Key Points
GARP efficiently represses proliferation of naïve and resting CD4+ T cells and is involved in the induction of adaptive regulatory T cells.
In vivo, GARP prevents T cell–mediated destructive inflammation in a preclinical humanized mouse model of GVHD.
Abstract
Glycoprotein A repetitions predominant (GARP) is expressed on the surface of activated human regulatory T cells (Treg) and regulates the bioavailability of transforming growth factor-β (TGF-β). GARP has been assumed to require membrane anchoring. To investigate the function of GARP in more detail, we generated a soluble GARP protein (sGARP) and analyzed its impact on differentiation and activation of human CD4+ T cells. We demonstrate that sGARP efficiently represses proliferation and differentiation of naïve CD4+ T cells into T effector cells. Exposure to sGARP induces Foxp3, decreases proliferation and represses interleukin (IL)-2 and interferon-γ production, resulting in differentiation of naïve T cells into induced Treg. This is associated with Smad2/3 phosphorylation and partially inhibited by blockade of TGF-β signaling. Furthermore, in the presence of the proinflammatory cytokines IL-6 and IL-23, sGARP facilitates the differentiation of naïve T cells into Th17 cells. More important, in a preclinical humanized mouse model of xenogeneic graft-versus-host disease (GVHD), sGARP prevents T cell–mediated destructive inflammation by enhancing Treg and inhibiting T effector cell activity. These results demonstrate a crucial role of sGARP in modulation of peripheral tolerance and T effector cell function, opening the possibility to use sGARP as a potent immunomodulator of inflammatory diseases including transplant rejection, autoimmunity, and allergy.
Introduction
Suppressive mechanisms of Treg include a variety of effector molecules and signaling pathways. Among these, a role of membrane-bound transforming growth factor (TGF)-β has been suggested from the very beginning. TGF-β exerts great influence on T-cell function by affecting their proliferation, differentiation and survival.1-3 Many cell types synthesize latent TGF-β consisting of a homodimer of latency-associated protein (LAP) noncovalently associated with a homodimer of mature TGF-β.4 Latent TGF-β lacks biological activity and has to be released from LAP to become biologically active. Herein, LAP degradation5,6 and/or conformational changes of LAP7-10 have been reported to be important. Release of TGF-β from LAP represents a critical step for TGF-β function and signaling.11
Recently, glycoprotein A repetitions predominant (GARP), an orphan toll-like receptor structure-related protein expressed by activated platelets and Treg,12,13 was shown to associate with LAP,4,14 forming an alternative cell surface platform for latent TGF-β presentation. On the surface of activated Treg, GARP participates in their regulatory function,15-17 demonstrated by impaired suppressive activity upon GARP silencing.15
GARP has been generally assumed to require membrane anchoring to exert its regulatory function, because its soluble TGF-β–associated form was found to be unable to support αVβ6- or αVβ8-mediated TGF-β activation.16 To address the cell-independent regulatory capacity of GARP, we generated a soluble human GARP protein (sGARP) and analyzed its influence on T-cell differentiation and T cell–mediated inflammation. We show that sGARP increases Foxp3 expression, decreases proliferation, and represses production of interleukin (IL)-2 and interferon (IFN)-γ in naïve CD4+ T cells but not in differentiated CD4+CD45RO+ T cells. Both Foxp3 induction and cytokine repression by sGARP are abrogated after TGF-β receptor blockade, demonstrating participation of TGF-β in sGARP activity. Naïve CD4+ T cells stimulated in the presence of sGARP differentiated into induced Treg that suppress the activation of CD4+ T effector cells in coculture. Furthermore, in the presence of the proinflammatory cytokines IL-6 and IL-23, sGARP promotes Th17 differentiation.
sGARP activity was additionally analyzed in a xenogeneic graft-versus-host disease (GVHD) mouse model.18 Upon transfer of human peripheral blood mononuclear cells (PBMC) into newborn Rag2−/−γc−/− mice, the animals develop a lethal GVHD, induced by human xenoreactive T-effector cells. Cotransfer of human Treg prevents GVHD onset in a dose-dependent manner. Importantly, administration of sGARP synergizes with Treg activity in vivo and results in repression of GVHD.
Methods
Human sGARP
The ectodomain of GARP (aa 20-627) fused to the Fc-domain of rabbit IgG was produced in transfected CHO cells using the Flip-In transfection system (Invitrogen) (supplemental Figure 1). GARP (aa 1-627), with a C-terminal 6 His tag, was purchased from R&D Systems (#6055-LR); recombinant GARP recLRRC32(hu):Fc(hu) (aa 20-627) fused to the Fc domain of human IgG1 was obtained from Enzo (#ALX-522-117).
Isolation and stimulation of human T-cell populations
Buffy coats were obtained from healthy volunteers, and cord blood samples from the obstetric department (University Hospital Mainz) after informed written consent, both with approval by the local ethical committee (Landesaerztekammer Rheinland-Pfalz). This study was conducted in accordance with the Declaration of Helsinki. Untouched CD4+CD25– T cells, as well as CD4+CD45RA+ or CD4+CD45RO+ T cells, were isolated as previously described.17-20 For some experiments, PBMC were depleted of T cells using CD3-Dynabeads (0.5 beads/cell; Invitrogen).
Cord blood–derived CD4+ T cells, CD4+CD45RA+, or CD4+CD45RO+ T cells were Treg-depleted with CD25-Dynabeads (0.5 beads/cell), resulting in a purity of CD4+CD25-Foxp3– T cells >98%. Cells were labeled with carboxyfluoroscein succinimidyl ester (CFSE) and cultured in 48 well plates at 106 cells/mL, stimulated with 0.5 µg/mL anti-CD3 mAb (clone OKT3) plus 1 µg/mL anti-CD28 mAb (clone 28.2, eBioscience) in the presence of 1 ng/mL TGF-β1 (#240-B, R&D Systems, referred to hereafter as TGF-β) or 1 to 10 µg/mL sGARP as indicated. Blocking anti–TGF-β RII (#AF-241-NA, R&D Systems) mAb was added at a concentration of 10 µg/mL.
To analyze the effect of sGARP on proliferation, CD4+ T cells were stimulated with anti-CD3 mAb (0.5 µg/mL) and anti-CD28 mAb (1 µg/mL) with or without sGARP (1 µg/mL). At day 7, T cells were harvested and restimulated with anti-CD3 mAb (0.5 µg/mL) plus irradiated (90 Gy) T cell–depleted PBMC. After 4 days, proliferation was measured by additional 16-hour pulse with [3H] TdR (37 kBq/well) using a liquid β-scintillation counter. In some experiments, T cells were stimulated in the presence of sGARP (1 µg/mL) with or without 200 IU/mL IL-6 (#1404, CellGenix, Freiburg, Germany) and 100 ng/mL IL-23 (#1290-IL; R&D Systems).
Enzyme-linked immunosorbent assay
TGF-β was analyzed by enzyme-linked immunosorbent assay according to the manufacturer’s protocol (DRG Instruments, Marburg, Germany).
Treg induction and analysis
Naïve cord blood–derived CD4+ T cells (Donor 1) were cultured in 48-well plates (106 cells/mL) and stimulated with 0.5 µg/mL anti-CD3 mAb plus 1 µg/mL anti-CD28 mAb in the presence or absence of 1 µg/mL sGARP. Peripheral CD4+ T cells of healthy volunteers (Donor 2, responder cells) were stimulated under the same conditions without sGARP. At day 7, both T-cell subsets were harvested, cultured either alone or together at different ratios as indicated, and stimulated with anti-CD3 mAb plus irradiated (90 Gy) T cell–depleted human PBMC (Donor 3) as feeder cells. Proliferation was measured after 4 days and an additional 16-hour pulse with [3H] TdR (37 kBq/well).
RT-PCR and quantitative RT-PCR
RNA was extracted from 106 cells using the RNeasy Kit (Qiagen) according to the manufacturer’s instructions. cDNA was generated by reverse transcription using the Sensiscript RT Kit (Qiagen). Gene expression levels were determined by quantitative real-time polymerase chain reaction (RT-PCR) (Applied Biosystems) and the QuantiFAST PCR Kit (Qiagen). TGF-β (QuantiTect Primer; Qiagen), RORγt (forward: 5′-agagccaaggcatgag; reverse: 5′-caggtgataagtggat-3′), and IL-17A (forward: 5′-tgccttcaagactgaacaa-3′; reverse: 5′-tctctgaggggccttaatc-3′), normalized to the housekeeping gene EF1α (forward: 5′-gattacagggacatctcaggctg-3′; reverse: 5′-tatctcttctggctgtagggtgg-3′). Relative mRNA expression was calculated in reference to untreated samples using the δ-δ Ct method.
Flow cytometry
Flow cytometric analyses were performed using the following antibodies: anti-CD45 (HI30), anti-CD4 (RPAT4), anti-CD25 (M-A25), anti-CD27 (L128), anti-CD45RA (HI100) (all from BD Biosciences); anti-CD62L (DREG-56; BD Pharmingen); and anti-CCR7 (150503; R&D Systems). TGF-β receptor and membrane-bound TGF-β were stained using biotinylated TGF-β1 or anti-LAP mAb (BAF 246) according to the manufacturer’s instructions (R&D Systems). For Smad2/3 phosphorylation, cord blood cells were stimulated with 0.5 µg/mL anti-CD3 mAb for 30 minutes and stained with anti-CD4 and anti–phospho-Smad2/3 mAb (O72-670; BD Phosflow) according to the manufacturer’s instructions. For intracellular staining of Foxp3, cells were fixed and permeabilized using a Fix/Permeabilization kit (eBioscience) and anti-Foxp3 mAb (259D/C7; BD Biosciences). Cytokine expression was analyzed in T cells restimulated with 5 µg/mL phytohaemagglutinin plus 1 µg/mL Ionomycin for 5 hours in the presence of Monensin (1.3 µM) 10 days after in vitro primary stimulation, permeabilized (perm/fix solution; BD Biosciences), and stained with anti–IL-2 and anti–IFN-γ mAb (BD Biosciences). For ex vivo analysis, cells were reisolated from spleens and restimulated as described with phytohaemagglutinin/Ionomycin in the presence of Monensin for 5 hours. Flow cytometry was performed on LSRII (BD Biosciences), using FlowJo software (Tree star).
Cell sorting
For purification of CD4+CD45RA+CCR7+CD62Lhigh and CD4+CD45RA+CCR7+CD62Llow T cells, CD4+CD45RA+ T cells were isolated as described.17-20 Cells were stained with anti-CD4, anti-CD45RA, anti-CD62, and anti-CCR7 mAb. CD62LhighCCR7+ and CD62LlowCCR7+ subsets were sorted from the CD4+CD45RA+ population, with a purity of 94.7 ± 4.6% (CD62LhighCCR7+, n = 4) and 99.1 ± 0.5% (CD62LlowCCR7+, n = 4). Sorting was performed on a FACSJazz using fluorescence-activated cell sorting software and analyzed by with FACSDiva software (all BD Biosciences).
Mice and GVHD induction
Rag2−/−γc−/− mice21 were obtained at 1 to 4 days after birth (central animal facility, Johannes Gutenberg University). Experiments were performed in accordance with relevant laws and institutional guidelines. GVHD was induced as described before.22 Briefly, 5 × 106 PBMC were injected intraperitoneally into newborn mice alone or together with isolated Treg at different ratios with or without 5 µg sGARP or control protein (rabbit IgG). Mice without PBMC transfer served as controls. Weight changes were monitored every third day. Results are presented as percent mean body weight ± SEM based on initial weight.
For ex vivo analysis of effector cells, mice were sacrificed 7 days after treatment initiation. Spleens were isolated, red blood cells were lysed, and the remaining cells were washed (in RPMI 1640). Cell suspension was resuspended in X-VIVO-15 (Lonza, Belgium). Expression of CD45, CD4, CD25, Foxp3, CD27, and CD45RA, and production of IL-2 and IFN-γ were determined by flow cytometry. Glutamate pyruvate transaminase (GPT) was quantified in murine sera using Reflovet Plus (Roche) 20 days after PBMC transfer.
Statistics
Results represent means ± SEM. Statistical significance was determined using the Student t test with *P < .05, **P < .01, ***P < .001, and n.s. (not significant) as indicated.
Results
sGARP enhances Foxp3 expression and represses proliferation and effector cytokine production in naïve CD4+ T cells
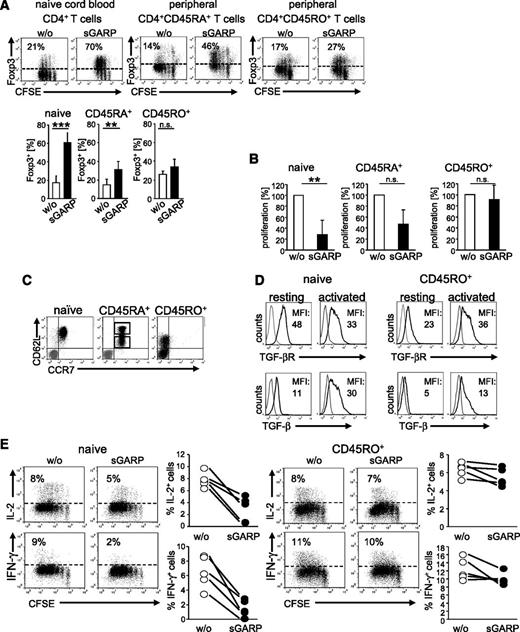
It has been previously shown that GARP expression marks a population of activated human Treg, and further work suggested that GARP may participate in Treg-suppressive activity.13-15,23,24 To address its Treg-independent immune regulatory potential, we generated a recombinant sGARP consisting of the GARP ectodomain (aa 20- 627) fused to the Fc-domain of rabbit IgG (supplemental Figure 1). Activation of cord blood–derived naïve CD4+ T cells and peripheral CD4+CD45RA+ T cells in the presence of sGARP resulted in a significant Foxp3 upregulation, whereas Foxp3 expression in CD4+CD45RO+ T cells remained nearly unaffected (Figure 1A). This effect on naïve CD4+ T cells was most pronounced at day 3. Nevertheless, we observed Foxp3 induction as early as day 1, with a maximum at day 3 and lasting up to 7 days (supplemental Figure 2A). In addition to Foxp3 induction, sGARP-treated naïve CD4+ T cells showed a significant reduction in proliferation compared with peripheral CD4+CD45RA+ (moderate effect) and CD4+CD45RO+ T cells (no effect, Figure 1B).
sGARP enhances Foxp3 expression and represses proliferation and cytokine production in CD4+ effector T cells. (A) sGARP increases Foxp3 expression. CFSE-labeled T cells were stimulated with anti-CD3 (0.5 µg/mL) and anti-CD28 mAb (1 µg/mL) in the presence of sGARP (1 µg/mL). Foxp3 expression was analyzed on day 3 by flow cytometry. Dot blots show one representative result for each T-cell subset (upper part). Diagrams (lower part) display summarized data of 7 independent experiments. Data represent the mean ± SEM of experiments (n = 7, **P < .01, ***P < .001; n.s., not significant). (B) sGARP inhibits proliferation. Each T-cell subset was stimulated under the same conditions described in (A) in the presence or absence of sGARP (1 µg/mL). Proliferation was analyzed on day 12 by 3H-TdR incorporation. Bar diagrams show pooled data of 3 independent experiments (percentage of proliferation) in the presence of sGARP normalized to proliferation of cells without sGARP (n = 3, mean ± SEM, **P < .01; n.s., not significant). (C) Each T-cell subset was analyzed for CD62L and CCR7 expression by flow cytometry as described. Peripheral CD4+CD45RA+ T cells displayed a mixed population of CD62Lhigh and CD62Llow cells (gray represents isotype control). Dot blot shows one representative out of 8 experiments (n = 8). (D) TGF-βR and membrane-bound TGF-β (TGF-β) expression on resting and activated (16 hour) naïve CD4+ T cells and CD4+CD45RO+ T cells. Histograms show one representative experiment of 4 independent experiments. Numbers indicate mean fluorescence intensities (MFI) of TGF-βR (naïve resting: 51.9 ± 19, activated: 34.5 ± 6; CD4+CD45RO+ resting: 22.1 ± 5, activated: 27.8 ± 14) or TGF-β (naïve resting: 15.2 ± 9, activated: 27.0 ± 4; CD4+CD45RO+ resting: 5.13 ± 0.5, activated: 13.3 ± 7) expression are indicated. (E) CFSE-labeled naïve CD4+ T cells and CD4+CD45RO+ T cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence or absence of sGARP (1 µg/mL). Cytokines were analyzed on day 10 upon restimulation. Dot blots show one representative result of 5. Diagrams show summarized data of 5 independent experiments.
sGARP enhances Foxp3 expression and represses proliferation and cytokine production in CD4+ effector T cells. (A) sGARP increases Foxp3 expression. CFSE-labeled T cells were stimulated with anti-CD3 (0.5 µg/mL) and anti-CD28 mAb (1 µg/mL) in the presence of sGARP (1 µg/mL). Foxp3 expression was analyzed on day 3 by flow cytometry. Dot blots show one representative result for each T-cell subset (upper part). Diagrams (lower part) display summarized data of 7 independent experiments. Data represent the mean ± SEM of experiments (n = 7, **P < .01, ***P < .001; n.s., not significant). (B) sGARP inhibits proliferation. Each T-cell subset was stimulated under the same conditions described in (A) in the presence or absence of sGARP (1 µg/mL). Proliferation was analyzed on day 12 by 3H-TdR incorporation. Bar diagrams show pooled data of 3 independent experiments (percentage of proliferation) in the presence of sGARP normalized to proliferation of cells without sGARP (n = 3, mean ± SEM, **P < .01; n.s., not significant). (C) Each T-cell subset was analyzed for CD62L and CCR7 expression by flow cytometry as described. Peripheral CD4+CD45RA+ T cells displayed a mixed population of CD62Lhigh and CD62Llow cells (gray represents isotype control). Dot blot shows one representative out of 8 experiments (n = 8). (D) TGF-βR and membrane-bound TGF-β (TGF-β) expression on resting and activated (16 hour) naïve CD4+ T cells and CD4+CD45RO+ T cells. Histograms show one representative experiment of 4 independent experiments. Numbers indicate mean fluorescence intensities (MFI) of TGF-βR (naïve resting: 51.9 ± 19, activated: 34.5 ± 6; CD4+CD45RO+ resting: 22.1 ± 5, activated: 27.8 ± 14) or TGF-β (naïve resting: 15.2 ± 9, activated: 27.0 ± 4; CD4+CD45RO+ resting: 5.13 ± 0.5, activated: 13.3 ± 7) expression are indicated. (E) CFSE-labeled naïve CD4+ T cells and CD4+CD45RO+ T cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence or absence of sGARP (1 µg/mL). Cytokines were analyzed on day 10 upon restimulation. Dot blots show one representative result of 5. Diagrams show summarized data of 5 independent experiments.
Because the impact of sGARP seemed to be most pronounced in naïve CD4+ T cells, with a gradual reduction correlated to the differentiation state of the T-cell populations analyzed, we investigated the populations of naïve CD4+ T cells and CD4+CD45RA+ and CD4+CD45RO+ T cells in more detail. Interestingly we found a striking difference in their expression of CD62L and CCR7. Whereas cord blood–derived CD4+ T cells displayed a homogenous population of CD62LhighCCR7+ cells, peripheral CD4+CD45RA+ T cells showed a mixed population of CD62Lhigh and CD62Llow cells (Figure 1C). Moreover, CD4+CD45RO+ T cells were CCR7-negative and only the minority expressed CD62L. Sorting of CD4+CD45RA+CCR7+ cells into CD62Lhigh- and CD62Llow-expressing cells indicated that only CD62Lhigh-expressing cells significantly upregulated Foxp3 in the presence of sGARP (supplemental Figure 2B). Nevertheless, Foxp3 induction in the presence of sGARP at day 3 after cell sort was less pronounced compared with the unsorted population as a result of the sorting procedure.
Based on these findings, following experiments were focused on the influence of sGARP on CD62LhighCCR7+ naïve CD4+ T cells compared with CD62Llow/–CCR7– CD4+CD45RO+ T cells. We detected significant differences of TGF-βR expression and membrane-bound TGF-β on naïve CD4+ T cells compared with differentiated CD4+CD45RO+ T cells (Figure 1D).
Taken together, these results suggest that the effect of sGARP is most pronounced on naïve T cells and decreases with the differentiation state of T cells, as was recently shown for modulating the Treg-inducing effects of TGF-β.25
We further investigated the functional impact of sGARP on effector-cell differentiation in more detail. We analyzed the acquisition of cytokine production by differentiating T cells in the presence or absence of sGARP by comparing naïve cord blood–derived CD4+ T cells with differentiated peripheral CD4+CD45RO+ T cells. The addition of sGARP repressed IFN-γ- and IL-2-production in naïve CD4+ T cells, but not in CD4+CD45RO+ T cells (Figure 1E). In addition, we excluded Fc domain–mediated effects in sGARP regulatory function. As exemplified on the level of Foxp3 upregulation, sGARP protein from 3 independent sources with and without different Fc domains featured comparable activity (supplemental Figure 2C).
Taken together, sGARP differentially modulates naïve CD4+ T cells and their maturation compared with already differentiated effector T cells by converting them into a peripheral Treg-like phenotype.26 Already differentiated effector T cells cannot be affected by sGARP.
sGARP cooperates with TGF-β in modulating T-cell differentiation
In accordance with its function as a receptor for latent TGF-β,13-15,23,24,27 we next assessed the role of TGF-β in the regulatory activity of sGARP. TGF-β production in naïve CD4+ T cells in the presence of sGARP was analyzed on protein and mRNA levels (Figure 2A). We observed a significant increase of TGF-β production in the presence of sGARP compared with untreated or control protein-treated cells. However, in conjunction with the inability of naïve human CD4+ T cells to generate the biologically active form, only latent TGF-β could be detected in response to sGARP. In addition, because we cannot exclude culture medium containing TGF-β binding to sGARP, we performed qRT-PCR of sGARP-treated T-cell populations. Herein, we revealed that the addition of sGARP to naïve CD4+ T cells indeed resulted in a strong induction of TGF-β on mRNA levels.
sGARP protein induces TGF-β production in naïve CD4+ T cells and signals through the TGF-β receptor. (A) Induction of TGF-β in naïve CD4+ T cells by sGARP. Cells were stimulated with anti-CD3 and anti-CD28 mAb with and without sGARP (1 µg/mL) or control protein. After 16 hours, supernatants were analyzed for TGF-β by enzyme-linked immunosorbent assay. TGF-β mRNA expression was analyzed 16 hours after stimulation normalized to EF1α. Relative expression of TGF-β mRNA is shown in percent normalized to untreated cells. Bar diagrams represent pooled data of 4 independent experiments (mean ± SEM, *P < .05). (B-C) sGARP binds to the TGF-β receptor. (B) CFSE-labeled naïve T cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence of sGARP (1 µg/mL) or TGF-β (1 ng/mL) with and without blocking anti–TGF-βRII mAb (10 µg/mL) or were left untreated. Numbers indicate percentage of Foxp3+ cells 24 hours after stimulation. Dot blots of one representative experiment of 8 are shown (left). Bar diagrams (right) show pooled data (mean ± SEM, *P < .05, **P < .01). (C) GARP-mediated cytokine repression. Naïve T cells were stimulated as indicated, and intracellular cytokine staining was performed 10 days after primary stimulation. Numbers indicate percentage of cytokine-producing cells. Dot blots of one representative experiment are shown in the upper part. The bar diagrams (lower part) show pooled data (n = 8, mean ± SEM, *P < .05). (D) Mononuclear cord blood cells were stimulated with 0.5 µg/mL anti-CD3 mAb with and without sGARP in the presence or absence of blocking anti–TGF-βRII mAb. Smad2/3 phosphorylation in gated CD4+ T cells is shown 30 minutes after stimulation. A histogram of one representative experiment of 3 is shown. The bar diagram shows pooled data of 3 independent experiments (n = 3, mean ± SEM). (E) sGARP-mediated Treg induction. Naïve CD4+ T cells (Donor 1) were stimulated with anti-CD3 and anti-CD28 mAb and cultured in the presence and absence of sGARP (1 µg/mL) for 7 days before being used as suppressor cells in a coculture experiment. Allogeneic CD4+ T cells (Donor 2) simultaneously stimulated for 7 days with anti-CD3 and anti-CD28 mAb served as responder cells. Cocultures (105 responder cells, Donor 2) plus titrated suppressor cells (Donor 1) were stimulated with 0.5 µg/mL anti-CD3 mAb in the presence of 3 × 105 irradiated T cell–depleted PBMC feeder cells (Donor 3). As a control, CD4+ T cells from Donor 1 cultured without sGARP were cocultured at a ratio of 1:1 with CD4+ T cells of Donor 2. Proliferation was determined on day 4 by a 16-hour [3H] TdR pulse. Results show the pooled data of 3 independent experiments (mean ± SEM).
sGARP protein induces TGF-β production in naïve CD4+ T cells and signals through the TGF-β receptor. (A) Induction of TGF-β in naïve CD4+ T cells by sGARP. Cells were stimulated with anti-CD3 and anti-CD28 mAb with and without sGARP (1 µg/mL) or control protein. After 16 hours, supernatants were analyzed for TGF-β by enzyme-linked immunosorbent assay. TGF-β mRNA expression was analyzed 16 hours after stimulation normalized to EF1α. Relative expression of TGF-β mRNA is shown in percent normalized to untreated cells. Bar diagrams represent pooled data of 4 independent experiments (mean ± SEM, *P < .05). (B-C) sGARP binds to the TGF-β receptor. (B) CFSE-labeled naïve T cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence of sGARP (1 µg/mL) or TGF-β (1 ng/mL) with and without blocking anti–TGF-βRII mAb (10 µg/mL) or were left untreated. Numbers indicate percentage of Foxp3+ cells 24 hours after stimulation. Dot blots of one representative experiment of 8 are shown (left). Bar diagrams (right) show pooled data (mean ± SEM, *P < .05, **P < .01). (C) GARP-mediated cytokine repression. Naïve T cells were stimulated as indicated, and intracellular cytokine staining was performed 10 days after primary stimulation. Numbers indicate percentage of cytokine-producing cells. Dot blots of one representative experiment are shown in the upper part. The bar diagrams (lower part) show pooled data (n = 8, mean ± SEM, *P < .05). (D) Mononuclear cord blood cells were stimulated with 0.5 µg/mL anti-CD3 mAb with and without sGARP in the presence or absence of blocking anti–TGF-βRII mAb. Smad2/3 phosphorylation in gated CD4+ T cells is shown 30 minutes after stimulation. A histogram of one representative experiment of 3 is shown. The bar diagram shows pooled data of 3 independent experiments (n = 3, mean ± SEM). (E) sGARP-mediated Treg induction. Naïve CD4+ T cells (Donor 1) were stimulated with anti-CD3 and anti-CD28 mAb and cultured in the presence and absence of sGARP (1 µg/mL) for 7 days before being used as suppressor cells in a coculture experiment. Allogeneic CD4+ T cells (Donor 2) simultaneously stimulated for 7 days with anti-CD3 and anti-CD28 mAb served as responder cells. Cocultures (105 responder cells, Donor 2) plus titrated suppressor cells (Donor 1) were stimulated with 0.5 µg/mL anti-CD3 mAb in the presence of 3 × 105 irradiated T cell–depleted PBMC feeder cells (Donor 3). As a control, CD4+ T cells from Donor 1 cultured without sGARP were cocultured at a ratio of 1:1 with CD4+ T cells of Donor 2. Proliferation was determined on day 4 by a 16-hour [3H] TdR pulse. Results show the pooled data of 3 independent experiments (mean ± SEM).
Because we could detect differences in Foxp3 induction as early as on day 1 (supplemental Figure 2A), we next investigated early signaling events in the presence of sGARP. Functionally, sGARP and biologically active TGF-β revealed comparable effects in regard to Foxp3 induction (Figure 2B) and cytokine repression (Figure 2C). Interestingly, the blockade of TGF-β signaling equally diminished sGARP and TGF-β effects on Foxp3 regulation and IL-2 production. These results demonstrate that the T-cell modulating impact of sGARP is at least in part associated with TGF-β signaling. This is further strengthened by the finding that sGARP induced phosphorylation of TGF-βR downstream targets such as Smad2/3 (Figure 2D). Surprisingly, TGF-β receptor blockade neither restored TGF-β– nor sGARP-repressed IFN-γ production, demonstrating differences in the sensitivity of IFN-γ and IL-2 regulation.
Because Foxp3 is critical for the differentiation and function of Treg we asked whether Foxp3 upregulation in naïve CD4+ T cells by sGARP was associated with the acquisition of anergy and suppressive activity. Cord blood–derived naïve CD4+ T cells repetitively stimulated in the presence of sGARP showed an anergic state and developed into induced Treg that suppressed activation and proliferation of allogeneic CD4+ T cells in coculture. Naïve CD4+ T cells cultured without sGARP had no suppressive capacity (Figure 2E). These results further confirm the findings that sGARP modulates the maturation process of naïve CD4+ T cells and promotes their differentiation into induced Treg.
In vivo application of sGARP prevents lethal GVHD induction
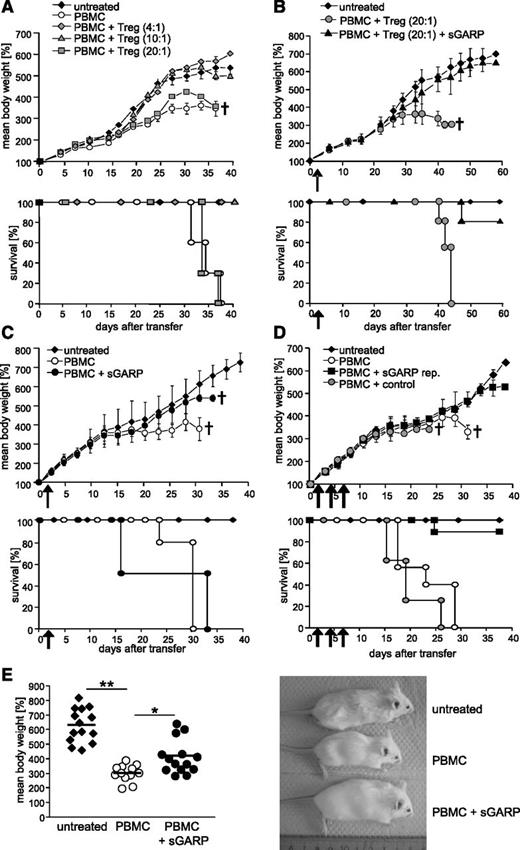
Suppression of CD4+ T effector cell differentiation and induction of induced Treg suggested an immunoregulatory potential of sGARP in inflammatory T-cell responses. To study the impact of sGARP on human T cell–mediated destructive inflammation in vivo, we used a model of xenogeneic GVHD in immunodeficient mice.22 Upon intraperitoneal engraftment with human PBMC, newborn Rag2−/−γc−/− mice developed lethal GVHD characterized by decelerated growth and reduced body weight and died within 2 months. This massive immune response is based on aggressive human T-cell reactivity against murine tissue because injection of T cell–depleted PBMC did not result in GVHD symptoms (supplemental Figure 3A). Transfer of increased numbers of human Treg suppresses disease onset in a dose-dependent manner19 (Figure 3A). Interestingly, the singular administration of sGARP in the presence of a nonprotective number of Treg (PBMC:Treg ratio 20:1) prevented development of GVHD in the majority of animals (Figure 3B), signifying that sGARP can synergize with Treg activity in repression of an inflammatory human T-cell response. More importantly, whereas a single injection of sGARP alone, in the absence of Treg, did not result in protection from GVHD (Figure 3C), a repetitive administration of sGARP prevented GVHD onset, even without additional Treg transfer (Figure 3D-E) demonstrating the potential of sGARP to prevent aggressive T-cell activity in vivo.
sGARP prevents GVHD development. (A) Protection from GVHD by human Treg. Newborn Rag2−/−γc−/− mice were transferred with 5 × 106 human PBMC with or without titrated numbers of Treg. Untreated mice served as controls. Percent mean body weight is based on initial weight. Upper panel: One representative experiment (3 mice per group, n = 3) is shown. Percent mean body weight data ± SEM. Lower panel: Kaplan-Meier plots of 9 mice per individual group. (B) Protection by a single GARP injection in the presence of Treg. 5 × 106 PBMC were intraperitoneally injected into newborn Rag2−/−γc−/− mice containing a nonprotective number of Treg (PBMC:Treg ratio 20:1) with and without 5 µg sGARP. Untreated mice served as controls. Upper panel: One representative experiment (4 mice per group, n = 3) is shown. Percent mean body weight data ± SEM. Lower panel: Kaplan-Meier plots of 12 mice per individual group. (C) Single injection of sGARP did not protect against GVHD. PBMC were injected with and without 5 µg sGARP into newborn Rag2−/−γc−/− mice. Untreated mice served as controls. Upper panel: One representative experiment (5 mice per group, n = 2) is shown. Percent mean body weight data ± SEM. Lower panel: Kaplan-Meier plots with 10 mice per individual group. (D) Protection by repetitive injection of sGARP. PBMC were injected with 5 µg sGARP or control protein into newborn Rag2−/−γc−/− mice. sGARP was administered 3 times every other day (sGARP rep.). Untreated mice served as controls. Upper panel: One typical experiment (6 mice per group, n = 4) is shown. Percent mean body weight data ± SEM. Lower panel: Kaplan-Meier plots with 24 mice per individual group. (E) Mice 27 days after transfer of PBMC with and without repetitive injection of sGARP compared with untreated mice (14 mice per group, mean ± SEM, *P < .05, **P < .01).
sGARP prevents GVHD development. (A) Protection from GVHD by human Treg. Newborn Rag2−/−γc−/− mice were transferred with 5 × 106 human PBMC with or without titrated numbers of Treg. Untreated mice served as controls. Percent mean body weight is based on initial weight. Upper panel: One representative experiment (3 mice per group, n = 3) is shown. Percent mean body weight data ± SEM. Lower panel: Kaplan-Meier plots of 9 mice per individual group. (B) Protection by a single GARP injection in the presence of Treg. 5 × 106 PBMC were intraperitoneally injected into newborn Rag2−/−γc−/− mice containing a nonprotective number of Treg (PBMC:Treg ratio 20:1) with and without 5 µg sGARP. Untreated mice served as controls. Upper panel: One representative experiment (4 mice per group, n = 3) is shown. Percent mean body weight data ± SEM. Lower panel: Kaplan-Meier plots of 12 mice per individual group. (C) Single injection of sGARP did not protect against GVHD. PBMC were injected with and without 5 µg sGARP into newborn Rag2−/−γc−/− mice. Untreated mice served as controls. Upper panel: One representative experiment (5 mice per group, n = 2) is shown. Percent mean body weight data ± SEM. Lower panel: Kaplan-Meier plots with 10 mice per individual group. (D) Protection by repetitive injection of sGARP. PBMC were injected with 5 µg sGARP or control protein into newborn Rag2−/−γc−/− mice. sGARP was administered 3 times every other day (sGARP rep.). Untreated mice served as controls. Upper panel: One typical experiment (6 mice per group, n = 4) is shown. Percent mean body weight data ± SEM. Lower panel: Kaplan-Meier plots with 24 mice per individual group. (E) Mice 27 days after transfer of PBMC with and without repetitive injection of sGARP compared with untreated mice (14 mice per group, mean ± SEM, *P < .05, **P < .01).
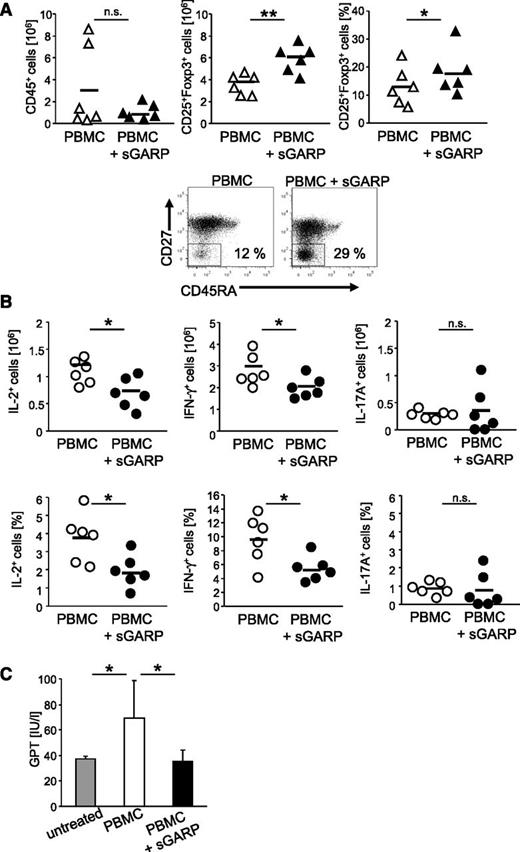
To further support the antiinflammatory effect of sGARP in this GVHD model, the ex vivo analyses of T effector cells revealed a reduced cellular infiltration, associated with a significant increase in CD4+CD25+Foxp3+ T cells in sGARP-treated animals (Figure 4A, upper panel), suggesting a Treg induction also in vivo. In addition, a strong shift in the activation state of CD4+ T effector cells from central memory (CD45RO+CD27+) to effector memory cells (CD45RO+CD27–) (Figure 4A, lower panel), associated with significantly decreased numbers of IL-2 and IFN-γ–producing T cells, was observed ex vivo. However, we observed only few IL-17–producing T cells, and their ratio remained unchanged upon sGARP treatment (Figure 4B).
Administration of sGARP decreases effector cytokine production in vivo. Rag2−/−γc−/− mice (6 mice per group) were injected with 5 × 106 PBMC and were treated either 3 times with 5 µg sGARP every other day starting from day 1 or were left untreated. (A) On day 7 after treatment initiation, human T cells were reisolated from the spleens. sGARP administration decreased the infiltration with human CD45+ cells while increasing the frequency of CD4+CD25+Foxp3+ T cells (relative ratio and absolute Treg number, horizontal bars represent mean values, n = 6, **P < .01, *P < .05; upper panel). sGARP shifted CD4+ T cells from central (CD45RO+CD27+) to effector memory cells (CD45RO+CD27–). Dot blots (lower panel) show one representative result of 3 independent experiments (17.6% ± 5.9 and 28.9% ± 3.7 in the absence and presence of sGARP, respectively, n = 3). (B) sGARP reduces the number of IL-2 and IFN-γ–producing CD4+ T cells without affecting IL-17 production. Intracellular cytokine staining of reisolated human T cells on day 7 after treatment initiation was performed. Numbers of cytokine-producing cells (relative ratio and absolute numbers) in 6 individual mice (mean ± SEM, *P < .05) are shown. (C) GPT serum levels of mice from different treatment groups analyzed 20 days after PBMC transfer (8 mice per group, mean ± SEM, *P < .05).
Administration of sGARP decreases effector cytokine production in vivo. Rag2−/−γc−/− mice (6 mice per group) were injected with 5 × 106 PBMC and were treated either 3 times with 5 µg sGARP every other day starting from day 1 or were left untreated. (A) On day 7 after treatment initiation, human T cells were reisolated from the spleens. sGARP administration decreased the infiltration with human CD45+ cells while increasing the frequency of CD4+CD25+Foxp3+ T cells (relative ratio and absolute Treg number, horizontal bars represent mean values, n = 6, **P < .01, *P < .05; upper panel). sGARP shifted CD4+ T cells from central (CD45RO+CD27+) to effector memory cells (CD45RO+CD27–). Dot blots (lower panel) show one representative result of 3 independent experiments (17.6% ± 5.9 and 28.9% ± 3.7 in the absence and presence of sGARP, respectively, n = 3). (B) sGARP reduces the number of IL-2 and IFN-γ–producing CD4+ T cells without affecting IL-17 production. Intracellular cytokine staining of reisolated human T cells on day 7 after treatment initiation was performed. Numbers of cytokine-producing cells (relative ratio and absolute numbers) in 6 individual mice (mean ± SEM, *P < .05) are shown. (C) GPT serum levels of mice from different treatment groups analyzed 20 days after PBMC transfer (8 mice per group, mean ± SEM, *P < .05).
To examine the development of clinical symptoms of GVHD, we analyzed the concentration of GPT levels as an indicator of GVHD-mediated liver injury in the serum of mice at an early time point.22 Herein, mice engrafted with PBMC showed a significant increase in GPT levels after onset of disease, whereas repetitive administration of sGARP prevented GVHD-mediated liver inflammation, as displayed by normal GPT levels (Figure 4C). In addition, GVHD induction was associated with splenomegaly (supplemental Figure 3B) as a result of infiltration with human effector T cells. Administration of sGARP prevented splenomegaly, reduced cellular infiltration, and inhibited effector cytokine production of CD4+ T cells, associated with enhanced ratios of CD25+Foxp3+ cells (supplemental Figure 3B, Figure 4A-B).
Taken together, these in vivo observations reveal the potency of sGARP as an antiinflammatory immunosuppressive drug that prevents the induction of destructive T-cell responses.
sGARP promotes Th17 differentiation under inflammatory conditions
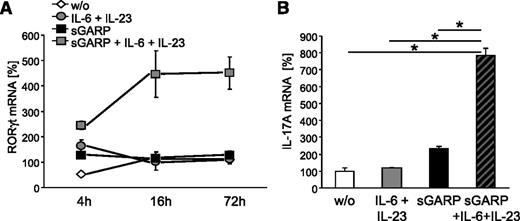
Our results demonstrated that sGARP mediates immunosuppressive effects under nonpolarizing conditions. These findings are similar to results described for the modulating properties of TGF-β.28,29 However, under inflammatory conditions TGF-β is involved in the promotion of Th17 differentiation.28 To determine the contribution of sGARP to induction of Th17 cells, we cultured naïve CD4+ T cells in the presence of sGARP and IL-6 plus IL-23. This resulted in a significant and long-lasting induction of RORγt mRNA (Figure 5A), the key transcription factor of Th17.30,31 Furthermore, sGARP together with IL-6 and IL-23 led to a significant IL-17A induction (Figure 5B). These findings indicate that sGARP facilitates the differentiation of naïve CD4+ T cells into Th17 cells under inflammatory conditions.
sGARP induces RORγt and IL-17 expression in naïve CD4+ T cells. Naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence or absence of sGARP (1 µg/mL) and with and without a combination of IL-6 (200 IU/mL) and IL-23 (100 ng/mL). RORγt mRNA expression 4 hours, 16 hours, and 72 hours (A) and IL-17A mRNA expression 72 hours (B) after stimulation was analyzed and normalized to EF1α and untreated cells calculated by δ-δ cycle threshold method. Results are representative of 3 independent experiments (mean ± SEM, *P < .05, n = 3).
sGARP induces RORγt and IL-17 expression in naïve CD4+ T cells. Naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence or absence of sGARP (1 µg/mL) and with and without a combination of IL-6 (200 IU/mL) and IL-23 (100 ng/mL). RORγt mRNA expression 4 hours, 16 hours, and 72 hours (A) and IL-17A mRNA expression 72 hours (B) after stimulation was analyzed and normalized to EF1α and untreated cells calculated by δ-δ cycle threshold method. Results are representative of 3 independent experiments (mean ± SEM, *P < .05, n = 3).
Discussion
In this study, we demonstrate that sGARP exerts potent immunoregulatory activity by inhibiting the development of effector functions in naïve T cells, both in vitro and in vivo. The presence of sGARP in differentiation cultures of naïve CD4+ T cells resulted in upregulation of Foxp3, reduced proliferation and cytokine production, and facilitated the differentiation into induced Treg. Importantly, modulation of T-cell function by sGARP did not require membrane anchoring as previously proposed,16 showing also a Treg cell–independent function of this molecule. T effector cell suppression by sGARP and induction of Treg, as well as promotion of Th17 cells in presence of IL-6 and IL-23, demonstrated similarities to the well-known biological activity of TGF-β.32
A possible role of GARP expressed on the surface of activated Treg is to act as a receptor for the latent TGF-β/LAP complex,16 allowing Treg to deliver TGF-β to sites of inflammation. Even though GARP recently gained interest through its role in Treg suppression, it has been originally identified on the surface of platelets,22,27 which form a rich source of TGF-β and release it from intracellular stores early in inflammatory responses.33 Here we show that sGARP stimulates TGF-β production in activated T cells. Hence, in addition to its passive role as a TGF-β/LAP transporter,16 GARP induces TGF-β production and thus contributes to the mechanism of infectious tolerance.34
As discussed, GARP as a surface molecule on activated human Treg binds to latent TGF-β and LAP.4,14 Stockis et al verified this also for sGARP. Therefore, we postulate that sGARP forms a LAP/TGF-β/GARP complex that allows signal transduction through TGF-βR and favors the induction of Treg. sGARP function depends on TGF-βR signaling and is associated with Smad2/3 phosphorylation. TGF-β, as well as sGARP, inhibited IFN-γ production even when TGF-βR signaling was blocked, indicating that additional receptors and molecules may be involved. Although receptor-mediated binding is usually the major mechanism of early signaling events, TGF-β can bind through other proteins such as biglycan or heparan sulfate as well.35 We postulate that sGARP forms a LAP/TGF-β/GARP complex and uses TGF-β signaling pathways, including these alternative receptors, that might be involved in sGARP function.
Also comparable with TGF-β, in the presence of inflammatory cytokines IL-6/IL-23 sGARP is a potent enhancer of Th17 differentiation. These results are in agreement with a recent study in mice28 showing that membrane-bound GARP/TGF-β complex is involved in the induction of Th17 cells under inflammatory conditions. However, Th17 cells are not an essential Teff subset in this in vivo model, at least at early time points of GVHD induction. In contrast, our results suggest that IFN-γ–producing CD4+ T cells play a crucial role in this setting, in agreement with data published recently36 showing in humans no difference in IL-17–producing T cells in the blood of patients with acute GVHD but IFN-γ–producing cells as initiators of acute GVHD.
The use of TGF-β as an immunosuppressive drug has several limitations. Multiplicity of actions and the nearly ubiquitous expression of TGF-β and its receptors makes it difficult for a controlled use in vivo. Regulation of TGF-β activation is the crucial step in controlling its function. Thus, administration of sGARP is a possible way to control and limit TGF-β function because “enzymatic activity” of sGARP seems to be limited to early activation processes of naïve T cells.25 In addition, sGARP enhances the suppressive properties of activated thymus-derived Treg.
To identify the functional impact of GARP in vivo, we used a xenogeneic GVHD model in humanized mice, as described before.22 Acute GVHD is known to be a T cell–dependent disease36 whose incidence, onset and severity have been related inter alia to the level of donor T cell–derived TGF-β.37,38 Previously, we demonstrated that transfer of PBMC into newborn immunodeficient mice represents a robust and highly reproducible model and thus is an ideal tool to analyze T cell–regulating biologicals such as anti-CD4 mAb or gp120 in vivo. In this setting, a single application of sGARP did not affect the onset of GVHD in the absence of Treg, which is in contrast to the Treg activator gp120.22 However, coinjection of sGARP with nonprotective numbers of Treg inhibited GVHD onset by enhancing Treg activity significantly, and repetitive application of sGARP prevented disease onset completely, supporting the idea of sGARP as a potent Treg enhancer and inhibitor of T effector cell induction. Therapeutic application of sGARP in disease models such as allergy,39 asthma,40 or rheumatoid arthritis should be investigated in future studies.
One of the critical mechanisms preventing GVHD seems to be direct attenuation of IFN-γ–producing T effector cells.41-43 Whereas induced Treg have been described to be ineffective in preventing acute GVHD,43 possibly because of different antigen specificities, migratory properties, and localization of transferred iTreg vs Teff, systemic administration of sGARP may reach all tissues where T cells are activated and thus might block their differentiation into IFN-γ–producing T effector cells at very early time points.
Taken together, our results indicate a crucial role for GARP in modulation of peripheral T-cell activity and open the possibility to use sGARP as an immune modifier in the treatment of transplant rejection and inflammatory disorders such as autoimmunity and allergy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Hubo, K. Steinbrink, B. Trinschek, and W. Rist for discussion and critically reading of the manuscript. They are grateful to L. Paragnik, A. Balli, and P. Hoelter for their expert technical assistance, and J. Kirberg for providing Rag2−/−γc−/− mice.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) grant A2TR52 (H.J. and A.T.).
The study was partially funded by Boehringer Ingelheim. The funder was involved in study design and the decision to publish the results (and to make them publicly available).
Authorship
Contribution: S.A.H. performed research and analyzed data; H.F.S. designed and characterized sGARP and edited the manuscript; C.B. analyzed data and wrote the paper; A.C. performed research; F.J.S. designed research and analyzed data; A.T. performed research, analyzed data, and wrote the paper; and H.J. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: H.F.S. and F.J.S. are employees of Boehringer Ingelheim Pharma GmbH & Co. KG. The remaining authors declare no competing financial interests.
Correspondence: Helmut Jonuleit, Department of Dermatology, University Medical Center, Johannes Gutenberg-University Mainz, Langenbeckstr.1, 55131 Mainz, Germany; e-mail: helmut.jonuleit@unimedizin-mainz.de.
References
Author notes
S.A.H. and H.F.S. contributed equally to this study.
A.T. and H.J. are considered co-senior authors of this study.

![Figure 2. sGARP protein induces TGF-β production in naïve CD4+ T cells and signals through the TGF-β receptor. (A) Induction of TGF-β in naïve CD4+ T cells by sGARP. Cells were stimulated with anti-CD3 and anti-CD28 mAb with and without sGARP (1 µg/mL) or control protein. After 16 hours, supernatants were analyzed for TGF-β by enzyme-linked immunosorbent assay. TGF-β mRNA expression was analyzed 16 hours after stimulation normalized to EF1α. Relative expression of TGF-β mRNA is shown in percent normalized to untreated cells. Bar diagrams represent pooled data of 4 independent experiments (mean ± SEM, *P < .05). (B-C) sGARP binds to the TGF-β receptor. (B) CFSE-labeled naïve T cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence of sGARP (1 µg/mL) or TGF-β (1 ng/mL) with and without blocking anti–TGF-βRII mAb (10 µg/mL) or were left untreated. Numbers indicate percentage of Foxp3+ cells 24 hours after stimulation. Dot blots of one representative experiment of 8 are shown (left). Bar diagrams (right) show pooled data (mean ± SEM, *P < .05, **P < .01). (C) GARP-mediated cytokine repression. Naïve T cells were stimulated as indicated, and intracellular cytokine staining was performed 10 days after primary stimulation. Numbers indicate percentage of cytokine-producing cells. Dot blots of one representative experiment are shown in the upper part. The bar diagrams (lower part) show pooled data (n = 8, mean ± SEM, *P < .05). (D) Mononuclear cord blood cells were stimulated with 0.5 µg/mL anti-CD3 mAb with and without sGARP in the presence or absence of blocking anti–TGF-βRII mAb. Smad2/3 phosphorylation in gated CD4+ T cells is shown 30 minutes after stimulation. A histogram of one representative experiment of 3 is shown. The bar diagram shows pooled data of 3 independent experiments (n = 3, mean ± SEM). (E) sGARP-mediated Treg induction. Naïve CD4+ T cells (Donor 1) were stimulated with anti-CD3 and anti-CD28 mAb and cultured in the presence and absence of sGARP (1 µg/mL) for 7 days before being used as suppressor cells in a coculture experiment. Allogeneic CD4+ T cells (Donor 2) simultaneously stimulated for 7 days with anti-CD3 and anti-CD28 mAb served as responder cells. Cocultures (105 responder cells, Donor 2) plus titrated suppressor cells (Donor 1) were stimulated with 0.5 µg/mL anti-CD3 mAb in the presence of 3 × 105 irradiated T cell–depleted PBMC feeder cells (Donor 3). As a control, CD4+ T cells from Donor 1 cultured without sGARP were cocultured at a ratio of 1:1 with CD4+ T cells of Donor 2. Proliferation was determined on day 4 by a 16-hour [3H] TdR pulse. Results show the pooled data of 3 independent experiments (mean ± SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/7/10.1182_blood-2012-12-474478/4/m_1182f2.jpeg?Expires=1769835429&Signature=bOowmffH3FATDG0ZgxYfFm1zYxQsywPb-vHwjbgywCqG88slsrbmHNf6h04BvPGQTk0Ay3a469l2fcZTCp3~hpUzv3sua26lmBEmjabEbEdjRAQ~ko1aW7mjZ1V2UMIXLvLCis44D974zUirwc7K9AEOqi-09b37aYu7JIRqBxNCPnr~90bZ1vF4h27Xh7CFhM6dkhNuwgJquNzCSOZIZHsK0AMQTcmRmhe1t3YqKvfbbiykfcnu3oYbqKDQVASPUyw2MsGlWl02qPnh23n9oHnTKsEFb4m2asNHjp7U5tyuK~uXzxA8q8fnoT7DWU3-6v~iP2Tr03xy6bGhJilyhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal