binding activity, should not provide hemostasis in the hemophilia B mouse; if, however, mFVIIa works directly on platelet surfaces, then the molecules with reduced TF binding (such as mFIXgla-egf1FVIIa) should stop bleeding as well as FVIIa.
Study design
Mouse complementary DNAs were cloned and proteins purified as previously described.8,-10 Insertion of the short amino acid sequence RKRRKR between R193 and I194 was performed as described previously so that secreted FVIIa is activated when secreted.11
TF-dependent FX activation used human TF (Innovin; DADE/ Behring, Deerfield, IL) or mouse TF–containing microparticles.12 Binding was inferred by the ability of the complex to convert FX to FXa, as measured by its ability to cleave Spectrozyme FXa (American Diagnostica).8
Clotting activity used a standard curve with NovoSeven (Novo Nordisk, Bagsvaerd, Denmark). Prothrombin time (PT) assays used human FVII–deficient plasma; activated partial thromboplastin time (APTT) assays used human FIX–deficient plasma (both from George King Bio-Medical, Overland Park, KS) using a START 4 Coagulation Analyzer (Diagnostica Stago, Asnieres, France).
Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina. All experiments used 6- to 10-week-old hemophilia B mice on a C57BL/6 background. Saphenous vein bleeding was performed as described elsewhere.13 The saphenous vein model is sensitive to TF. The average time to hemostasis (ATTH) in homozygous mice with low TF (Parry et al)14 was doubled, relative to either wild-type (WT) mice or heterozygous littermates (data not shown). This result is consistent with tail-bleeding studies in low-TF mice.15
Results and discussion
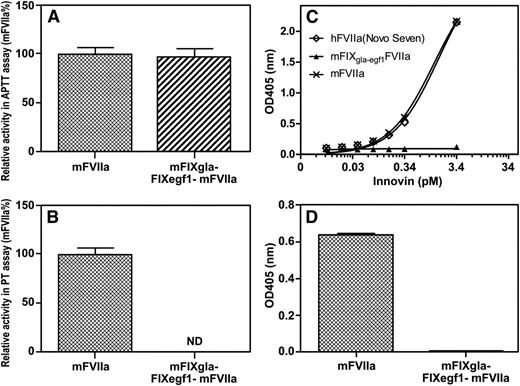
We did 2 tests to ensure that FIXgla-egf1FVIIa is not TF dependent. First, we examined the intrinsic and extrinsic coagulation activity of mFVIIa and mFIXgla-egf1FVIIa in TF-independent APTT assays (Figure 1A) and TF-dependent PT assays (Figure 1B). Mouse FIXgla-egf1FVIIa has the same APTT activity as does mFVIIa (P = .63) (Figure 1A); however, mFIXgla-egf1FVIIa has no PT activity when compared with mFVIIa (Figure 1B), which indicates that mFIXgla-egf1FVIIa does not bind to TF.
Characterization of mFIXgla-egf1FVIIa and hFIXgla-egf1FVIIa. (A) Relative coagulant activity of mFIXgla-egf1FVIIa compared with recombinant mFVIIa in an APTT assay; 25 µL of mFVIIa or mFIXgla-egf1FVIIa (5 µg/mL) was added to 25 µL FIX -deficient human plasma and 25 µL APTT reagent, and the assay initiated with 25 µL CaCl. (B) Relative activity of mFIXgla-egf1FVIIa compared with recombinant mFVIIa in a PT assay; 25 µL of either mFVIIa or mFIXgla-egf1FVIIa (0.5 µg/mL) was added to 25 µL FVII-deficient plasma, and 100 µL Innovin was added to initiate the reaction. The Innovin concentration was determined by titration with human FVIIa. Data were derived from at least 3 experimental points. Data are shown as average ± 1 standard deviation (SD). (C) Functional binding of 5000 ng/mL mFVIIa and mFIXgla-egf1FVIIa to Innovin (human TF), measured by FXa generation and detected with an FXa chromogenic substrate. Absorbance was measured after 15 minutes. Backgrounds were subtracted. (D) Relative functional binding of 5000 ng/mL mFIXgla-egf1FVIIa compared with mFVIIa to mouse TF–positive microparticles. Substrate (S-2288, 2 mM final concentration) was added to the reaction and the absorbance at 405 nm was determined after 15 minutes. Backgrounds were subtracted. The Mann-Whitney U test was used for all statistical analysis. ND, not detectable; OD, optical density.
Characterization of mFIXgla-egf1FVIIa and hFIXgla-egf1FVIIa. (A) Relative coagulant activity of mFIXgla-egf1FVIIa compared with recombinant mFVIIa in an APTT assay; 25 µL of mFVIIa or mFIXgla-egf1FVIIa (5 µg/mL) was added to 25 µL FIX -deficient human plasma and 25 µL APTT reagent, and the assay initiated with 25 µL CaCl. (B) Relative activity of mFIXgla-egf1FVIIa compared with recombinant mFVIIa in a PT assay; 25 µL of either mFVIIa or mFIXgla-egf1FVIIa (0.5 µg/mL) was added to 25 µL FVII-deficient plasma, and 100 µL Innovin was added to initiate the reaction. The Innovin concentration was determined by titration with human FVIIa. Data were derived from at least 3 experimental points. Data are shown as average ± 1 standard deviation (SD). (C) Functional binding of 5000 ng/mL mFVIIa and mFIXgla-egf1FVIIa to Innovin (human TF), measured by FXa generation and detected with an FXa chromogenic substrate. Absorbance was measured after 15 minutes. Backgrounds were subtracted. (D) Relative functional binding of 5000 ng/mL mFIXgla-egf1FVIIa compared with mFVIIa to mouse TF–positive microparticles. Substrate (S-2288, 2 mM final concentration) was added to the reaction and the absorbance at 405 nm was determined after 15 minutes. Backgrounds were subtracted. The Mann-Whitney U test was used for all statistical analysis. ND, not detectable; OD, optical density.
Second, we measured whether mFIXgla-egf1FVIIa catalyzes the conversion of FX to FXa. Mouse FVIIa binds to human TF in a concentration-dependent manner to catalyze the conversion of FX to FXa. However, mFIXgla-egf1FVIIa exhibits no measurable TF-dependent FXa generation (Figure 1C). Similarly, mFVIIa is functional with mouse TF–bearing microparticles, but mFIXgla-egf1FVIIa is not (Figure 1D).
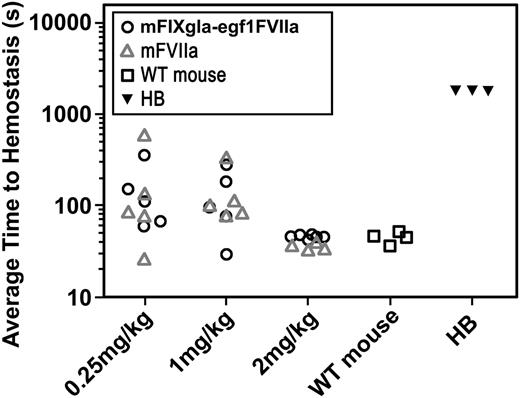
The effect of mFVIIa on hemostasis in the hemophilia B mouse was examined by the saphenous vein bleeding model (Figure 2B).13 Three different concentrations of mFVIIa or mFIXgla-egf1FVIIa were infused into hemophilia B mice 10 minutes before saphenous vein incision; the time to hemostasis for each bleeding event was recorded over a 30-minute time period and the ATTH for each mouse was calculated. Control WT C57BL/6 mice (n = 4) had a median ATTH of 44 seconds; hemophilia B mice (n = 3) failed to achieve hemostasis within the 30-minute experimental time frame. The median ATTH remained similar for mFVIIa- and mFIXgla-egf1FVIIa-treated hemophilia B mice at the 3 tested doses.
Effect of FIXgla-egf1FVIIa chimera on saphenous vein bleeding in the hemophilia B mouse. Wild-type (control group) or hemophilia B (experimental group) mice were subjected to saphenous vein incision. After initial clot formation, the clot was disrupted repeatedly for 30 minutes and the time for each clot to form was measured; the average time to clot formation was calculated for each mouse. Ten minutes before incision, mFVIIa or mFIXgla-egf1FVIIa was injected into Hemophilia B mice. The hemophilic mice bled continuously during the entire 1800-second duration of the experiment; WT mice have an ATTH of 44 seconds. Each point represents the average of multiple bleeding times in each mouse during the 30-minute observation time. For 2 mg/kg, median ATTH was 37 seconds (n = 4) for mFVIIa-treated hemophilia B mice and 47 seconds for mFIXgla-egf1FVIIa-treated mice (n = 6). The difference in ATTH was not statistically significant (P = .24); both are significantly shorter than untreated hemophilia B mice (P < .05). For 1 mg/kg, mFVIIa-treated hemophilia B mice (n = 5) clotted in an average of 108 seconds; the ATTH was 97 seconds for mFIXgla-egf1FVIIa-treated hemophilia B mice (n = 6) (P = .75). At a dose of 0.25 mg/kg, the median ATTH for mFVIIa-treated mice (n = 5) was 86 seconds, whereas for mFIXgla-egf1FVIIa–treated mice (n = 5) it was 111 seconds (P = .83). All P values were calculated using the nonparametric Mann-Whitney U test. HB, hemophilia B.
Effect of FIXgla-egf1FVIIa chimera on saphenous vein bleeding in the hemophilia B mouse. Wild-type (control group) or hemophilia B (experimental group) mice were subjected to saphenous vein incision. After initial clot formation, the clot was disrupted repeatedly for 30 minutes and the time for each clot to form was measured; the average time to clot formation was calculated for each mouse. Ten minutes before incision, mFVIIa or mFIXgla-egf1FVIIa was injected into Hemophilia B mice. The hemophilic mice bled continuously during the entire 1800-second duration of the experiment; WT mice have an ATTH of 44 seconds. Each point represents the average of multiple bleeding times in each mouse during the 30-minute observation time. For 2 mg/kg, median ATTH was 37 seconds (n = 4) for mFVIIa-treated hemophilia B mice and 47 seconds for mFIXgla-egf1FVIIa-treated mice (n = 6). The difference in ATTH was not statistically significant (P = .24); both are significantly shorter than untreated hemophilia B mice (P < .05). For 1 mg/kg, mFVIIa-treated hemophilia B mice (n = 5) clotted in an average of 108 seconds; the ATTH was 97 seconds for mFIXgla-egf1FVIIa-treated hemophilia B mice (n = 6) (P = .75). At a dose of 0.25 mg/kg, the median ATTH for mFVIIa-treated mice (n = 5) was 86 seconds, whereas for mFIXgla-egf1FVIIa–treated mice (n = 5) it was 111 seconds (P = .83). All P values were calculated using the nonparametric Mann-Whitney U test. HB, hemophilia B.
Clearly, FIXgla-egf1FVIIa does not productively bind TF; however, there are other differences in these molecules that may affect their function. For example, FIX’s Gla domain alone is sufficient for binding to collagen IV in the subendothelium.16,17 Thus, it is possible that modified pharmacokinetics of FVIIa compared with FIXgla-egf1FVIIa due to additional interactions within the mouse circulation may explain some of our data.
Hemophilia B mice require much higher doses of FVIIa than do humans to correct bleeding. Our 2-mg/kg dose is higher than the 90- to 270-µg/kg dose currently recommended for human use; however, it is consistent with others’ observations.18 For example, Ivanciu et al report that 5 mg/kg of mouse or human FVIIa prevents bleeding equally well in a hemophilia B mouse.18 Because the affinity of human FVIIa for mouse TF is 1000-fold less than that of mFVIIa for mouse TF, this is further evidence that pharmacologic use of FVIIa does not require TF for hemostasis.19
In summary, FIXgla-egf1 does not productively bind TF but is as effective as mFVIIa in promoting hemostasis in a hemophilia B mouse. Blood-cell–derived TF is the decisive trigger for the massive fibrin deposition of deep vein thrombosis.20 Moreover, long-term high levels of FVIIa expression in mice cause premature death related to thrombosis in the lung and heart, both tissues rich in TF.11 Thus, because FIXgla-egf1FVIIa does not bind TF, it is likely a safer and more effective molecule than FVIIa, especially for those cases where FVIIa is used off-label for uncontrolled bleeding.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Nigel Mackman and his colleagues in the University of North Carolina at Chapel Hill Department of Medicine for providing mouse microparticles.
This work was supported by National Institutes of Health, National Heart Lung and Blood Institute grant HL006350.
Authorship
Contribution: All authors contributed to the writing and correction of the manuscript, and most of the experimental work was done by D.F.
Conflict-of-interest disclosure: The authors declare no competing financial interests. A patent has been filed for the use of the chimera for treating patients with inhibitors.
Correspondence: Darrel W. Stafford, Department of Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3280; e-mail: dws@e-mail.unc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal