Key Points
IKZF1 deletions are predictive of an unfavorable outcome in childhood BCR-ABL1–positive ALL.
Good-risk BCR-ABL1–positive patients with wild-type IKZF1 have good outcomes when treated with imatinib.
Childhood BCR-ABL1–positive B-cell precursor acute lymphoblastic leukemia (BCP-ALL) has an unfavorable outcome and shows high frequency of IKZF1 deletions. The prognostic value of IKZF1 deletions was evaluated in 2 cohorts of BCR-ABL1–positive BCP-ALL patients, before tyrosine kinase inhibitors (pre-TKI) and after introduction of imatinib (in the European Study for Philadelphia–Acute Lymphoblastic Leukemia [EsPhALL]). In 126/191 (66%) cases an IKZF1 deletion was detected. In the pre-TKI cohort, IKZF1-deleted patients had an unfavorable outcome compared with wild-type patients (4-year disease-free survival [DFS] of 30.0 ± 6.8% vs 57.5 ± 9.4%; P = .01). In the EsPhALL cohort, the IKZF1 deletions were associated with an unfavorable prognosis in patients stratified in the good-risk arm based on early clinical response (4-year DFS of 51.9 ± 8.8% for IKZF1-deleted vs 78.6 ± 13.9% for IKZF1 wild-type; P = .03), even when treated with imatinib (4-year DFS of 55.5 ± 9.5% for IKZF1-deleted vs 75.0 ± 21.7% for IKZF1 wild-type; P = .05). In conclusion, the highly unfavorable outcome for childhood BCR-ABL1–positive BCP-ALL with IKZF1 deletions, irrespective of imatinib exposure, underscores the need for alternative therapies. In contrast, good-risk patients with IKZF1 wild-type responded remarkably well to imatinib-containing regimens, providing a rationale to potentially avoid hematopoietic stem-cell transplantation in this subset of patients.
Introduction
The introduction of risk-adjusted treatment protocols has significantly improved the overall prognosis of childhood acute lymphoblastic leukemia (ALL).1 A highly unfavorable abnormality is the translocation of the BCR gene at chromosome region 22q11 to ABL1 gene at 9q34, resulting in the t(9;22)(q34;q11) translocation or “Philadelphia chromosome” (with BCR-ABL1 fusion transcript). This translocation occurs in 2% to 3% of children and 25% of adults with B-cell precursor ALL (BCP-ALL).2 Both adults and children are frequently treated with intensive chemotherapy followed by hematopoietic stem cell transplantation (HSCT). However, despite this intensive therapy, the prognosis of BCR-ABL1–positive BCP-ALL remains poor with a 5-year event-free survival (EFS) of <50% in both children and adults.3,,-6 The introduction of tyrosine kinase inhibitors (TKIs), such as imatinib and dasatinib, targeting the BCR-ABL1–fusion protein has significantly improved treatment outcome.7,,-10 However, BCR-ABL1–positive ALL is a biological and clinical heterogeneous disease with large individual differences in response to chemotherapy.11,-13 Therefore, studies that elucidate the biological and prognostic variation in BCR-ABL1–positive ALL may help to rationally assign patients to more appropriate treatment modalities.
Recent studies correlate deletions in the gene encoding Ikaros (IKZF1) with an unfavorable outcome in BCR-ABL1–negative BCP-ALL.14,,,,-19 IKZF1 is involved in B-cell proliferation and differentiation processes, most likely by modulating the rearrangement of the immunoglobulin heavy chain locus and signaling of the immature B-cell receptor.20 Deletions of the whole gene and partial deletions that affect at least the starting codon located in exon 2 result in haploinsufficiency. Deletions that affect the DNA-binding domain in exons 4-7 (known as isoform 6) exert a dominant negative effect over the unaffected allele resulting into a loss of the tumor suppressor function attributed to wild-type IKZF1.21,22 Together, deletion variants have been considered as indicators of poor prognosis in previous studies.14,,,,-19,23 In contrast to the relatively low frequency of deletions (15%) in BCR-ABL1–negative BCP-ALL, the frequency of deletions is high (70%) in BCR-ABL1–positive BCP-ALL,19,23,,-26 suggesting that the unfavorable prognosis of BCR-ABL1–positive ALL is correlated with IKZF1 deletions. All IKZF1 deletion variants taken together were shown to be highly predictive of an adverse clinical outcome in adults with BCR-ABL1–positive ALL.23 However, the prognostic value in children with BCR-ABL1–positive ALL is unknown.
In this international multicenter study, we investigated the prognostic role of IKZF1 deletions in 191 BCR-ABL1–positive childhood BCP-ALL patients before TKI (pre-TKI) and after the introduction of imatinib. Our data showed that deletions of IKZF1 are predictive of a poor outcome in both the pre-TKI era and in imatinib-treated patients. Remarkably, good risk-stratified patients with wild-type IKZF1 who received imatinib had a favorable outcome, which is comparable to the outcome results for BCR-ABL1–negative BCP-ALL patients treated with contemporary protocols. Recently, it was demonstrated that HSCT does not improve the prognosis of BCR-ABL1–positive patients who are treated with chemotherapy and TKIs.8 Together with the present study, these findings provide a strong rationale to avoid HSCT in IKZF1 wild-type BCR-ABL1–positive patients who are stratified as good-risk patients based on a good early clinical response to a therapeutic window with prednisone or induction therapy at day 21.
Methods
Patients
Leukemic cells of 191 BCR-ABL1–positive BCP-ALL patients who achieved complete remission were analyzed for the presence of IKZF1 deletions. These cases were collected from the “Ponte di Legno” (pre-TKI) and the European Study for Philadelphia–positive ALL (EsPhALL) cohorts, and were representative for the total cohort with respect to outcome (supplemental Figure 1, available on the Blood Web site) and clinical variables (supplemental Table 1). Additional data on 11 pre-TKI patients who did not achieve complete remission are not included in the analysis: 7 had an IKZF1 deletion and 4 wild-type (no induction failures were observed in the EsPhALL cohort).
Bone marrow samples from these patients were collected from the Associazione Italiana di Ematologia Pediatrica (Italy), the German Berlin-Frankfurt-Munster study group (Germany), the Childhood Leukemia Investigation Prague (Czech Republic), the Dutch Childhood Oncology Group (The Netherlands), the European Organization for Research and Treatment of Cancer Children’s Leukemia Group, the French Acute Lymphoblastic Leukemia Study Group (France), and the Children’s Cancer and Leukemia Group (United Kingdom). All experimental tests were performed in the national reference laboratories. Written informed consent according to the Declaration of Helsinki was obtained from patients and/or parents/guardians to use excess of diagnostic material for research purposes, as approved by the Erasmus University Medical Center institutional review board.
Pre-TKI cohort. There were 351 patients diagnosed between 1995 and 2005, who entered the national study protocols and achieved complete remission with induction therapy in the 7 countries participating in the Ponte di Legno study group. DNA for analysis of IKZF1 status analysis was available for 84 patients, of whom 18 received imatinib due to compassionate use at the discretion of the treating physicians, but it was not standardized in terms of frequency, duration, and dosage.
EsPhALL cohort. DNA of the patients enrolled in this study was available for 107 of 213 patients registered between 2005 and 2010, in the 7 participating countries. These patients were assigned to the good-risk (n = 63) or the poor-risk group (n = 44) according to their early clinical response. A good early clinical response was defined as blast cell count of <1000 cells/μL in peripheral blood after 7 days of treatment with prednisone and a single intrathecal dose of methotrexate, or ≤5% leukemic blast cells in the bone marrow at day 21, depending on national induction protocols. Good-risk patients were initially randomized for imatinib treatment (EudraCT: 2004-001647-30). Following the amendment of the protocol in December 2009, all patients received imatinib. All patients stratified as poor-risk received imatinib. This study was monitored by the EsPhALL International Trial Data Centre (University of Milano-Bicocca, Monza, Italy).
Sample preparation
BCR-ABL1–positive BCP-ALL bone-marrow samples were collected prior to initial treatment. Mononuclear fraction of cells was isolated by Ficoll gradient and DNA isolated according to local laboratory procedures.
IKZF1-status analysis
The presence of IKZF1 deletions was investigated by the Multiplex Ligation-dependent Probe Amplification (MLPA) assay SALSA p335 kit (MRC-Holland, Amsterdam, The Netherlands) using 125 ng of genomic DNA. The assays were performed according to the manufacturers’ protocol. Electrophoresis and quantification of fluorescein amidite–labeled amp icons were performed on an ABI-3130 genetic analyzer (Applied Biosystems, Carlsbad, CA). The resulting peak intensities were normalized to the manufacturers’ control probes and to normal DNA as a reference. An intensity ratio between 0.75 and 1.3 was considered to represent a normal copy number, a ratio between 0.25 and 0.75 a monoallelic deletion, and a ratio <0.25 a biallelic deletion. Deletions were validated by either an independent MLPA SALSA p335, SALSA MLPA p202, array comparative genomic hybridization (Sureprint G3 Human CG 180K arrays; Agilent Technologies, Santa Clara, CA) as previously described,24 or by single nucleotide polymorphism array analysis (GeneChip Human Mapping, 100K Array Set; Affymetrix, Santa Clara, CA), as previously performed.27
Deletions were classified by assumed effect on protein function in 3 different groups: the “dominant negative group,” including all cases with at least one exons 4-7 deleted allele22 ; the “haploinsufficiency group,” including whole gene deletions and deletions affecting exon 213,22 ; and the “miscellaneous group,” representing all remaining variants.
Genomic aberrations in other B-cell differentiation genes
Aberrations in other B-cell genes (PAX5, ETV6, RB1, BTG1, EBF1, CDKN2A, CDKN2B, and P2RY8-CRLF2 were investigated by the same MLPA SALSA p335 kit as used for the detection of IKZF1 deletions. Data were interpreted as they were defined for IKZF1 deletions.
Statistical analysis
Disease-free survival (DFS) was calculated from date of first remission to the date of event, which included relapse, death in complete remission, or second malignancy, whichever occurred first. Overall survival (OS) was calculated from the date of first remission to the date of death from any cause. Observations of patients were censored at the date of last contact when no events were observed. Follow-up was made on December 31, 2008, for the pre-TKI cohort, and on December 31, 2010, for the EsPhALL cohort, with a median (interquartile range) follow-up of 5.0 years (2.8-6.0) and 2.8 years (1.5-3.7), respectively. DFS was chosen as the primary end point for both cohorts, as EsPhALL-treated patients were registered at the end of the national induction treatment. The Kaplan-Meier method was used to estimate the probabilities of DFS and OS, with standard errors (SE) calculated according to Greenwood’s method. Curves were compared using the log-rank test. Cumulative incidence of relapse (CIR) was estimated, adjusting for competing risks of death, and it was statistically analyzed by the Gray test.28 The Cox model was used to investigate the prognostic role of IKZF1 status. The χ2 test was used to assess the association between IKZF1 status and clinical features. All tests were two-sided. Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC) at the EsPhALL Trial Center.
Results
The characteristics of IKZF1-deleted vs wild-type patients are shown in Table 1. IKZF1-deletions were identified in 126 of 191 (66%) BCR-ABL1–positive BCP-ALL samples, confirming the frequency observed in previous studies.19,23,,-26 The frequency of IKZF1 deletion did not differ between the pre-TKI (65%) and EsPhALL trials (66%). Together, all IKZF1-deleted cases had a higher white blood cell count than wild-type patients (P = .006) (Table 1) The “haploinsufficient” group accounted for 36.5%, the dominant negative group for 52.4%, and the miscellaneous group for 11.1% of all IKZF1-deleted cases (Table 1). This distribution was different from what was found in other studies in BCR-ABL1–negative BCP-ALL (P < .001)18,19,29 with a twofold higher frequency of the dominant negative variant and a 1.7-fold lower frequency of haploinsufficient variants in a representative cohort of Dutch BCR-ABL1–positive BCP-ALL cases (supplemental Figure 2).
Patients’ characteristics
| . | IKZF1 wild-type . | IKZF1-deleted . | . | ||
|---|---|---|---|---|---|
| . | (n = 65) . | (n = 126) . | . | ||
| . | N . | % . | N . | % . | P value . |
| Gender | |||||
| Male | 44 | 67.7 | 85 | 67.5 | .97 |
| Female | 21 | 32.3 | 41 | 32.5 | |
| Age (years) | |||||
| <10 | 43 | 66.2 | 71 | 56.4 | .19 |
| ≥10 | 22 | 33.8 | 55 | 43.6 | |
| White blood cell count (cells × 109/L)* | |||||
| <50 | 42 | 65.6 | 58 | 46.0 | .006 |
| 50-100 | 10 | 15.6 | 15 | 11.9 | |
| ≥100 | 12 | 18.8 | 53 | 42.1 | |
| Early clinical response† | |||||
| Yes | 38 | 63.3 | 81 | 66.4 | .68 |
| No | 22 | 36.7 | 41 | 33.6 | |
| IKZF1 status | |||||
| Wild-type | 65 | 100.0 | NA | NA | |
| Haploinsufficient | NA | NA | 46 | 36.5 | |
| Dominant negative | NA | NA | 66 | 52.4 | |
| Miscellaneous | NA | NA | 14 | 11.1 | |
| . | IKZF1 wild-type . | IKZF1-deleted . | . | ||
|---|---|---|---|---|---|
| . | (n = 65) . | (n = 126) . | . | ||
| . | N . | % . | N . | % . | P value . |
| Gender | |||||
| Male | 44 | 67.7 | 85 | 67.5 | .97 |
| Female | 21 | 32.3 | 41 | 32.5 | |
| Age (years) | |||||
| <10 | 43 | 66.2 | 71 | 56.4 | .19 |
| ≥10 | 22 | 33.8 | 55 | 43.6 | |
| White blood cell count (cells × 109/L)* | |||||
| <50 | 42 | 65.6 | 58 | 46.0 | .006 |
| 50-100 | 10 | 15.6 | 15 | 11.9 | |
| ≥100 | 12 | 18.8 | 53 | 42.1 | |
| Early clinical response† | |||||
| Yes | 38 | 63.3 | 81 | 66.4 | .68 |
| No | 22 | 36.7 | 41 | 33.6 | |
| IKZF1 status | |||||
| Wild-type | 65 | 100.0 | NA | NA | |
| Haploinsufficient | NA | NA | 46 | 36.5 | |
| Dominant negative | NA | NA | 66 | 52.4 | |
| Miscellaneous | NA | NA | 14 | 11.1 | |
NA, not applicable.
White blood cell count was unknown in 1 patient (EsPhALL cohort).
Early clinical response was defined as <1000 cells/μL in peripheral blood after 7 days of treatment with prednisone and a single intrathecal dose of methotrexate, or ≤5% leukemic blast cells in the bone marrow at day 21 (depending on national induction protocols). Early clinical response was unknown for 5 wild-type and 4 IKZF1-deleted patients (all from the pre-TKI cohort).
Prognostic value of all types of IKZF1 deletions together
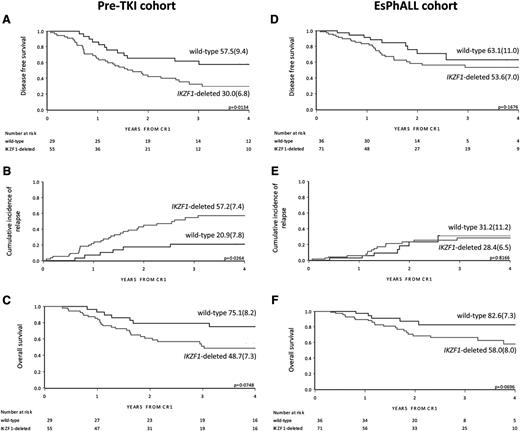
In the pre-TKI cohort, IKZF1-deleted patients had a lower DFS compared with wild-type patients (4-year DFS 30.0% [SE 6.8] vs 57.5% [SE 9.4]; P = .013) (Figure 1A). This was mainly because of relapses, as indicated by the 4-year CIR of 57.2% (SE 7.4) for IKZF1-deleted patients and 20.9% (SE 7.8) for IKZF1 wild-type patients (P = .026) (Figure 1B and Table 2). This resulted in a trend for superior OSfor IKZF1 wild-type patients with a 4-year OS of 75.1% (SE 8.2) vs 48.7% (SE 7.3) for IKZF1-deleted patients (P = .075) (Figure 1C). There were 19 of 29 (65.5%) of the IKZF1 wild-type patients who received an HSCT that did not significantly differ from the 28 of 55 (50.9%) of IKZF1-deleted patients who received HSCT (supplemental Table 2).
Outcome by cohort and IKZF1 status. DFS, CIR, and OS curves by IKZF1 status in the pre-TKI cohort (A, B, and C, respectively) and in the EsPhALL cohort (D, E, and F, respectively), with 4-year estimates (SE). Three additional events occurred after more than 4 years from CR1 (2 relapses in IKZF1-deleted patients and 1 relapse in wild-type), and are therefore not depicted in the plots of A and B. Two deaths that occurred after more than 4 years from CR1 in wild-type, relapsed pre-TKI patients are not depicted in C. CR1, first complete remission.
Outcome by cohort and IKZF1 status. DFS, CIR, and OS curves by IKZF1 status in the pre-TKI cohort (A, B, and C, respectively) and in the EsPhALL cohort (D, E, and F, respectively), with 4-year estimates (SE). Three additional events occurred after more than 4 years from CR1 (2 relapses in IKZF1-deleted patients and 1 relapse in wild-type), and are therefore not depicted in the plots of A and B. Two deaths that occurred after more than 4 years from CR1 in wild-type, relapsed pre-TKI patients are not depicted in C. CR1, first complete remission.
Clinical events by cohort and IKZF1 status
| . | IKZF1 wild-type . | IKZF1-deleted . | ||
|---|---|---|---|---|
| . | N . | % . | N . | % . |
| Pre-TKI | ||||
| No. of patients | 29 | 100.0 | 55 | 100.0 |
| Relapses (deaths after relapse) | 7 (3) | 24.1 | 31 (19) | 56.4 |
| BM involvement | 7 | 25 | ||
| Isolated extramedullary | 0 | 6 | ||
| Deaths in CCR | 6 | 20.7 | 7 | 12.7 |
| Deaths after HSCT | 5 | 4 | ||
| EsPhALL overall | ||||
| No. of patients | 36 | 100.0 | 71 | 100.0 |
| Relapses (deaths after relapse) | 7 (3) | 19.4 | 15 (10) | 21.1 |
| BM involvement | 6 | 12 | ||
| Isolated extramedullary | 1 | 3 | ||
| Deaths in CCR | 2 | 5.6 | 11 | 15.5 |
| Deaths after HSCT | 2 | 8 | ||
| EsPhALL good-risk | ||||
| No. of patients | 20 | 100.0 | 43 | 100.0 |
| Relapses (deaths after relapse) | 2 (0) | 10.0 | 11 (7) | 25.6 |
| BM involvement | 1 | 8 | ||
| Isolated extramedullary | 1 | 3 | ||
| Deaths in CCR | 0 | 0.0 | 6 | 14.0 |
| Deaths after HSCT | 0 | 4 | ||
| EsPhALL poor-risk | ||||
| No. of patients | 16 | 100.0 | 28 | 100.0 |
| Relapses (deaths after relapse) | 5 (3) | 31.3 | 4 (3) | 14.3 |
| BM involvement | 5 | 4 | ||
| Isolated extramedullary | 0 | 0 | ||
| Deaths in CCR | 2 | 12.5 | 5 | 17.9 |
| Deaths after HSCT | 2 | 4 | ||
| . | IKZF1 wild-type . | IKZF1-deleted . | ||
|---|---|---|---|---|
| . | N . | % . | N . | % . |
| Pre-TKI | ||||
| No. of patients | 29 | 100.0 | 55 | 100.0 |
| Relapses (deaths after relapse) | 7 (3) | 24.1 | 31 (19) | 56.4 |
| BM involvement | 7 | 25 | ||
| Isolated extramedullary | 0 | 6 | ||
| Deaths in CCR | 6 | 20.7 | 7 | 12.7 |
| Deaths after HSCT | 5 | 4 | ||
| EsPhALL overall | ||||
| No. of patients | 36 | 100.0 | 71 | 100.0 |
| Relapses (deaths after relapse) | 7 (3) | 19.4 | 15 (10) | 21.1 |
| BM involvement | 6 | 12 | ||
| Isolated extramedullary | 1 | 3 | ||
| Deaths in CCR | 2 | 5.6 | 11 | 15.5 |
| Deaths after HSCT | 2 | 8 | ||
| EsPhALL good-risk | ||||
| No. of patients | 20 | 100.0 | 43 | 100.0 |
| Relapses (deaths after relapse) | 2 (0) | 10.0 | 11 (7) | 25.6 |
| BM involvement | 1 | 8 | ||
| Isolated extramedullary | 1 | 3 | ||
| Deaths in CCR | 0 | 0.0 | 6 | 14.0 |
| Deaths after HSCT | 0 | 4 | ||
| EsPhALL poor-risk | ||||
| No. of patients | 16 | 100.0 | 28 | 100.0 |
| Relapses (deaths after relapse) | 5 (3) | 31.3 | 4 (3) | 14.3 |
| BM involvement | 5 | 4 | ||
| Isolated extramedullary | 0 | 0 | ||
| Deaths in CCR | 2 | 12.5 | 5 | 17.9 |
| Deaths after HSCT | 2 | 4 | ||
No second malignant neoplasm was observed in the 2 cohorts. EsPhALL cases were stratified in good-risk or poor-risk, based on their early clinical response. Fifteen of 20 IKZF1 wild-type EsPhALL good-risk patients received imatinib: 1 relapsed in BM and testis and none died in CCR. Thirty-six of 43 IKZF1-deleted EsPhALL good-risk patients received imatinib: 8 suffered from a relapse (5 in BM and 3 isolated extramedullary) and 5 died in CCR (4 after HSCT in CR1).
BM, bone marrow; CCR, continuous complete remission.
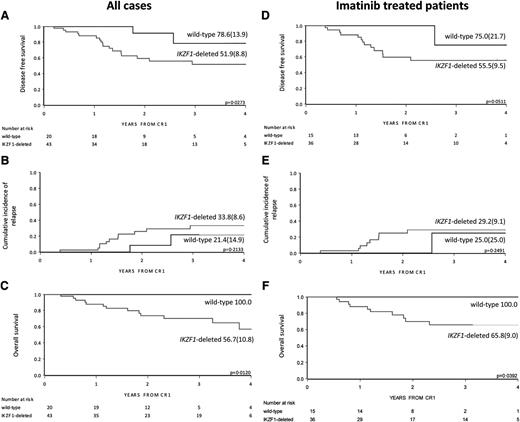
In the EsPhALL study, IKZF1-deleted patients had a trend for inferior DFS and OS compared with wild-type patients, but not an inferior CIR (Figure 1D-F and Table 2). Within the good-risk stratum of the EsPhALL protocol (N = 63), the 20 IKZF1 wild-type patients had a highly favorable prognosis (4-year DFS 78.6%; SE 13.9) compared with that of the 43 IKZF1-deleted patients (4-year DFS 51.9%; SE 8.8; P = .027) (Figure 2A), although the difference in CIR did not reach statistical significance (Figure 2B). Interestingly, only 2 of 20 IKZF1 wild-type patients relapsed at 1.5 and 2.5 years from achieving complete remission compared with 11 out of 43 IKZF1-deleted patients who relapsed at a median time of 1.4 years (interquartile range, 1.2-1.9) (Table 2). All deaths, whether in continuous complete remission or after relapse, occurred in the IKZF1-deleted group (4-year OS 56.7%, SE 10.8; Figure 2C) (P = .012). HSCT was given in 12 of 20 (60.0%) of the wild-type patients and in 29 of 43 (67.4%) of the IKZF1-deleted patients (supplemental Table 2; P > .05).
Outcome by IKZF1 status in EsPhALL good-risk patients. DFS, CIR, and OS by IKZF1 status of all EsPhALL good-risk patients (A, B, and C, respectively) and DFS, CIR, and OS of EsPhALL good-risk patients treated with imatinib (D, E, and F, respectively), with 4-year estimates (SE). CR1, first complete remission.
Outcome by IKZF1 status in EsPhALL good-risk patients. DFS, CIR, and OS by IKZF1 status of all EsPhALL good-risk patients (A, B, and C, respectively) and DFS, CIR, and OS of EsPhALL good-risk patients treated with imatinib (D, E, and F, respectively), with 4-year estimates (SE). CR1, first complete remission.
Fifty-one good-risk EsPhALL patients were treated with imatinib. The IKZF1 wild-type good-risk patients had a 4-year DFS of 75.0% (SE 21.7) compared with 55.5% (SE 9.5) for IKZF1-deleted patients (P = .051) (Figure 2D). Although only 1 of 15 IKZF1 wild-type good-risk patients relapsed in contrast to 8 of 36 IKZF1-deleted good-risk patients, this difference in CIR did not reach statistical significance (Figure 2E). IKZF1-deleted good-risk patients had an OS of 65.8% (SE 9.0) compared with 100% for wild-type good-risk patients (Figure 2F) (P = .039). There were 25 of 36 (69.4%) IKZF1-deleted patients who received HSCT vs 9 of 15 (60.0%) of the wild-type patients (supplemental Table 2) (P > .05). Multivariate analysis including age, white blood cell count, and treatment with imatinib, which indicated that IKZF1 deletions were of independent unfavorable prognostic value in good-risk EsPhALL patients (hazard ratio, 4.30; 95% CI 0.98-19.0; P = .05) (Table 3).
Multivariate analysis of DFS including potential risk factors and IKZF1 status in BCR-ABL1–positive ALL
| . | HR . | 95% CI . | P value . |
|---|---|---|---|
| EsPhALL good-risk (n = 62) | |||
| Age at diagnosis, years | |||
| <10 | 1 | ||
| ≥10 | 1.69 | 0.61-4.68 | .31 |
| WBC at diagnosis (× 109/L) | |||
| <50 | 1 | ||
| ≥50 | 1.53 | 0.62-3.78 | .36 |
| Imatinib exposure | |||
| Yes | 1 | ||
| No | 1.52 | 0.51-4.51 | .45 |
| IKZF1 status | |||
| Wild-type | 1 | ||
| Deleted | 4.30 | 0.98-19.0 | .05 |
| . | HR . | 95% CI . | P value . |
|---|---|---|---|
| EsPhALL good-risk (n = 62) | |||
| Age at diagnosis, years | |||
| <10 | 1 | ||
| ≥10 | 1.69 | 0.61-4.68 | .31 |
| WBC at diagnosis (× 109/L) | |||
| <50 | 1 | ||
| ≥50 | 1.53 | 0.62-3.78 | .36 |
| Imatinib exposure | |||
| Yes | 1 | ||
| No | 1.52 | 0.51-4.51 | .45 |
| IKZF1 status | |||
| Wild-type | 1 | ||
| Deleted | 4.30 | 0.98-19.0 | .05 |
For 62 of EsPhALL good-risk patients all covariates were known. EsPhALL-treated patients with a good early clinical response were stratified into the good-risk arm.
95% CI, 95% confidence interval; HR, hazard ratio; WBC, white blood cell count.
There was no significant difference in DFS, CIR, or in OS between IKZF1-deleted and wild-type patients observed in the EsPhALL poor-risk group treated with imatinib (supplemental Figure 3).
Additional genetic lesions in B-cell differentiation genes (P2RY8-CRLF2, CDKN2A, CDKN2B, PAX5, ETV6, BTG1, RB1, and/or EBF1) were found in 73% (92 of 126) of IKZF1-deleted samples compared with 48% (28 of 65) of the wild-type samples (P < .001). Furthermore, among imatinib-treated good-risk patients, 47% (7 of 15) of IKZF1 wild-type patients had additional genetic lesions vs 86% (31 of 6) of IKZF1-deleted (P = .0058). Although these are significant differences, they did not impact outcome. Indeed, none of the imatinib-treated good-risk and IKZF1 wild-type patients suffered from a relapse, despite having additional genetic lesions in 1 or more of the above-mentioned genes. Therefore, although the number of patients is limited, the good prognosis of imatinib-treated IKZF1 wild-type cases cannot be explained by absence of lesions in other B-cell maturation-related genes.
Prognosis according to the functional type of IKZF1 deletion variant
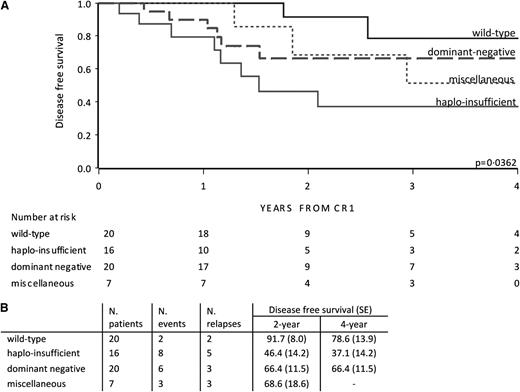
In the EsPhALL good-risk group, outcome depended on the type of deletion (P = .036; Figure 3). The outcome of patients with deletions in IKZF1 resulting in the dominant negative variant and miscellaneous variants did not statistically differ from wild-type patients. Interestingly, IKZF1 haploinsufficient patients showed an unfavorable outcome (4-year DFS of 37.1% [SE 14.2]) compared with wild-type patients vs (4-year DFS of 78.6% [SE 13.9]) (P = .0042; Figure 3). Taking both poor- and good-risk EsPhALL patients together, haploinsufficient patients showed an unfavorable outcome compared with wild-type patients (P = .010) (supplemental Figure 4A). The poor outcome of haploinsufficient patients was sustained by the outcome of patients with whole gene or exon 2-7 deletions, which were the most frequent variants in the haploinsufficient group compared with those with the dominant-negative isoform 6 (supplemental Figure 4B).
DFS by IKZF1 deletion variant in EsPhALL good-risk patients. DFS curves of EsPhALL good-risk patients by IKZF1 wild-type and deletion variants (A), with 2- and 4-year estimates (SE) (B). CR1, first complete remission; dominant negative, samples with exons 4-7 deletions involving at least 1 allele; haploinsufficient, samples with total deletions plus exons 2-7 deletions, plus samples with exon 2 involving 1 allele; miscellaneous, samples not classified in previous groups.
DFS by IKZF1 deletion variant in EsPhALL good-risk patients. DFS curves of EsPhALL good-risk patients by IKZF1 wild-type and deletion variants (A), with 2- and 4-year estimates (SE) (B). CR1, first complete remission; dominant negative, samples with exons 4-7 deletions involving at least 1 allele; haploinsufficient, samples with total deletions plus exons 2-7 deletions, plus samples with exon 2 involving 1 allele; miscellaneous, samples not classified in previous groups.
Discussion
The overall prognosis of childhood BCP-ALL has improved enormously throughout recent decades, reaching 80% to 85% EFS rates at 5 years from diagnosis.1 However, the prognosis of BCR-ABL1–positive BCP-ALL remained highly unfavorable in the pre-TKI era with 5-year EFS <50%.3,-5 Generally, until mid-2000, these patients were treated according to high-risk protocols of individual national study groups. The introduction of TKIs (such as imatinib and dasatinib) has markedly improved the prognosis of BCR-ABL1–positive BCP-ALL.7,,-10
Recent studies have shown that BCR-ABL1–positive BCP-ALL is characterized by a very high frequency (70%) of IKZF1 deletions.19,23,,-26 In BCR-ABL1–negative childhood BCP-ALL, these deletions of IKZF1 were fourfold less frequent, but predictive of an unfavorable outcome in children, and recent studies have showed that this predictive value is independent of other known risk factors.14,,,,-19 As the frequency of BCR-ABL1–positive BCP-ALL in children is low (<5% of the newly diagnosed BCP-ALL patients), the predictive value of IKZF1 deletions remained unaddressed. Moreover, the recent introduction of TKIs may modify the predictive value of IKZF1 deletions in BCR-ABL1–positive ALL. Therefore, this international collaborative study was undertaken to address the prognostic value of IKZF1 deletions in children with BCR-ABL1–positive ALL, both in patients treated in the pre-TKI era and in patients treated with imatinib.
Here, we show that IKZF1 deletions are related to a poor outcome in BCR-ABL1–positive BCP-ALL. With the introduction of imatinib, the 4-year DFS of IKZF1-deleted patients improved to ∼50% compared with ∼30% for IKZF1-deleted patients in the pre-TKI cohort. Of interest, IKZF1 deletions remain associated with an unfavorable clinical outcome, even in imatinib-containing therapies. Importantly, this difference in poor outcome could not be explained by a difference in the frequencies of HSCT between IKZF1-deleted and wild-type patients. In the pre-TKI cohort, a lower frequency of HSCT was observed in the IKZF1-deleted group, because many IKZF1-deleted patients suffered from an early relapse excluding the possibility for a first-line HSCT.
As the BCR-ABL1–translocation itself is a strong adverse risk factor, it is even more remarkable that the IKZF1 deletions can further dissect patients out who are at high risk of relapse. This implies that IKZF1-deleted patients should receive more intensive therapy and/or alternative therapy. IKZF1 deletions were shown to be a second hit in BCR-ABL1–positive BCP-ALL, thereby inducing a more aggressive type of leukemia.11,30 Deletions in IKZF1 may lead to activated JAK-STAT pathway13 and an immature B-cell receptor signaling via BTK activation.31 If confirmed, JAK and/or BTK inhibitors may offer an alternative strategy for treatment in BCR-ABL1–positive ALL with IKZF1 deletions.
The frequency of deletions in exons 4-7 of IKZF1, resulting in the dominant negative isoform 6, is higher in BCR-ABL1–positive than in BCR-ABL1–negative BCP-ALL. BCR-ABL1–positive BCP-ALL cells were demonstrated to have an increased activity of RAG1/RAG2 recombination genes.25,26,32 Heptamer recombination signal sequences have been localized to the break points of exons 4 and 7 of IKZF1.23,25 Therefore, the hyperactivity of RAG enzymes may be related to the increased frequency of exons 4 to 7 deletions in BCR-ABL1–positive BCP-ALL. Intriguingly, we observed that the less-frequent haploinsufficient patients who had a poorer prognosis than the more frequent dominant negative patients. Interestingly, loss of chromosome 7, which carries the IKZF1-gene, was shown to predict an unfavorable prognosis in BCR-ABL1–positive BCP-ALL.33 A recent study also indicated that the leukemic cells of BCR-ABL1–positive with monosomy 7 have a different gene expression profile compared with leukemic cells from patients with the dominant negative isoform 6 and those with IKZF1 wild-type.13 Further studies are needed to understand whether this aberrant expression profile represents a different biology and how this could be associated with the difference in clinical outcome.
The most striking observation in our study is the highly favorable clinical outcome for EsPhALL good-risk patients with IKZF1 wild-type. The majority (>60%) of the patients received HSCT. A recent study showed that BCR-ABL1–positive patients treated with TKI and chemotherapy have a comparable outcome to patients treated with TKI, chemotherapy, and HSCT.8 Taken together with our data, this suggests that IKZF1 wild-type patients with a good early clinical response should be spared from HSCT, thereby reducing treatment-related morbidity. Then HSCT may be used as salvage therapy for those IKZF1 wild-type patients who suffer from a relapse.
In conclusion, as in children with BCR-ABL1–negative BCP-ALL, IKZF1 deletions are a strong adverse prognostic factor in BCR-ABL1–positive patients, even if treated with imatinib. Studies on the pathobiological role of IKZF1 deletions in BCR-ABL1–positive ALL should yield new clues for alternative treatment. Imatinib-treated good-risk patients with wild-type IKZF1 may be spared from HSCT because their prognosis is as good as for BCR-ABL1–negative BCP-ALL cases. All together, we propose to implement the IKZF1 status in the risk stratification of childhood BCR-ABL1–positive BCP-ALL in future treatment protocols.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Dutch Cancer Society (grant EMCR 2007-3718) (M.L.d.B. and R.P.); the Sophia Foundation for Scientific Research (grant 658) (A.v.d.V. and M.L.d.B.); the Pediatric Oncology Foundation Rotterdam (M.L.d.B. and R.P.); the GAČR Centre of Excellence (grant P302/12/G101) (M.Z.); the project for conceptual development of research organization, Ministry of Health, Czech Republic (grant 00064203) (M.Z.); the University Hospital Motol, Prague, Czech Republic (grant UNCE204012) (M.Z.); the Italian Association for Cancer Research (G.C.); the Italian Ministry of University and Research (G.C.); and the Fondazione Cariplo (G.C.). The research leading to these results has received funding from the European Union's Seventh Framework Program (FP7/2007-2013) under the European Network of Cancer Research in Children and Adolescents project grant agreement HEALTH-F2-2011-261474 (M.G.V., G.t.K., G.C., and M.L.d.B.). These funding sources had no role in the collection, analysis, or interpretation of the results, or in writing the manuscript or the decision for submission of this manuscript.
Authorship
Contribution: A.v.d.V., M.Z., F.M., M. Schrappe, A.B., R.P., M. Stanulla, M.L.d.B., and G.C. designed this study and the experimental setup; A.v.d.V., M.Z., F.M., G.t.K., C.J.H., H.C., J.T., M.L.d.B., and V.S. performed and/or analyzed the MLPA assays; statistics were done by A.v.d.V., P.D.L., and M.G.V.; and all authors wrote and approved of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M. Stanulla is Pediatric Hematology/Oncology, Hannover Medical School, Hannover, Germany.
Correspondence: Monique L. den Boer, Molewaterplein 60, Rotterdam, 3015GJ The Netherlands; e-mail: m.l.denboer@erasmusmc.nl; and Giovanni Cazzaniga, Centro Ricerca Tettamanti, Via Pergolesi 33, Monza, 20900 Italy; e-mail: gianni.cazzaniga@hsgerardo.org.
References
Author notes
A.v.d.V., M.Z., F.M., M. Stanulla, M.L.d.B., and G.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal